True Diaphragmatic Hernia (Morgagni Hernia) Incidentally Diagnosed with Positive Contrast Peritoneography in a Cat: A Rare Case Report and a Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Case Presentation
2.1. Physical Examination
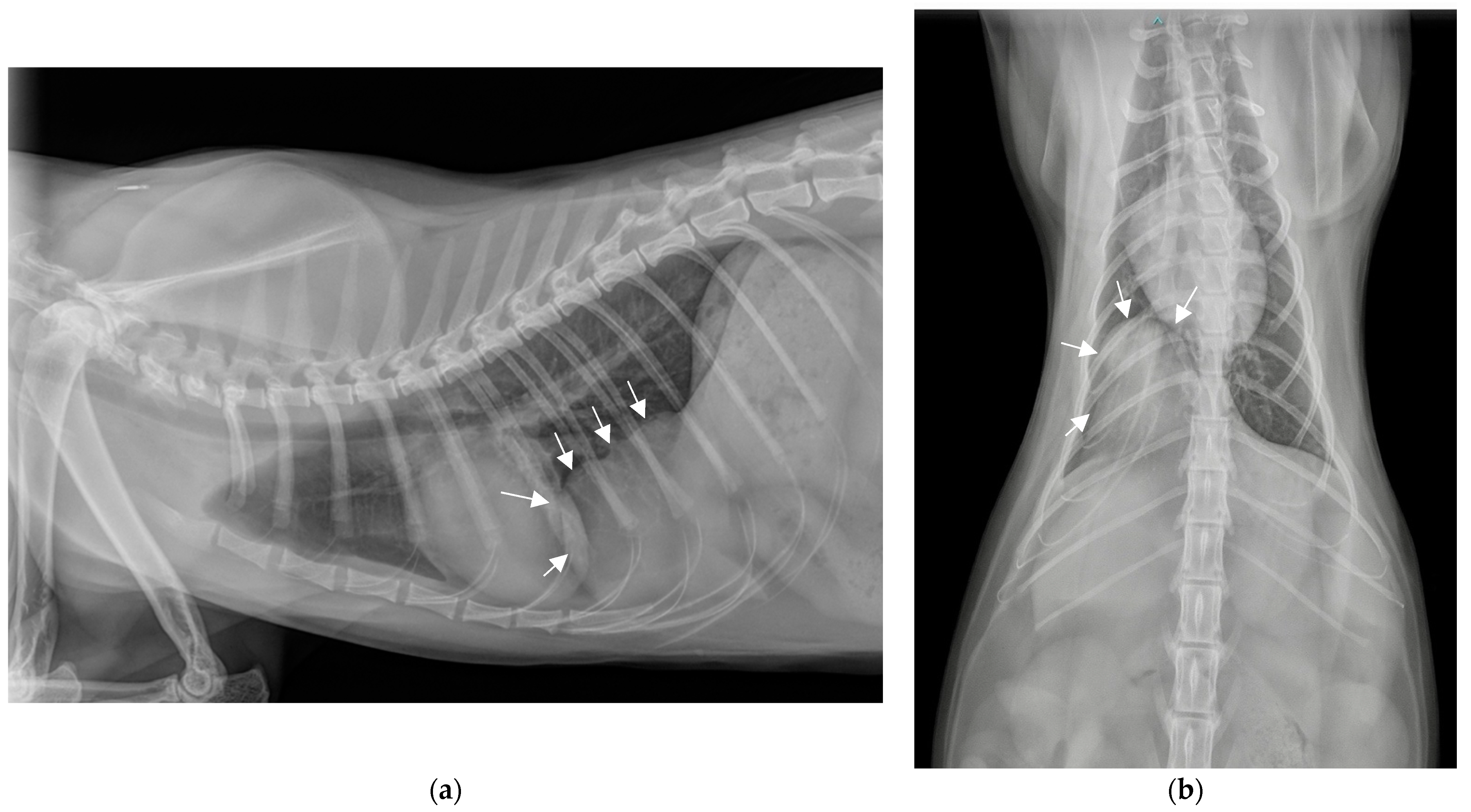
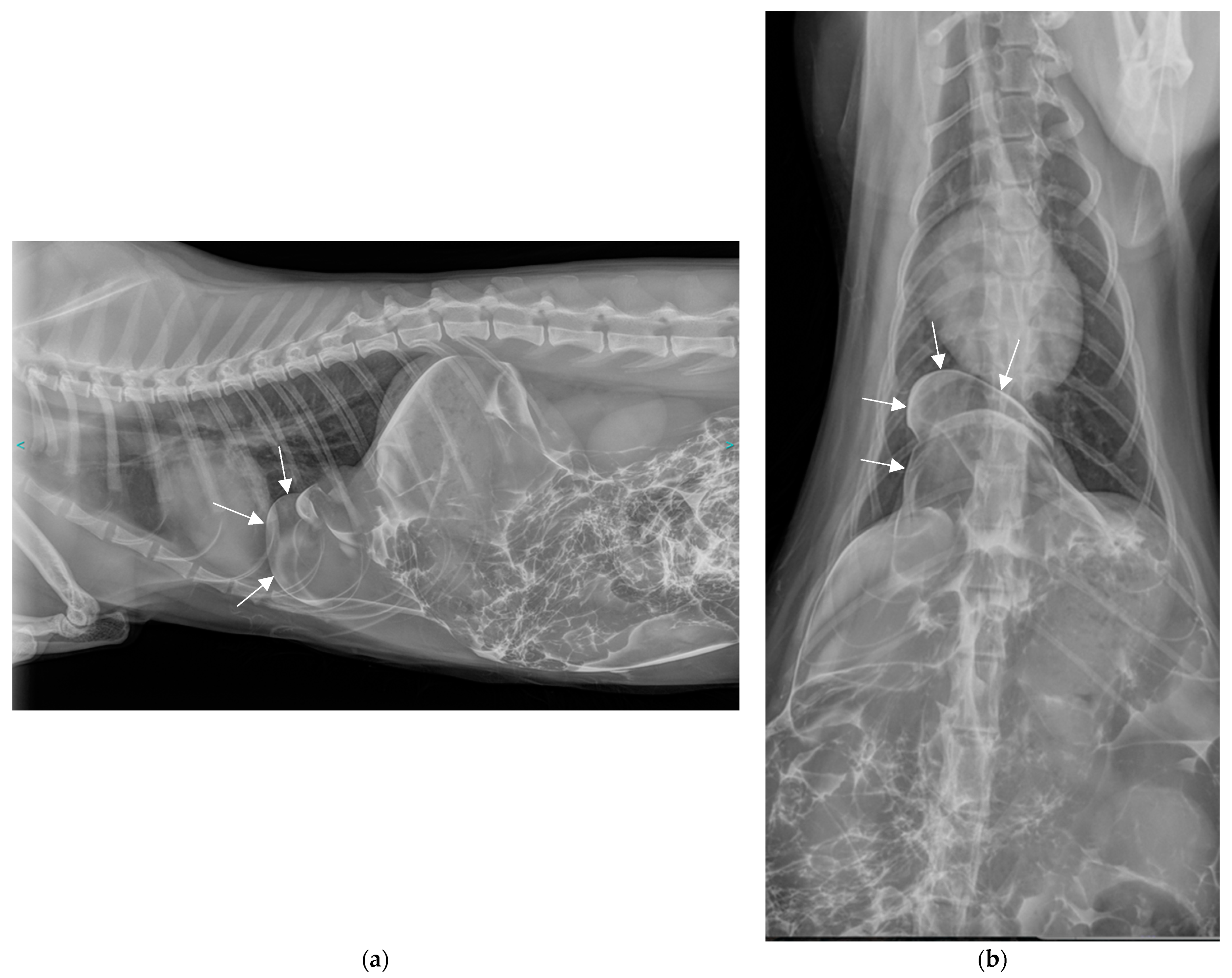
2.2. Additional Tests
2.3. Diagnosis
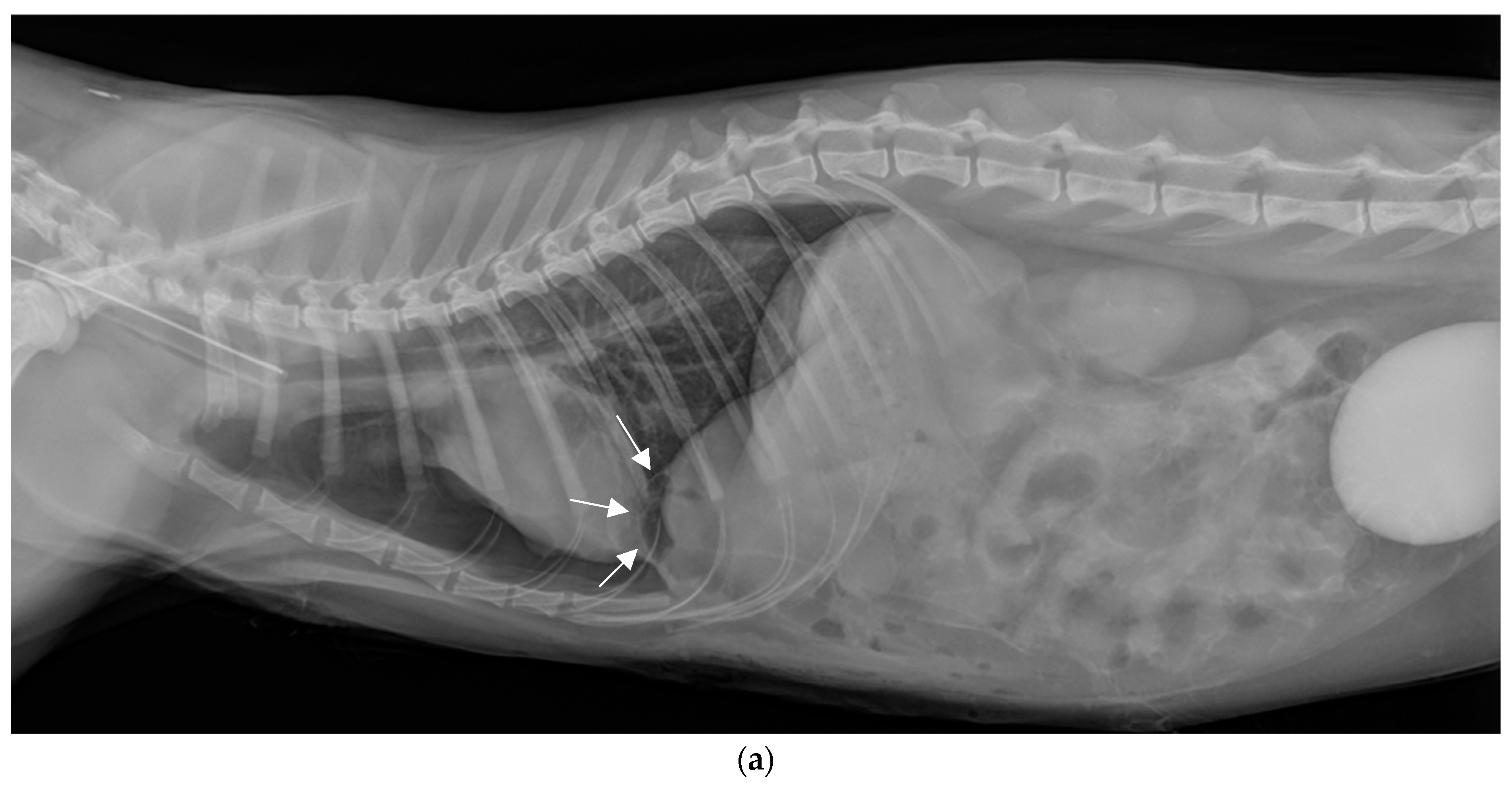
2.4. Surgical Treatment
2.5. Postoperative Phase
3. Discussion
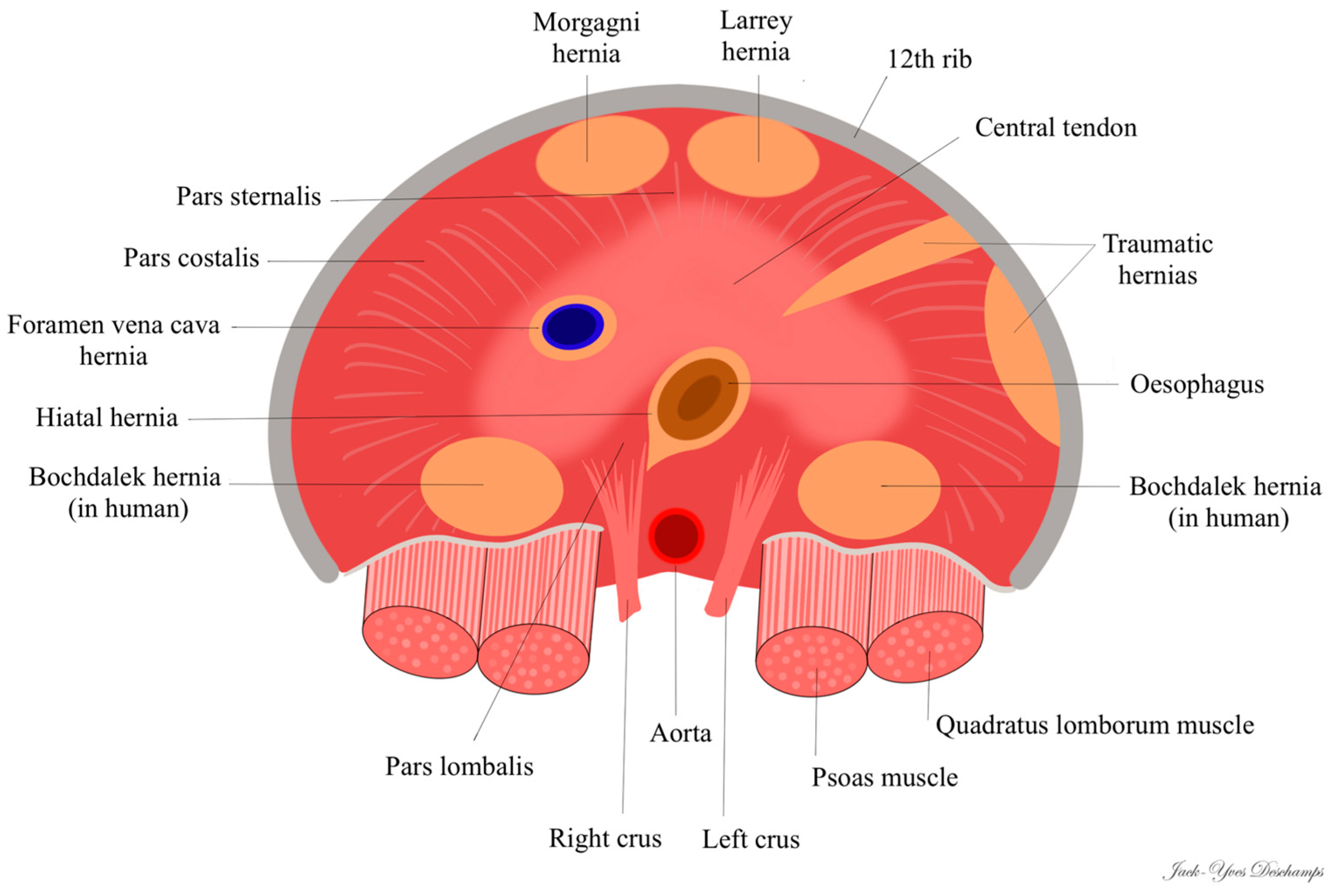
3.1. True Diaphragmatic Hernia
3.2. Location of the Congenital Hernias
3.3. Serendipitous Discoveries
3.4. Herniated Organs
3.5. Positive Contrast Peritoneography
3.6. Surgery
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frye, F.L. Hiatal diaphragmatic hernia and tricholithiasis in a Golden cat (a case history). Vet. Med. Small Anim. Clin. 1972, 67, 391–392. [Google Scholar] [PubMed]
- Peterson, S.L. Esophageal hiatal hernia in a cat. J. Am. Vet. Med. Assoc. 1983, 183, 325–326. [Google Scholar] [PubMed]
- Prymak, C.; Saunders, H.M.; Washabau, R.J. Hiatal hernia repair by restoration and stabilization of normal anatomy. An evaluation in four dogs and one cat. Vet. Surg. 1989, 18, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Owen, M.C.; Morris, P.J.; Bateman, R.S. Concurrent gastro-oesophageal intussusception, trichobezoar and hiatal hernia in a cat. N. Z. Vet. J. 2005, 53, 371–374. [Google Scholar] [CrossRef] [PubMed]
- DeSandre-Robinson, D.M.; Madden, S.N.; Walker, J.T. Nasopharyngeal stenosis with concurrent hiatal hernia and megaesophagus in an 8-year-old cat. J. Feline Med. Surg. 2011, 13, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Meixner, M.; Strohmeyer, K. Hiatal hernia in a cat. Tierarztl Prax Ausg K Kleintiere Heimtiere 2012, 40, 278–282. [Google Scholar]
- Gambino, J.M.; Sivacolundhu, R.; DeLucia, M.; Hiebert, E. Repair of a sliding (type I) hiatal hernia in a cat via herniorrhaphy, esophagoplasty and floppy Nissen fundoplication. J. Feline Med. Surg. Open Rep. 2015, 1, 2055116915602498. [Google Scholar] [CrossRef]
- Siow, J.W.; Hoon, Q.J.; Jenkins, E.; Heblinski, N.; Makara, M. Caval foramen hernia in a cat. J. Feline Med. Surg. Open Rep. 2020, 6, 2055116920964021. [Google Scholar] [CrossRef]
- Kvitka, D.; Juodzente, D.; Rudenkovaite, G.; Burbaite, E.; Laukute, M. Successful early diagnosis and surgical treatment of congenital caval foramen hernia in an 8-month-old mixed breed cat. Braz. J. Vet. Med. 2023, 45, e005622. [Google Scholar] [CrossRef]
- Mann, F.A.; Aronson, E.; Keller, G. Surgical correction of a true congenital pleuroperitoneal diaphragmatic hernia in a cat. J. Am. An. Hosp Assoc. 1991, 27, 501–507. [Google Scholar]
- Voges, A.K.; Bertrand, S.; Hill, R.C.; Neuwirth, L.; Schaer, M. True diaphragmatic hernia in a cat. Vet. Radiol. Ultrasound 1997, 38, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A.; Goggin, J.M.; Kraft, S.L. What is your diagnosis? A 2-cm rounded structure similar in opacity to adjacent falciform fat located between the apex of the heart and the cupula of the diaphragm along the ventral midline of the thorax. J. Am. Vet. Med. Assoc. 1999, 214, 479–480. [Google Scholar] [CrossRef]
- Green, E.; Thamm, D. What is your diagnosis? A soft-tissue mass in the thoracic cavity between the heart and the right crus of the diaphragm. J. Am. Vet. Med. Assoc. 2000, 216, 23–24. [Google Scholar] [CrossRef]
- White, J.D.; Tisdall, P.L.; Norris, J.M.; Malik, R. Diaphragmatic hernia in a cat mimicking a pulmonary mass. J. Feline Med. Surg. 2003, 5, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Cariou, M.P.; Shihab, N.; Kenny, P.; Baines, S.J. Surgical management of an incidentally diagnosed true pleuroperitoneal hernia in a cat. J. Feline Med. Surg. 2009, 11, 873–877. [Google Scholar] [CrossRef]
- Parry, A. Positive contrast peritoneography in the diagnosis of a pleuroperitoneal diaphragmatic hernia in a cat. J. Feline Med. Surg. 2010, 12, 141–143. [Google Scholar] [CrossRef]
- Gombač, M.; Vrecl, M.; Svara, T. Congenital diaphragmatic eventration in two closely related British Shorthair cats. J. Feline Med. Surg. 2011, 13, 276–279. [Google Scholar] [CrossRef] [PubMed]
- Rose, A.M.; Ryan, S.D.; Johnstone, T.; Beck, C. Imaging Diagnosis-the Computed Tomography Features of a Pleuroperitoneal Hernia in a Cat. Vet. Radiol. Ultrasound 2017, 58, E55–E59. [Google Scholar] [CrossRef]
- Lee, S.-K.; Jeong, W.; Choi, J. Remnant parietal serosa detection in a cat with true diaphragmatic hernia using computed tomography. Korean J. Vet. Res. 2019, 59, 105–108. [Google Scholar] [CrossRef]
- Pilli, M.; Ozgencil, F.E.; Seyrek-Intas, D.; Gultekin, C.; Turgut, K. Pleuroperitoneal true diaphragmatic hernia of the liver in a cat. Tierärztliche Prax. Ausg. K. Kleintiere/Heimtiere 2020, 48, 292–296. [Google Scholar] [CrossRef]
- Choi, J.; Kim, H.; Kim, M.; Yoon, J. Imaging diagnosis--positive contrast peritoneographic features of true diaphragmatic hernia. Vet. Radiol. Ultrasound 2009, 50, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, H.F.; Basso, P.C.; Faria, K.L.; Oliveira, M.T.; Souza, F.W.; Garcia, É.V.; Feranti, J.P.S.; Silva, M.A.M.; Brun, M.V. Laparoscopic repair of congenital pleuroperitoneal hernia using a polypropylene mesh in a dog. Arq. Bras. Med. Vet. Zootec. 2015, 67, 1547–1553. [Google Scholar] [CrossRef]
- Devereux, E.A.; Scharf, V.F. Surgical technique and complications associated with laparoscopic pleuroperitoneal diaphragmatic herniorrhaphy in a dog. Vet. Rec. Case Rep. 2023, 11, e657. [Google Scholar] [CrossRef]
- Keep, J.M. Congenital diaphragmatic hernia in a cat. Aust. Vet. J. 1950, 26, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Bismuth, C.; Deroy, C. Congenital cranial ventral abdominal hernia, peritoneopericardial diaphragmatic hernia and sternal cleft in a 4-year-old multiparous pregnant queen. J. Feline Med. Surg. Open Rep. 2017, 3, 2055116917747741. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D.B.; Bree, M.M.; Cohen, B.J. Congenital diaphragmatic hernia in neonatal dogs. J. Am. Vet. Med. Assoc. 1968, 153, 942–944. [Google Scholar]
- Valentine, B.A.; Cooper, B.J.; Dietze, A.E.; Noden, D.M. Canine congenital diaphragmatic hernia. J. Vet. Intern. Med. 1988, 2, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Auger, J.M.; Riley, S.M. Combined hiatal and pleuroperitoneal hernia in a shar-pei. Can Vet. J. 1997, 38, 640–642. [Google Scholar]
- Rossanese, M.; Pivetta, M.; Pereira, N.; Burrow, R. Congenital pleuroperitoneal hernia presenting as gastrothorax in five cavalier King Charles spaniel dogs. J. Small Anim. Pract. 2019, 60, 701–704. [Google Scholar] [CrossRef]
- Adereti, C.; Zahir, J.; Robinson, E.; Pursel, J.; Hamdallah, I. A Case Report and Literature Review on Incidental Morgagni Hernia in Bariatric Patients: To Repair or Not to Repair? Cureus 2023, 15, e39950. [Google Scholar] [CrossRef]
- Svetanoff, W.J.; Rentea, R.M. Morgagni Hernia; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- White, J.J.; Suzuki, H. Hernia through the foramen of Bochdalek: A misnomer. J. Pediatr. Surg. 1972, 7, 60–61. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Khanna, R.; Panigrahy, P.; Meena, R.N.; Mishra, S.P.; Khanna, S. An Incidental Finding of Morgagni Hernia in an Elderly Female and Its Successful Management: A Rare Case Report and Review of Literature. Cureus 2023, 15, e42676. [Google Scholar] [CrossRef] [PubMed]
- Palma, R.; Angrisani, F.; Santonicola, A.; Iovino, P.; Ormando, V.M.; Maselli, R.; Angrisani, L. Case report: Laparoscopic nissen-sleeve gastrectomy in a young adult with incidental finding of Morgagni-Larrey hernia. Front. Surg. 2023, 10, 1227567. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Alabdrabalmeer, M.; Alealiwi, M.; Danan, O.A.; Alshomimi, S. Incidental Morgagni hernia found during laparoscopic repair of hiatal hernia: Case report & review of literature. Int. J. Surg. Case. Rep. 2019, 57, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Moon, H.S.; Jung, H.Y.; Sung, J.K.; Gang, S.H.; Kim, M.H. An Incidental Discovery of Morgagni Hernia in an Elderly Patient Presented with Chronic Dyspepsia. Korean J. Gastroenterol. Taehan Sohwagi Hakhoe Chi 2017, 69, 68–73. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shah, A.A.; Karass, M.; Page, A.J.; Shehata, B.M.; Durham, M.M. Extra-hepatic bile duct hamartoma in a 10-month-old with a morgagni hernia and multiple anatomical anomalies: A rare and incidental finding. Pediatr. Surg. Int. 2013, 29, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Gaco, S.; Krdzalic, G.; Krdzalic, A. Incarcerated Morgagni hernia in an octogenarian with incidental right-sided colonic malignancy. Med. Arch. 2013, 67, 73–74. [Google Scholar] [CrossRef] [PubMed]
- McCabe, A.; Watts, A.; Abdulrazak, A.; McCann, B. Incidental finding of a large Morgagni’s hernia in a 76-year-old lady. BMJ Case. Rep. 2012, 2012, 7175. [Google Scholar] [CrossRef] [PubMed]
- Zisa, M.; Pulvirenti, E.; Toro, A.; Mannino, M.; Reale, G.; Di Carlo, I. Seizure attack and Morgagni diaphragmatic hernia: Incidental diagnosis or direct correlation? Updates Surg. 2011, 63, 55–58. [Google Scholar] [CrossRef]
- Smith, J.; Ghani, A. Morgagni hernia: Incidental repair during laparoscopic cholecystectomy. J. Laparoendosc. Surg. 1995, 5, 123–125. [Google Scholar] [CrossRef]
- Al-Salem, H. Incidental bilateral Morgagni hernia in a traumatized child. Aust. N. Z. J. Surg. 1992, 62, 910–912. [Google Scholar] [CrossRef] [PubMed]
- Rendano Jr, V. Positive contrast peritoneography: An aid in the radiographic diagnosis of diaphragmatic hernia. Vet. Radiol. 1979, 20, 67–73. [Google Scholar] [CrossRef]
- Stickle, R.L. Positive-contrast celiography (peritoneography) for the diagnosis of diaphragmatic hernia in dogs and cats. J. Am. Vet. Med. Assoc. 1984, 185, 295–298. [Google Scholar] [PubMed]
- Kibar, M.E.; Bumïn, A.; Kaya, M.; Alkan, Z. Use of peritoneography (positive contrast che liography) and ultrasonography in the diagnosis of diaphragmatic hernia: Review of 35 cats. Rev. Med. Vet. 2006, 157, 331–335. [Google Scholar]
- Ferrante, S.L.; Schreiman, J.S.; Rouse, J.W.; Rysavy, J.A.; Cheng, S.C.; Frick, M.P. Iopamidol as a gastrointestinal contrast agent. Lack of peritoneal reactivity. Investig. Radiol. 1990, 25, 141–145. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deschamps, J.-Y.; Corbarieu, T.; Abboud, N.; Roux, F.A. True Diaphragmatic Hernia (Morgagni Hernia) Incidentally Diagnosed with Positive Contrast Peritoneography in a Cat: A Rare Case Report and a Review. Vet. Sci. 2024, 11, 159. https://doi.org/10.3390/vetsci11040159
Deschamps J-Y, Corbarieu T, Abboud N, Roux FA. True Diaphragmatic Hernia (Morgagni Hernia) Incidentally Diagnosed with Positive Contrast Peritoneography in a Cat: A Rare Case Report and a Review. Veterinary Sciences. 2024; 11(4):159. https://doi.org/10.3390/vetsci11040159
Chicago/Turabian StyleDeschamps, Jack-Yves, Théo Corbarieu, Nour Abboud, and Françoise A. Roux. 2024. "True Diaphragmatic Hernia (Morgagni Hernia) Incidentally Diagnosed with Positive Contrast Peritoneography in a Cat: A Rare Case Report and a Review" Veterinary Sciences 11, no. 4: 159. https://doi.org/10.3390/vetsci11040159
APA StyleDeschamps, J.-Y., Corbarieu, T., Abboud, N., & Roux, F. A. (2024). True Diaphragmatic Hernia (Morgagni Hernia) Incidentally Diagnosed with Positive Contrast Peritoneography in a Cat: A Rare Case Report and a Review. Veterinary Sciences, 11(4), 159. https://doi.org/10.3390/vetsci11040159






