Effects of Neuromuscular Electrical Stimulation and Therapeutic Ultrasound on Quadriceps Contracture of Immobilized Rats
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
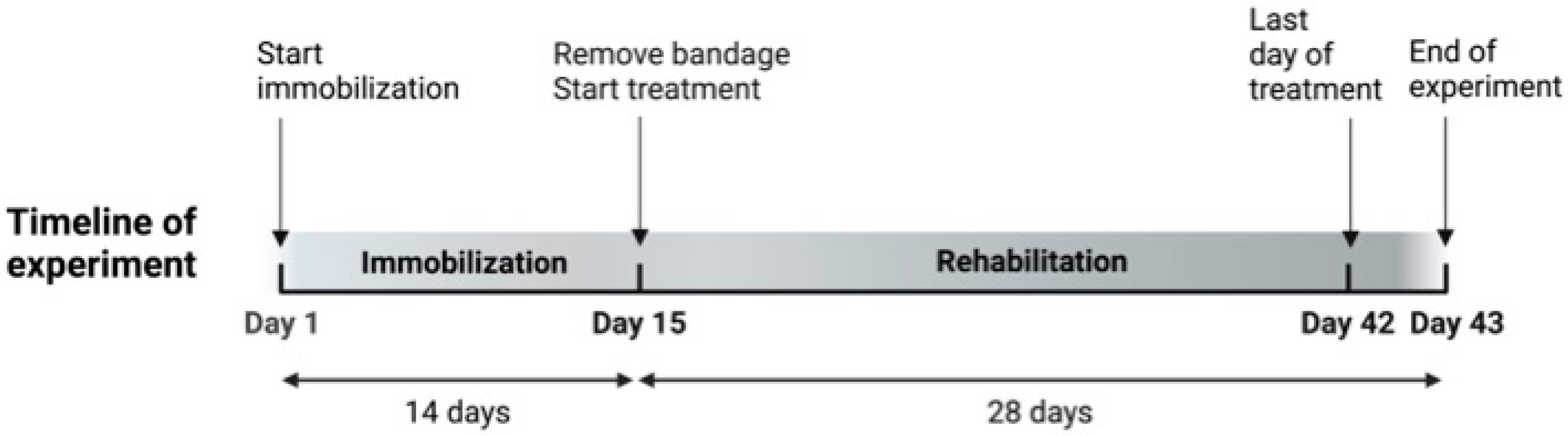
2.1. Animals and Study Design
2.2. Immobilization Procedure
2.3. Spontaneous Recovery
2.4. Therapeutic Ultrasound
2.5. Neuromuscular Electrical Stimulation (NMES)
2.6. ROM of the Stifle Joint
2.7. Body Weight and Food Intake
2.8. Evaluation of Creatinine Phosphokinase Level
2.9. Euthanasia, Muscle Collection, and Measurement
2.10. Histopathological Examination
2.11. Immunohistochemical Analysis
2.12. Real-Time RT-PCR to Measure Interleukin-1β (IL-1β), Transforming Growth factor-β1 (TGF-β1), Transforming Growth Factor-β3 (TGF-β3), and Hypoxia-Inducible Transcription Factor-1α (HIF-1α) mRNA
2.13. Statistical Analysis
3. Results
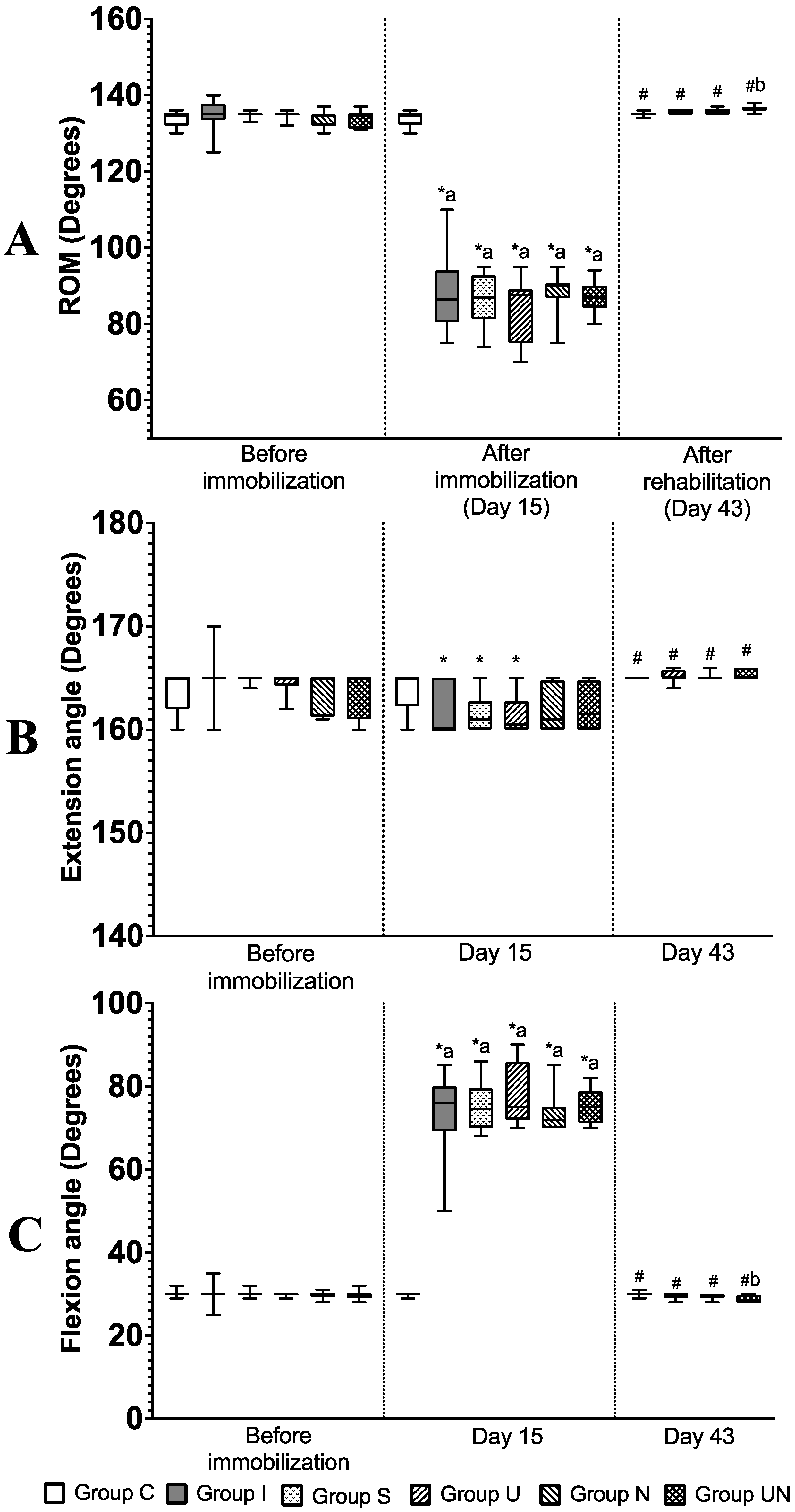
3.1. Stifle Joint Angle and ROM
3.2. Body Weight and Food Intake
3.3. Creatinine Phosphokinase Level
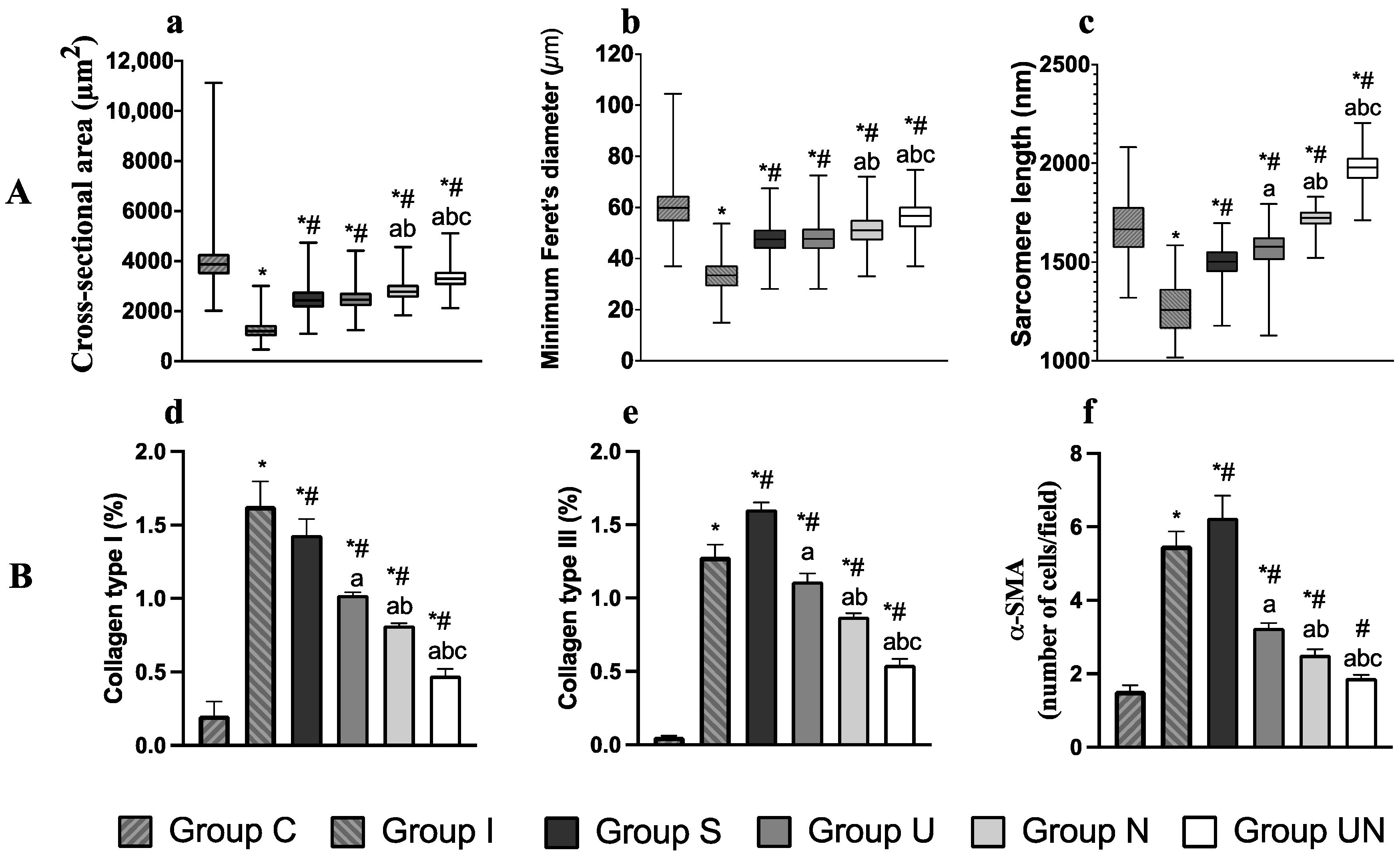
3.4. The Ratio of Quadriceps Muscle Weight to Body Weight and Quadriceps Muscle Measurements
3.5. Histological Evaluation of Quadriceps Muscle Fiber and Sarcomere Length
3.6. Measurement of -Smooth Muscle Actin (-SMA) Cells and Collagen Types I and III
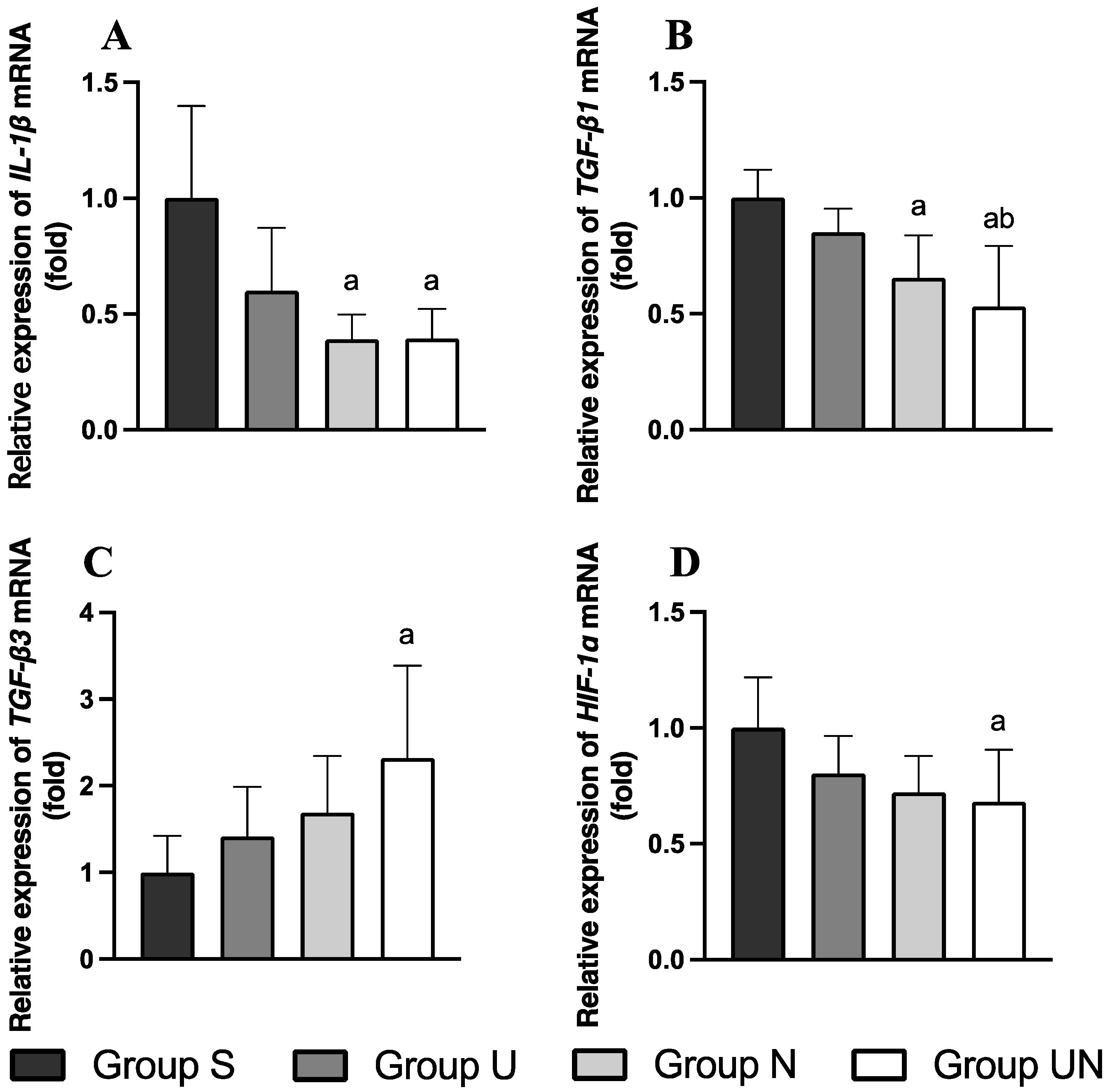
3.7. Relative Expression of IL-1, TGF-1, TGF-3, and HIF-1 mRNA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schulz, K.S. Management of muscle and tendon injury or disease. In Small Animal Surgery Textbook, 4th ed.; Fossum, T.W., Ed.; Elsevier Health Sciences: Philadelphia, PA, USA, 2013; pp. 1375–1390. [Google Scholar]
- Canapp, S.O.; Saunders, D.G. Common conditions and physical rehabilitation of the athletic patient. In Canine Rehabilitation and Physical Therapy, 2nd ed.; Millis, D., Levine, D., Eds.; Elsevier Health Sciences: Philadelphia, PA, USA, 2014; pp. 582–608. [Google Scholar]
- Carmichael, S.; Marshall, W.G. Muscle and tendon disorders. In Veterinary Surgery: Small Animal, 2nd ed.; Tobias, K.M., Johnston, S.A., Eds.; Elsevier Health Sciences: St. Louis, MI, USA, 2013; pp. 1316–1322. [Google Scholar]
- Millis, D. Quadriceps contracture. In Complications in Small Animal Surgery; Dominique, G., Annick, H., Eds.; John Wiley & Sons, Inc.: Ames, IA, USA, 2016; pp. 692–696. [Google Scholar]
- Taylor, J.; Tangner, C. Acquired muscle contractures in the dog and cat. A review of the literature and case report. Vet. Comp. Orthop. Traumatol. 2007, 2, 79–85. [Google Scholar]
- Wang, F.; Zhang, Q.B.; Zhou, Y.; Chen, S.; Huang, P.P.; Liu, Y.; Xu, Y.H. The mechanisms and treatments of muscular pathological changes in immobilization-induced joint contracture: A literature review. Chin. J. Traumatol. 2019, 22, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Järvinen, M.J.; Einola, S.A.; Virtanen, E.O. Effect of the position of immobilization upon the tensile properties of the rat gastrocnemius muscle. Arch. Phys. Med. Rehabil. 1992, 73, 253–257. [Google Scholar] [PubMed]
- Honda, Y.; Tanaka, M.; Tanaka, N.; Sasabe, R.; Goto, K.; Kataoka, H.; Sakamoto, J.; Nakano, J.; Okita, M. Relationship between extensibility and collagen expression in immobilized rat skeletal muscle. Muscle Nerve 2018, 57, 672–678. [Google Scholar] [CrossRef]
- Okita, M.; Yoshimura, T.; Nakano, J.; Motomura, M.; Eguchi, K. Effects of reduced joint mobility on sarcomere length, collagen fibril arrangement in the endomysium, and hyaluronan in rat soleus muscle. J. Muscle Res. Cell Motil. 2004, 25, 159–166. [Google Scholar] [CrossRef]
- Suwankanit, K.; Shimizu, M. Rat model of quadriceps contracture by joint immobilization. Biology 2022, 11, 1781. [Google Scholar] [CrossRef]
- Trudel, G.; Uhthoff, H.K. Contractures secondary to immobility: Is the restriction articular or muscular? An experimental longitudinal study in the rat knee. Arch. Phys. Med. Rehabil. 2000, 81, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Hoso, M.; Matsuzaki, T.; Kojima, S. Histopathological changes of joint capsule after joint immobility compared with aging in rats. J. Phys. Ther. Sci. 2010, 22, 369–374. [Google Scholar] [CrossRef]
- Hildebrand, K.A.; Zhang, M.; Hart, D.A. Joint capsule matrix turnover in a rabbit model of chronic joint contractures: Correlation with human contractures. J. Orthop. Res. 2006, 24, 1036–1043. [Google Scholar] [CrossRef]
- Roh, Y.H.; Choi, M.H.; Lee, J.H.; Mok, J.S.; Lee, H.B. Treatment of quadriceps contracture with femoral shortening ostectomy, rectus femoris muscle transposition and dynamic stifle flexion apparatus in a dog. J. Vet. Clin. 2020, 37, 163–167. [Google Scholar] [CrossRef]
- Harry, S.; Juan, M.; Philip, W. Feline Orthopaedics, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2022; p. 338. [Google Scholar]
- Ulusan, S.; Captug-Ozdemir, O.; Gul-Sancak, I.; Bilgili, H. Treatment techniques of femoral quadriceps muscle contracture in ten dogs and two cats. Kafkas Univ. Vet. Fak. Derg. 2011, 17, 401–408. [Google Scholar]
- Roy, S.H.; Klein, A.B. Physical Agents. In The Rehabilitation Specialist’s Handbook, 4th ed.; Roy, S.H., Wolf, S.L., Scalzitti, D.A., Eds.; F.A. Davis: Philadelphia, PA, USA, 2013; pp. 1079–1121. [Google Scholar]
- Wessling, K.C.; Devane, D.A.; Hylton, C.R. Effects of static stretch versus static stretch and ultrasound combined on triceps surae muscle extensibility in healthy women. Phys. Ther. 1987, 67, 674–679. [Google Scholar] [CrossRef]
- Brancalion Catapani, L.; da Costa Gonçalves, A.; Morano Candeloro, N.; Rossi, L.A.; Caldeira de Oliveira Guirro, E. Influence of therapeutic ultrasound on the biomechanical characteristics of the skin. J. Ther. Ultrasound 2016, 4, 21. [Google Scholar] [CrossRef]
- Monisha, R. To evaluate the effects of ultrasound therapy in increasing hamstring extensibility—A pilot study. Int. J. Musculoskelet. Disord. 2018, 10, 111380726. [Google Scholar]
- Morishita, K.; Karasuno, H.; Yokoi, Y.; Morozumi, K.; Ogihara, H.; Ito, T.; Fujiwara, T.; Fujimoto, T.; Abe, K. Effects of therapeutic ultrasound on intramuscular blood circulation and oxygen dynamics. J. Jpn. Phys. Ther. Assoc. 2014, 17, 1–7. [Google Scholar] [CrossRef]
- Belcik, J.T.; Davidson, B.P.; Xie, A.; Wu, M.D.; Yadava, M.; Qi, Y.; Liang, S.; Chon, C.R.; Ammi, A.Y.; Field, J. Augmentation of muscle blood flow by ultrasound cavitation is mediated by ATP and purinergic signaling. Circulation 2017, 135, 1240–1252. [Google Scholar] [CrossRef]
- Usuba, M.; Miyanaga, Y.; Miyakawa, S.; Maeshima, T.; Shirasaki, Y. Effect of heat in increasing the range of knee motion after the development of a joint contracture: An experiment with an animal model. Arch. Phys. Med. Rehabil. 2006, 87, 247–253. [Google Scholar] [CrossRef]
- Morishita, K.; Karasuno, H.; Yokoi, Y.; Morozumi, K.; Ogihara, H.; Ito, T.; Hanaoka, M.; Fujiwara, T.; Fujimoto, T.; Abe, K. Effects of therapeutic ultrasound on range of motion and stretch pain. J. Phys. Ther. Sci. 2014, 26, 711–715. [Google Scholar] [CrossRef][Green Version]
- Okita, M.; Nakano, J.; Kataoka, H.; Sakamoto, J.; Origuchi, T.; Yoshimura, T. Effects of therapeutic ultrasound on joint mobility and collagen fibril arrangement in the endomysium of immobilized rat soleus muscle. Ultrasound Med. Biol. 2009, 35, 237–244. [Google Scholar] [CrossRef]
- Cunha, D.; Nascimento, C.; Artifon, E.; Ferrari, D.; Ribeiro, L.; Fontanesi, L.; Bertolini, G. Evaluation of rats’ soleus muscle submitted to remobilization protocol with therapeutic ultrasound associated with static stretching. J. Morphol. Sci. 2012, 29, 53–57. [Google Scholar]
- Nussbaum, E.L.; Houghton, P.; Anthony, J.; Rennie, S.; Shay, B.L.; Hoens, A.M. Neuromuscular electrical stimulation for treatment of muscle impairment: Critical review and recommendations for clinical practice. Physiother. Can. 2017, 69, 1–76. [Google Scholar] [CrossRef]
- Lake, D.A. Neuromuscular electrical stimulation: An overview and its application in the treatment of sports injuries. Sports Med. 1992, 13, 320–336. [Google Scholar] [CrossRef]
- Azman, M.F.; Azman, A.W. The effect of electrical stimulation in improving muscle tone (clinical). In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Philadelphia, PA, USA, 2017. [Google Scholar]
- Moon, S.H.; Choi, J.H.; Park, S.E. The effects of functional electrical stimulation on muscle tone and stiffness of stroke patients. J. Phys. Ther. Sci. 2017, 29, 238–241. [Google Scholar] [CrossRef]
- Qin, L.; Appell, H.; Chan, K.; Maffulli, N. Electrical stimulation prevents immobilization atrophy in skeletal muscle of rabbits. Arch. Phys. Med. Rehabil. 1997, 78, 512–517. [Google Scholar] [CrossRef]
- Durigan, J.L.; Delfino, G.B.; Peviani, S.M.; Russo, T.L.; Ramírez, C.; Da Silva Gomes, A.D.; Salvini, T.F. Neuromuscular electrical stimulation alters gene expression and delays quadriceps muscle atrophy of rats after anterior cruciate ligament transection. Muscle Nerve 2014, 49, 120–128. [Google Scholar] [CrossRef]
- Yoshida, N.; Morimoto, Y.; Kataoka, H.; Sakamoto, J.; Nakano, J.; Okita, M. Effects of combination therapy of heat stress and muscle contraction exercise induced by neuromuscular electrical stimulation on disuse atrophy in the rat gastrocnemius. J. Phys. Ther. Sci. 2013, 25, 201–206. [Google Scholar] [CrossRef][Green Version]
- Baker, L.L.; Yeh, C.; Wilson, D.; Waters, R.L. Electrical stimulation of wrist and fingers for hemiplegic patients. Phys. Ther. 1979, 59, 1495–1499. [Google Scholar] [CrossRef]
- Yoshimura, A.; Sakamoto, J.; Honda, Y.; Kataoka, H.; Nakano, J.; Okita, M. Cyclic muscle twitch contraction inhibits immobilization-induced muscle contracture and fibrosis in rats. Connect. Tissue Res. 2017, 58, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.E.; Catanese, T.; Lucey, E.G.; Goldspink, G. The importance of stretch and contractile activity in the prevention of connective tissue accumulation in muscle. J. Anat. 1988, 158, 109–114. [Google Scholar]
- Devrimsel, G.; Metin, Y.; Serdaroglu Beyazal, M. Short-term effects of neuromuscular electrical stimulation and ultrasound therapies on muscle architecture and functional capacity in knee osteoarthritis: A randomized study. Clin. Rehabil. 2019, 33, 418–427. [Google Scholar] [CrossRef]
- Amjad, F.; Shahid, H.A.; Batool, S.; Ahmad, A.; Ahmed, I. A comparison on efficacy of transcutaneous electrical nerve stimulation and therapeutic ultrasound in treatment of myofascial trigger points. Khyber. Med. Univ. J. 2016, 8, 3–6. [Google Scholar]
- Boonhong, J.; Suntornpiyapan, P.; Piriyajarukul, A. Ultrasound combined transcutaneous electrical nerve stimulation (UltraTENS) versus phonophoresis of piroxicam (PhP) in symptomatic knee osteoarthritis: A randomized double-blind, controlled trial. J. Back. Musculoskelet. Rehabil. 2018, 31, 507–513. [Google Scholar] [CrossRef]
- Király, M.; Gömöri, E.; Kiss, R.; Nógrádi, N.; Nusser, N.; Hodosi, K.; Bender, T. Effects of various types of ultrasound therapy in hip osteoarthritis-a double-blind, randomized, controlled, follow-up study. Physiother. Theory Pract. 2022, 38, 1958–1968. [Google Scholar] [CrossRef]
- Jang, Y.; Park, Y.E.; Yun, C.W.; Kim, D.H.; Chung, H. The vest-collar as a rodent collar to prevent licking and scratching during experiments. Lab. Anim. 2016, 50, 296–304. [Google Scholar] [CrossRef]
- Honda, Y.; Tanaka, N.; Kajiwara, Y.; Kondo, Y.; Kataoka, H.; Sakamoto, J.; Akimoto, R.; Nawata, A.; Okita, M. Effect of belt electrode-skeletal muscle electrical stimulation on immobilization-induced muscle fibrosis. PLoS ONE 2021, 16, e0244120. [Google Scholar] [CrossRef]
- Millis, D.; Levine, D. Joint motions and ranges. In Canine Rehabilitation and Physical Therapy, 2nd ed.; Millis, D., David, L., Eds.; Elsevier Health Sciences: Philadelphia, PA, USA, 2014; pp. 730–735. [Google Scholar]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. In Guide for the Care and Use of Laboratory Animals, 8th ed.; National Acedemies Press: Washington, DC, USA, 2010.
- Cardiff, R.D.; Miller, C.H.; Munn, R.J. Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harb. Protoc. 2014, 2014, 655–658. [Google Scholar] [CrossRef]
- Wedderburn, L.R.; Varsani, H.; Li, C.K.; Newton, K.R.; Amato, A.A.; Banwell, B.; Bove, K.E.; Corse, A.M.; Emslie-Smith, A.; Harding, B. International consensus on a proposed score system for muscle biopsy evaluation in patients with juvenile dermatomyositis: A tool for potential use in clinical trials. Arthritis Care Res. 2007, 57, 1192–1201. [Google Scholar] [CrossRef]
- Wangkaew, S.; Suwansirikul, S.; Aroonrungwichian, K.; Kasitanon, N.; Louthrenoo, W. The correlation of muscle biopsy scores with the clinical variables in idiopathic inflammatory myopathies. Open Rheumatol. J. 2016, 10, 141–149. [Google Scholar] [CrossRef]
- Suwankanit, K.; Shimizu, M.; Suzuki, K.; Kaneda, M. Usefulness of ultrasound shear wave elastography for detection of quadriceps contracture in immobilized rats. Animals 2023, 14, 76. [Google Scholar] [CrossRef]
- Calvi, E.N.d.C.; Nahas, F.X.; Barbosa, M.V.; Calil, J.A.; Ihara, S.S.M.; Silva, M.d.S.; Franco, M.F.d.; Ferreira, L.M. An experimental model for the study of collagen fibers in skeletal muscle. Acta Cir. Bras. 2012, 27, 681–686. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Transforming growth factor–β in tissue fibrosis. J. Exp. Med. 2020, 217, e20190103. [Google Scholar] [CrossRef]
- Zhao, C.G.; He, X.J.; Lu, B.; Li, H.P.; Kang, A.J. Increased expression of collagens, transforming growth factor-β1, and-β3 in gluteal muscle contracture. BMC Musculoskelet. Disord. 2010, 11, 15. [Google Scholar] [CrossRef]
- Ismaeel, A.; Kim, J.S.; Kirk, J.S.; Smith, R.S.; Bohannon, W.T.; Koutakis, P. Role of Transforming Growth Factor-β in Skeletal Muscle Fibrosis: A Review. Int. J. Mol. Sci. 2019, 20, 2446. [Google Scholar] [CrossRef]
- Chang, Z.; Kishimoto, Y.; Hasan, A.; Welham, N.V. TGF-β3 modulates the inflammatory environment and reduces scar formation following vocal fold mucosal injury in rats. Dis. Models Mech. 2014, 7, 83–91. [Google Scholar] [CrossRef]
- Xue, K.; Zhang, J.; Li, C.; Li, J.; Wang, C.; Zhang, Q.; Chen, X.; Yu, X.; Sun, L.; Yu, X. The role and mechanism of transforming growth factor beta 3 in human myocardial infarction-induced myocardial fibrosis. J. Cell Mol. Med. 2019, 23, 4229–4243. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, P.; Gao, X.; Qian, W.; Xu, K. rAAV2-TGF-β 3 decreases collagen synthesis and deposition in the liver of experimental hepatic fibrosis rat. Dig. Dis. Sci. 2010, 55, 2821–2830. [Google Scholar] [CrossRef]
- Valle-Tenney, R.; Rebolledo, D.; Acuña, M.J.; Brandan, E. HIF-hypoxia signaling in skeletal muscle physiology and fibrosis. J. Cell Commun. Signal 2020, 14, 147–158. [Google Scholar] [CrossRef]
- Tzouvelekis, A.; Harokopos, V.; Paparountas, T.; Oikonomou, N.; Chatziioannou, A.; Vilaras, G.; Tsiambas, E.; Karameris, A.; Bouros, D.; Aidinis, V. Comparative expression profiling in pulmonary fibrosis suggests a role of hypoxia-inducible factor-1alpha in disease pathogenesis. Am. J. Respir. Crit. Care Med. 2007, 176, 1108–1119. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Nakano, J.; Oga, S.; Kataoka, H.; Honda, Y.; Sakamoto, J.; Okita, M. The non-thermal effects of pulsed ultrasound irradiation on the development of disuse muscle atrophy in rat gastrocnemius muscle. Ultrasound Med. Biol. 2014, 40, 1578–1586. [Google Scholar] [CrossRef][Green Version]
- Honda, Y.; Sakamoto, J.; Nakano, J.; Kataoka, H.; Sasabe, R.; Goto, K.; Tanaka, M.; Origuchi, T.; Yoshimura, T.; Okita, M. Upregulation of interleukin-1β/transforming growth factor-β1 and hypoxia relate to molecular mechanisms underlying immobilization-induced muscle contracture. Muscle Nerve 2015, 52, 419–427. [Google Scholar] [CrossRef]
- Abdel, M.P.; Morrey, M.E.; Barlow, J.D.; Kreofsky, C.R.; An, K.N.; Steinmann, S.P.; Morrey, B.F.; Sanchez-Sotelo, J. Myofibroblast cells are preferentially expressed early in a rabbit model of joint contracture. J. Orthop. Res. 2012, 30, 713–719. [Google Scholar] [CrossRef]
- Levine, D.; Watson, T. Therapeutic ultrasound. In Canine Rehabilitation and Physical Therapy, 2nd ed.; Millis, D., Levine, D., Eds.; Elsevier Health Sciences: Philadelphia, PA, USA, 2014; pp. 328–341. [Google Scholar]
- Carolina, M.; Wendy, D. Therapeutic ultrasound. In Physical Rehabilitation for Veterinary Technicians and Nurses, 1st ed.; Goldberg, M.E., Tomlinson, J.E., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; pp. 262–272. [Google Scholar]
- Watanabe, M.; Kojima, S.; Hoso, M. Effect of low-intensity pulsed ultrasound therapy on a rat knee joint contracture model. J. Phys. Ther. Sci. 2017, 29, 1567–1572. [Google Scholar] [CrossRef]
- Levine, D.; Millis, D.L.; Mynatt, T. Effects of 3.3-MHz ultrasound on caudal thigh muscle temperature in dogs. Vet. Surg. 2001, 30, 170–174. [Google Scholar] [CrossRef]
- Sakamoto, J.; Nakano, J.; Kataoka, H.; Origuchi, T.; Yoshimura, T.; Okita, M. Continuous therapeutic ultrasound inhibits progression of disuse atrophy in rat gastrocnemius muscles. J. Phys. Ther. Sci. 2012, 24, 443–447. [Google Scholar] [CrossRef][Green Version]
- Cho, S.; Park, M.; Won, J.; Kim, Y.; Hong, J.; Kim, S. The effect of therapeutic ultrasound and static stretching of the hamstring muscle on flexibility and static balance ability. J. Korean Phys. Ther. Sci. 2019, 26, 45–53. [Google Scholar] [CrossRef]
- Macedo, A.C.B.d.; Ywazaki, J.L.; Macedo, R.M.d.; Noronha, L.; Gomes, A.R.S. Morphologic study of different treatments for gastrocnemius muscle contusion in rats. Rev. Bras. Ortop. 2016, 51, 697–706. [Google Scholar] [CrossRef][Green Version]
- Aibara, Y.; Nakashima, A.; Kawano, K.; Yusoff, F.M.; Mizuki, F.; Kishimoto, S.; Kajikawa, M.; Maruhashi, T.; Higashi, Y. Daily low-intensity pulsed ultrasound ameliorates renal fibrosis and inflammation in experimental hypertensive and diabetic nephropathy. Hypertension 2020, 76, 1906–1914. [Google Scholar] [CrossRef]
- Zhao, K.; Weng, L.; Xu, T.; Yang, C.; Zhang, J.; Ni, G.; Guo, X.; Tu, J.; Zhang, D.; Sun, W.; et al. Low-intensity pulsed ultrasound prevents prolonged hypoxia-induced cardiac fibrosis through HIF-1α/DNMT3a pathway via a TRAAK-dependent manner. Clin. Exp. Pharmacol. Physiol. 2021, 48, 1500–1514. [Google Scholar] [CrossRef]
- Levine, D.; Bockstahler, B. Electrical stimulation. In Canine Rehabilitation and Physical Therapy, 2nd ed.; Millis, D., David, L., Eds.; Elsevier Health Sciences: Philadelphia, PA, USA, 2014; pp. 342–358. [Google Scholar]
- Sprague, S.; Goldberg, M.E. Electrotherapy and electromagnetic therapy. In Physical Rehabilitation for Veterinary Technicians and Nurses, 1st ed.; Goldberg, M.E., Tomlinson, J.E., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; pp. 241–261. [Google Scholar]
- Nakanishi, R.; Takeuchi, K.; Akizuki, K.; Nakagoshi, R.; Kakihana, H. Short-duration neuromuscular electrical stimulation increases range of motion following an increased tolerance for muscle extensibility in healthy subjects. J. Balneol. Climatol. Phys. Med. 2021, 2021, 2342. [Google Scholar]
- Uçar, N.; Öner, H.; Kuş, M.A.; Karaca, H.; Fırat, T. The effect of neuromuscular electrical stimulation applied at different muscle lengths on muscle architecture and sarcomere morphology in rats. Anat. Rec. 2023, 307, 356–371. [Google Scholar] [CrossRef]
- Susilaningsih, E.; Rahman, F. (Eds.) Case study: The influence of ultrasound and tens on increasing the range of motion of joint in frozen shoulder due to rotator cuff. In Academic Physiotherapy Conference Proceeding; Universitas Muhammadiyah Surakarta: Karangasem, Indonesia, 2021. [Google Scholar]
- Venosa, M.; Romanini, E.; Padua, R.; Cerciello, S. Comparison of high-intensity laser therapy and combination of ultrasound treatment and transcutaneous nerve stimulation in patients with cervical spondylosis: A randomized controlled trial. Lasers Med. Sci. 2019, 34, 947–953. [Google Scholar] [CrossRef]
- Hohlbaum, K.; Bert, B.; Dietze, S.; Palme, R.; Fink, H.; Thöne-Reineke, C. Severity classification of repeated isoflurane anesthesia in C57BL/6JRj mice—Assessing the degree of distress. PLoS ONE 2017, 12, e0179588. [Google Scholar] [CrossRef]
- Flecknell, P. Laboratory Animal Anaesthesia; Academic Press: London, UK, 2015. [Google Scholar]
- Council, N.R. Nutrient Requirements of Laboratory Animals; National Academy of Sciences: Washington, DC, USA, 1995. [Google Scholar]
- Pop, D.; Pop-Vancia, V.; Constantinescu, R.; Miresan, V.; Rusu, A.S.; Papuc, I. Physiological effects of human-animal positive interaction in dogs-review of the literature. Bull. Univ. Agric. Sci. Veter-Med. Cluj-Napoca Anim. Sci. Biotechnol. 2014, 71, 102–110. [Google Scholar] [CrossRef]
- Lloyd, J.K. Minimising stress for patients in the veterinary hospital: Why it is important and what can be done about it. Vet. Sci. 2017, 4, 22. [Google Scholar] [CrossRef]
- Robert, L.; Hall, A.H.S.B. Muscle. In Duncan and Prasse’s Veterinary Laboratory Medicine: Clinical Pathology, 5th ed.; Latimer, K.S., Ed.; John Wiley & Sons: West Sussex, UK, 2011; pp. 283–294. [Google Scholar]
- Kopp, J.; Loos, B.; Spilker, G.; Horch, R.E. Correlation between serum creatinine kinase levels and extent of muscle damage in electrical burns. Burns 2004, 30, 680–683. [Google Scholar] [CrossRef]
- Bardet, J. Quadriceps contracture and fracture disease. Vet. Clin. N. Am. 1987, 17, 957–973. [Google Scholar] [CrossRef]
- Carson, J.A.; Manolagas, S.C. Effects of sex steroids on bones and muscles: Similarities, parallels, and putative interactions in health and disease. Bone 2015, 80, 67–78. [Google Scholar] [CrossRef]

| Histopathological Parameters | Score | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Inflammation Inflammatory cell infiltration (leukocyte accumulation) | None (<10 cells at 20× high power field) | 1 cluster (≥10 cells at 20× high power field) | 2 clusters | ≥3 clusters |
| Vasculitis | None | 1 vessel | 2 vessels | 3 vessels |
| Fibrosis Collagen is present as pale eosinophilic fibrillar material in perimysium and endomysium | Normal level | Mild increase | Moderate increased deposition separating and surrounding adjacent fibers with attenuation of several muscle fibers | Marked presence of collagen deposits in large areas and loss of tissue architecture |
| Muscle fiber degeneration Degeneration is characterized by irregular shapes, cell swelling, or cell shrunken, hypereosinophilia (pale color), and necrosis | <5% of total area | 6–25% | 26–50% | >50% |
| Object Gene | F/R | Sequence |
|---|---|---|
| IL-1 | F | 5′-AATGACCTGTTCTTTGAGGCTGAC-3′ |
| R | 5′-CGAGATGCTGCTGTGAGATTTGAA-3′ | |
| TGF-1 | F | 5′-ATGCCAACTTCTGTCTGGGG-3′ |
| R | 5′-GGTTGTAGAGGGCAAGGACC-3′ | |
| TGF-3 | F | 5′-GGACTTCGGCCACATC-3′ |
| R | 5′-CGGGTGCTGTTGTAAA-3′ | |
| HIF-1 | F | 5′-TGCTGGCTCCCTATATCCCA-3′ |
| R | 5′-GGAGGGCTTGGAGAATTGCT-3′ | |
| β-actin | F | 5′-GCAGGAGTACGATGAGTCCG-3′ |
| R | 5′-ACGCAGCTCAGTAACAGTCC-3′ |
| Parameters | Day | Group | |||
|---|---|---|---|---|---|
| S | U | N | UN | ||
| Body weight | 15 | 250.3 (233.4–258.4) | 251.3 (241.5–262.8) | 256.5 (235.0–263.4) | 252.3 (247.0–266.6) |
| 22 | 231.0 (225.0–236.8) | 230.3 a (213.0–239.8) | 232.5 (223.5–235.1) | 237.8 (232.0–244.0) | |
| 29 | 246.0 (238.1–255.3) | 233.5 (227.1–248.0) | 244.5 (237.3–250.0) | 245.8 (242.5–260.3) | |
| 36 | 259.8 b (245.4–266.3) | 249.3 (236.8–256.4) | 253.5 (246.0–254.1) | 254.8 b (251.3–271.5) | |
| 43 | 269.3 b (254.8–275.1) | 254.0 bc (244.3–265.0) | 260.0 bc (254.6–265.0) | 260.3 bc (257.0–277.0) | |
| Food intake | 15 | 13.9 (10.0–16.3) | 14.7 (10.4–16.0) | 15.6 (12.9–18.1) | 14.0 (11.0–20.2) |
| 22 | 19.0 (17.1–21.5) | 15.4 (13.6–18.7) | 17.6 (14.3–21.6) | 18.8 (16.8–21.2) | |
| 29 | 19.3 (18.0–20.6) | 18.5 (18.0–19.4) | 18.6 (16.4–19.4) | 20.1 (16.9–22.6) | |
| 36 | 20.0 (17.1–21.3) | 17.5 (17.3–18.1) | 19.0 (17.4–19.5) | 18.4 (16.3–19.9) | |
| 43 | 16.6 (13.9–19.1) | 15.6 (13.4–19.4) | 17.3 (16.1–18.9) | 18.4 (17.2–19.1) | |
| Parameter | Group | |||
|---|---|---|---|---|
| S | U | N | UN | |
| Creatinine phosphokinase level (U/L) | 420 (268–964) | 534 (207–1061) | 300 (228–554) | 477 (297–852) |
| Quadriceps Muscle | Group | |||||
|---|---|---|---|---|---|---|
| C | I | S | U | N | UN | |
| Muscle weight/Body weight (mg/g) | 7.9 (7.8–8.0) | 6.1 (5.7–6.4) | 8.4 # (8.0–8.9) | 8.6 # (8.4–8.8) | 9.0 *# (8.8–9.0) | 9.1 *# (9.0–9.2) |
| Muscle measurement (mm) | ||||||
| Length | 29.3 (27.9–30.0) | 23.1 * (22.1–23.8) | 24.6 * (24.3–25.1) | 25.5* (25.3–26.1) | 27.5 #a (27.0–27.9) | 28.6 #ab (27.7–28.9) |
| Width | 14.2 (13.3–14.6) | 12.0 * (10.8–12.6) | 14.5 # (13.8–14.7) | 13.8 # (13.0–14.7) | 14.2 # (13.5–14.9) | 14.5 # (13.5–14.9) |
| Height | 11.9 (10.4–13.2) | 8.7 * (8.1–10.0) | 10.4 (9.4–11.0) | 10.5 (10.2–11.1) | 10.9 (10.7–11.4) | 11.7 # (10.9–12.0) |
| Histopathological Parameter | Group | |||||
|---|---|---|---|---|---|---|
| C | I | S | U | N | UN | |
| Inflammation | 0.0 (0.0–0.3) | 2.0 * (1.8–2.3) | 2.0 * (1.8–2.0) | 0.0 # (0.0–1.0) | 0.0 # (0.0–1.0) | 0.0 # (0.0–1.0) |
| Vasculitis | 0.0 (0.0–0.3) | 2.0 * (1.0–3.0) | 2.0 (0.8–3.0) | 0.0 # (0.0–0.3) | 0.0 # (0.0–0.3) | 0.0 # (0.0–0.3) |
| Fibrosis | 0.0 (0.0–1.0) | 3.0 * (2.0–3.0) | 1.0 (0.8–2.0) | 0.0 # (0.0–1.0) | 0.0 # (0.0–1.0) | 0.0 # (0.0–0.3) |
| Muscle degeneration | 1.0 (0.0–1.0) | 2.5 * (2.0–3.0) | 2.0 (1.0–2.0) | 1.0 # (0.0–1.3) | 1.0 # (0.8–1.0) | 1.0 # (0.0–1.0) |
| Total score | 0.5 (0.0–1.3) | 9.0 * (8.0–10.3) | 7.0 (5.0–7.3) | 1.0 # (0.8–3.3) | 1.5 # (1.0–2.3) | 1.0 # (0.8–2.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suwankanit, K.; Shimizu, M. Effects of Neuromuscular Electrical Stimulation and Therapeutic Ultrasound on Quadriceps Contracture of Immobilized Rats. Vet. Sci. 2024, 11, 158. https://doi.org/10.3390/vetsci11040158
Suwankanit K, Shimizu M. Effects of Neuromuscular Electrical Stimulation and Therapeutic Ultrasound on Quadriceps Contracture of Immobilized Rats. Veterinary Sciences. 2024; 11(4):158. https://doi.org/10.3390/vetsci11040158
Chicago/Turabian StyleSuwankanit, Kanokwan, and Miki Shimizu. 2024. "Effects of Neuromuscular Electrical Stimulation and Therapeutic Ultrasound on Quadriceps Contracture of Immobilized Rats" Veterinary Sciences 11, no. 4: 158. https://doi.org/10.3390/vetsci11040158
APA StyleSuwankanit, K., & Shimizu, M. (2024). Effects of Neuromuscular Electrical Stimulation and Therapeutic Ultrasound on Quadriceps Contracture of Immobilized Rats. Veterinary Sciences, 11(4), 158. https://doi.org/10.3390/vetsci11040158






