Effects of Clostridium butyricum on Production Performance and Bone Development of Laying Hens
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Experimental Design
2.3. Laying Performance
2.4. Egg Quality
2.5. Bone
2.6. Organ Index
2.7. Bone RNA Extraction
2.8. Real-Time PCR Analyses
2.9. Statistical Analysis
3. Results
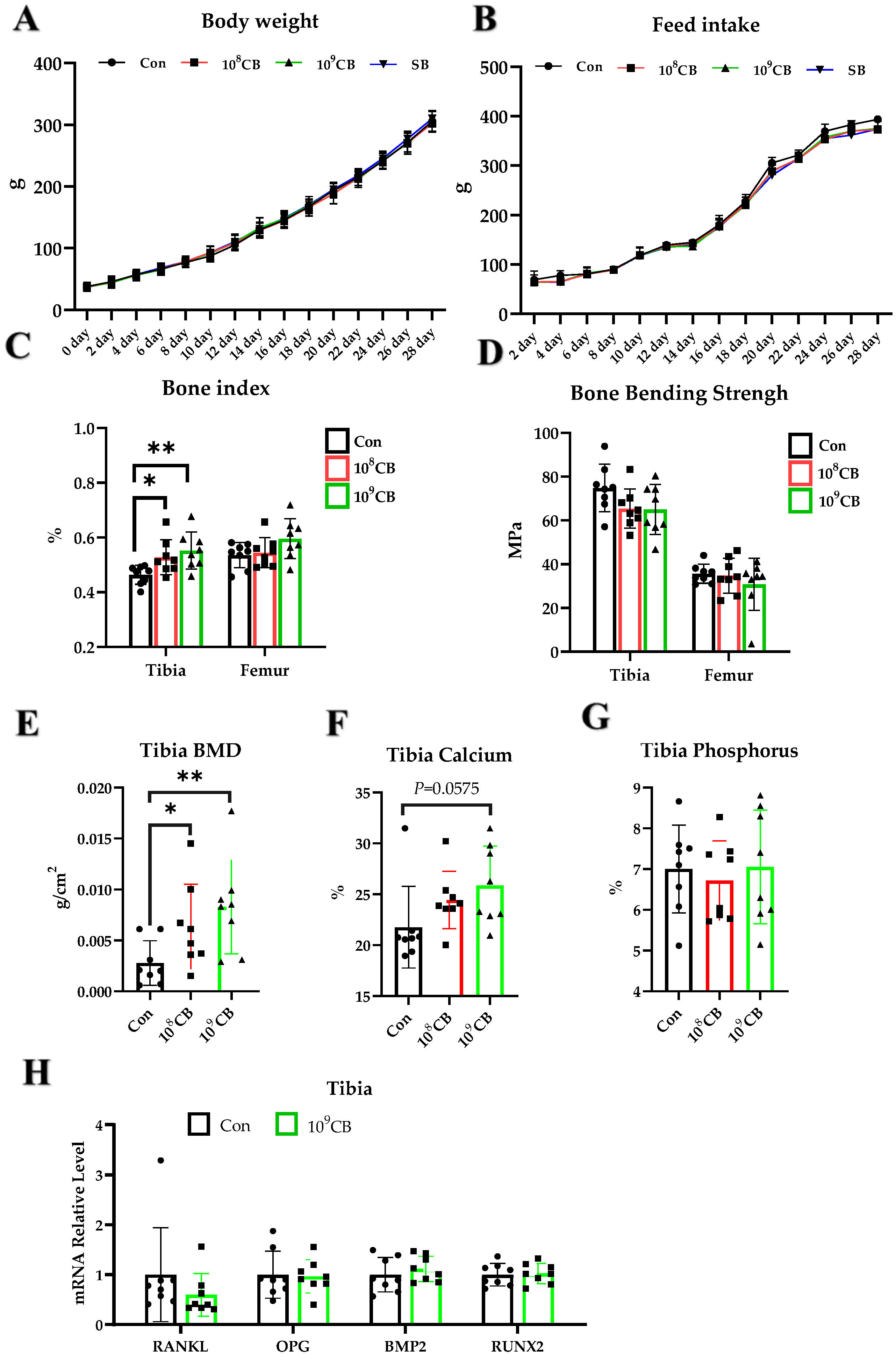
3.1. Effect of CB on Green-Shell Laying Hens
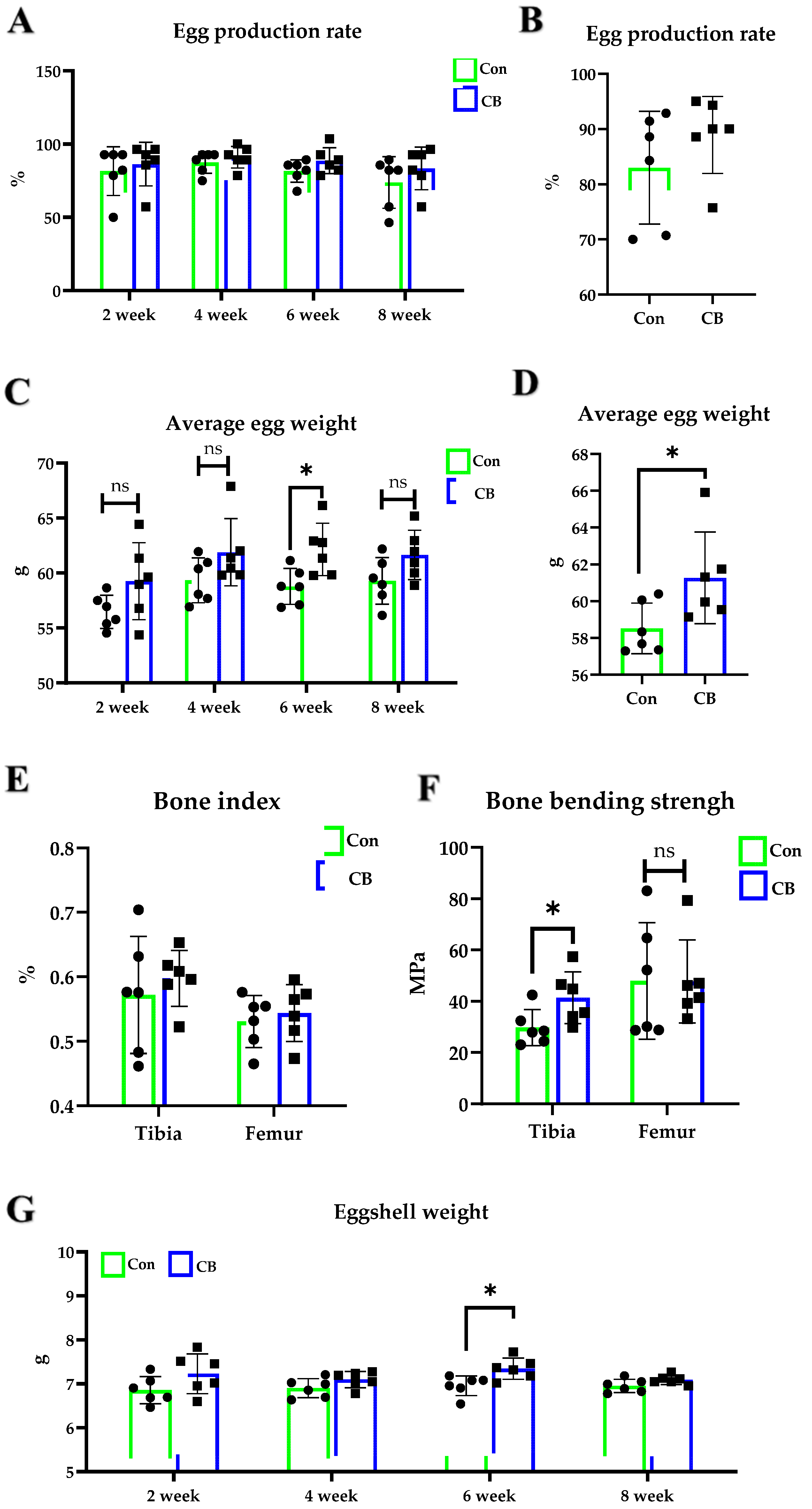
3.2. Effect of CB on Luhua Laying Hens
3.3. Effect of CB on Hy-Line Brown Laying Hens
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, H.; Lu, Y.; Li, D.; Yan, C.; Jiang, Y.; Hu, Z.; Zhang, Z.; Du, R.; Zhao, X.; Zhang, Y.; et al. Probiotic mediated intestinal microbiota and improved performance, egg quality and ovarian immune function of laying hens at different laying stage. Front. Microbiol. 2023, 14, 1041072. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Chen, B.; Zhu, Q.; Xu, Z.; Ning, C.; Yin, H.; Wang, Y.; Zhao, X.; Fan, X.; Yang, M.; et al. Transcriptome analysis reveals differentially expressed genes associated with high rates of egg production in chicken hypothalamic-pituitary-ovarian axis. Sci. Rep. 2020, 10, 5976. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Lin, X.; Zhao, J.; Wang, X.; Jiao, H.; Li, H.; Sun, S.; Lin, H. High frequency vaccination-induced immune stress reduces bone strength with the involvement of activated osteoclastogenesis in layer pullets. Poult. Sci. 2020, 99, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Zhang, X.; Hao, G.; Lin, H.; Sun, S. Clostridium butyricum Can Promote Bone Development by Regulating Lymphocyte Function in Layer Pullets. Int. J. Mol. Sci. 2023, 24, 1457. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Latif, M.A.; Abd El-Hack, M.E.; Swelum, A.A.; Saadeldin, I.M.; Elbestawy, A.R.; Shewita, R.S.; Ba-Awadh, H.A.; Alowaimer, A.N.; Abd El-Hamid, H.S. Single and Combined Effects of Clostridium butyricum and Saccharomyces cerevisiae on Growth Indices, Intestinal Health, and Immunity of Broilers. Animals 2018, 8, 184. [Google Scholar] [CrossRef]
- Liao, X.; Ma, G.; Cai, J.; Fu, Y.; Yan, X.; Wei, X.; Zhang, R. Effects ofClostridium butyricum on growth performance, antioxidation, and immune function of broilers. Poult. Sci. 2015, 94, 662–667. [Google Scholar] [CrossRef]
- Yu, Y.; Li, Q.; Zhang, H.; Wu, Y.; Zhang, R.; Yue, M.; Yang, C.; Cao, G. Clostridium butyricum alone or combined with 1, 25-dihydroxyvitamin D3 improved early-stage broiler health by modulating intestinal flora. J. Appl. Microbiol. 2022, 132, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xu, B.; Wang, L.; Sun, Q.; Deng, W.; Wei, F.; Ma, H.; Fu, C.; Wang, G.; Li, S. Effects of Clostridium butyricum on Growth Performance, Gut Microbiota and Intestinal Barrier Function of Broilers. Front. Microbiol. 2021, 12, 777456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yang, X.; Guo, Y.; Long, F. Effects of dietary lipids and Clostridium butyricum on the performance and the digestive tract of broiler chickens. Arch. Anim. Nutr. 2011, 65, 329–339. [Google Scholar] [CrossRef]
- Whitehead, C.C. Overview of bone biology in the egg-laying hen. Poult. Sci. 2004, 83, 193–199. [Google Scholar] [CrossRef]
- Kerschnitzki, M.; Zander, T.; Zaslansky, P.; Fratzl, P.; Shahar, R.; Wagermaier, W. Rapid alterations of avian medullary bone material during the daily egg-laying cycle. Bone 2014, 69, 109–117. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, M.; Zhang, Y.; Yuan, X.; Zhou, M.; Xu, X.; Zhang, T.; Song, J. Clostridium butyricum MIYAIRI 588 alleviates periodontal bone loss in mice with diabetes mellitus. Ann. N. Y. Acad. Sci. 2023, 1529, 84–100. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.L.; Lin, Y.Y.; Liu, S.C.; Tsai, Y.S.; Lin, S.W.; Chen, Y.L.; Chen, C.C.; Ko, C.Y.; Chen, H.T.; Chen, W.C.; et al. Oral Administration of Clostridium butyricum GKB7 Ameliorates Signs of Osteoarthritis in Rats. Cells 2022, 11, 2169. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, L.T.; Rodriguez, M.L.; Alzueta, C.; Rebole, A.; Trevino, J. Effect of inulin on growth performance, intestinal tract sizes, mineral retention and tibial bone mineralisation in broiler chickens. Br. Poult. Sci. 2009, 50, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Regulation of Proliferation, Differentiation and Functions of Osteoblasts by Runx2. Int. J. Mol. Sci. 2019, 20, 1694. [Google Scholar] [CrossRef] [PubMed]
- Udagawa, N.; Koide, M.; Nakamura, M.; Nakamichi, Y.; Yamashita, T.; Uehara, S.; Kobayashi, Y.; Furuya, Y.; Yasuda, H.; Fukuda, C.; et al. Osteoclast differentiation by RANKL and OPG signaling pathways. J. Bone Miner. Metab. 2021, 39, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Lin, X.; Gou, Z.; Fan, Q.; Jiang, S. Effects of Clostridium butyricum, Sodium Butyrate, and Butyric Acid Glycerides on the Reproductive Performance, Egg Quality, Intestinal Health, and Offspring Performance of Yellow-Feathered Breeder Hens. Front. Microbiol. 2021, 12, 657542. [Google Scholar] [CrossRef] [PubMed]
- Obianwuna, U.E.; Qiu, K.; Wang, J.; Zhang, H.J.; Qi, G.H.; Huang, L.L.; Wu, S.G. Effects of dietary Clostridium butyricum and fructooligosaccharides, alone or in combination, on performance, egg quality, amino acid digestibility, jejunal morphology, immune function, and antioxidant capacity of laying hens. Front. Microbiol. 2023, 14, 1125897. [Google Scholar] [CrossRef] [PubMed]
- Khogali, M.K.; Wen, K.; Jauregui, D.; Malik, H.E.E.; Liu, L.; Zhao, M.; Gong, D.; Geng, T. Probiotics-induced Changes in Intestinal Structure and Gut Microbiota Are Associated with Reduced Rate of Pimpled Eggs in the Late Laying Period of Hens. J. Poult. Sci. 2022, 59, 206–222. [Google Scholar] [CrossRef]
- Lv, J.; Guo, L.; Chen, B.; Hao, K.; Ma, H.; Liu, Y.; Min, Y. Effects of different probiotic fermented feeds on production performance and intestinal health of laying hens. Poult. Sci. 2022, 101, 101570. [Google Scholar] [CrossRef]
- Gunnarsson, S.; Yngvesson, J.; Keeling, L.J.; Forkman, B. Rearing without early access to perches impairs the spatial skills of laying hens. Appl. Anim. Behav. Sci. 2000, 67, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Bishop, S.C.; Fleming, R.H.; McCormack, H.A.; Flock, D.K.; Whitehead, C.C. Inheritance of bone characteristics affecting osteoporosis in laying hens. Br. Poult. Sci. 2000, 41, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Navarro, A.B.; McCormack, H.M.; Fleming, R.H.; Alvarez-Lloret, P.; Romero-Pastor, J.; Dominguez-Gasca, N.; Prozorov, T.; Dunn, I.C. Influence of physical activity on tibial bone material properties in laying hens. J. Struct. Biol. 2018, 201, 36–45. [Google Scholar] [CrossRef] [PubMed]

| Green-Shell Laying Hens | |
| Items | Composition, % |
| Ingredients | |
| Corn | 66 |
| Soybean meal | 29 |
| Limestone | 0 |
| NaCl | 0.3 |
| Choline chloride (50%) | 0.1 |
| Premix 1 | 4.60 |
| Total | 100 |
| Nutrient levels 2 | |
| Metabolizable energy (Kcal/kg) | 2900 |
| Crude protein (%) | 17.7 |
| Lysine (%) | 0.94 |
| Methionine (%) | 0.61 |
| Calcium (%) | 0.7 |
| Available phosphorus (%) | 0.47 |
| Luhua Laying Hens | |
| Items | Composition, % |
| Ingredients | |
| Corn | 65 |
| Soybean meal | 23 |
| Limestone | 7 |
| NaCl | 0.3 |
| Choline chloride (50%) | 0.1 |
| Premix 1 | 4.60 |
| Total | 100 |
| Nutrient levels 2 | |
| Metabolizable energy (Kcal/kg) | 2700 |
| Crude protein (%) | 15.0 |
| Lysine (%) | 0.77 |
| Methionine (%) | 0.53 |
| Calcium (%) | 3.20 |
| Available phosphorus (%) | 0.45 |
| Hy-line Brown Laying Hens | |
| Items | Composition, % |
| Ingredients | |
| Corn | 63 |
| Soybean meal | 24 |
| Limestone | 8 |
| NaCl | 0.3 |
| Choline chloride (50%) | 0.1 |
| Premix 1 | 4.60 |
| Total | 100 |
| Nutrient levels 2 | |
| Metabolizable energy (Kcal/kg) | 2700 |
| Crude protein (%) | 15.3 |
| Lysine (%) | 0.79 |
| Methionine (%) | 0.53 |
| Calcium (%) | 3.50 |
| Available phosphorus (%) | 0.45 |
| Gene Name | Genbank Number | Primer Position | Primer Sequences (5′→3′) |
|---|---|---|---|
| RANKL | NM001083361.2 | Forward | TGTTGGCTCTGATGCTTGTC |
| Reverse | TCCTGCTTCTGGCTCTCAAT | ||
| OPG | DQ098013.1 | Forward | CGCTTGTGCTCTTGGACATT |
| Reverse | GCTGCTTTACGTAGCTCCCA | ||
| BMP2 | NM001398170.1 | Forward | CCTTCGGAAGACGTCCTCAG |
| Reverse | CTGAGTGCCTGCGGTACAGA | ||
| RUNX2 | NM204128.1 | Forward | TTTTTCCTGCCCGTATTCTG |
| Reverse | GCTTGGTGCTGGAGAGTCTT | ||
| β-actin | L08165 | Forward | GAGAAATTGTGCGTGACATCAAGG |
| Reverse | CACCTGAACCTCTCATTGCCA |
| Items | Con | 107 CB | 108 CB | p = | F = |
|---|---|---|---|---|---|
| 8 Week | |||||
| Eggshell thickness, 0.01 mm | 30.31 ± 0.60 b | 33.3 ± 0.67 a | 31.26 ± 0.50 b | 0.0033 | F (2,15) = 8.54 |
| Haugh unit | 68.13 ± 1.75 a | 68.37 ± 1.94 a,b | 61.98 ± 1.44 b | 0.0722 | F (2,15) = 3.15 |
| Items | Con | CB | p = | F = |
|---|---|---|---|---|
| 2 Week | ||||
| 4 Week | ||||
| Egg shape index, % | 1.3 ± 0.003 a | 1.27 ± 0.006 b | 0.0003 | F (1,10) = 30.30 |
| 6 Week | ||||
| Egg shape index, % | 1.3 ± 0.005 a | 1.27 ± 0.007 b | 0.0200 | F (1,9) = 7.64 |
| 8 Week | ||||
| Egg shape index, % | 1.3 ± 0.004 a | 1.28 ± 0.008 b | 0.0468 | F (1,10) = 5.14 |
| Haugh unit | 79.88 ± 1.10 b | 84.90 ± 2.02 a | 0.0207 | F (1,10) = 7.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Cui, L.; Lin, H.; Song, M.; Sun, S. Effects of Clostridium butyricum on Production Performance and Bone Development of Laying Hens. Vet. Sci. 2024, 11, 160. https://doi.org/10.3390/vetsci11040160
Huang J, Cui L, Lin H, Song M, Sun S. Effects of Clostridium butyricum on Production Performance and Bone Development of Laying Hens. Veterinary Sciences. 2024; 11(4):160. https://doi.org/10.3390/vetsci11040160
Chicago/Turabian StyleHuang, Jiaqi, Lulu Cui, Hai Lin, Mengze Song, and Shuhong Sun. 2024. "Effects of Clostridium butyricum on Production Performance and Bone Development of Laying Hens" Veterinary Sciences 11, no. 4: 160. https://doi.org/10.3390/vetsci11040160
APA StyleHuang, J., Cui, L., Lin, H., Song, M., & Sun, S. (2024). Effects of Clostridium butyricum on Production Performance and Bone Development of Laying Hens. Veterinary Sciences, 11(4), 160. https://doi.org/10.3390/vetsci11040160





