Identification of Sarcocystis and Trichinella Species in Muscles of Gray Wolf (Canis lupus) from Lithuania
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Morphological Examination for the Presence of Sarcocystis spp. and Trichinella spp.
2.3. Molecular Analysis of Sarcocystis spp. and Trichinella spp.
2.4. Data Analysis
3. Results
3.1. Prevalence and Morphology of Sarcocysts of Sarcocystis spp.
3.2. Genetic Characterisation and Phylogeny of S. arctica and S. svanai
3.3. Microscopical and Molecular Examination of Trichinella spp.
4. Discussion
4.1. Pathogenic Impact of Parasites Identified in Gray Wolf
4.2. Host Specificity of Sarcocystis Species from Canids
4.3. Morphological and Molecular Characteristics of Identified Sarcocystis Species
4.4. Prevalence and Species Composition of Trichinella in Gray Wolf
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boitani, L.P.; Kaczensky, F.; Alvares, H.; Andrén, V.; Balys, J.; Blanco, G.; Chapron, S.; Chiriac, D.; Cirovic, N.; Drouet-Houguet, C.; et al. Assessment of the Conservation Status of the Wolf (Canis Lupus) in Europe; Prepared for the Berne Convention on the Conservation of European Wildlife and Natural Habitats and the Council of Europe: Strasbourg, France, 2022; pp. 1–25. Available online: https://rm.coe.int/inf45e-2022-wolf-assessment-bern-convention-2791-5979-4182-1-2/1680a7fa47 (accessed on 20 November 2023).
- Nowak, R.M. A perspective on the taxonomy of wolves in North America. In Wolves in Canada and Alaska: Their Status, Biology, and Management; Carbyn, L.N., Ed.; Canadian Wildlife Service: Ottawa, ON, Canada, 1983; pp. 10–19. [Google Scholar]
- Paquet, P.C.; Carbyn, L.N. Gray wolf. In Wild Mammals of North America: Biology, Management, and Conservation, 2nd ed.; Feldhamer, A.G., Thompson, B.C., Joseph, A., Chapman, J.A., Eds.; John Hopkins University Press: Baltimore, MD, USA, 2003; pp. 482–510. [Google Scholar]
- Mech, L.D.; Boitani, L. Wolves: Behavior, Ecology, and Conservation; The University of Chicago Press: Chicago, IL, USA, 2003. [Google Scholar]
- Ciucci, P.; Reggioni, W.; Maiorano, L.; Boitani, L. Long-distance dispersal of a rescued wolf from the northern Apennines to the western Alps. J. Wildl. Manage 2009, 73, 1300–1306. [Google Scholar] [CrossRef]
- Hindrikson, M.; Remm, J.; Pilot, M.; Godinho, R.; Stronen, A.V.; Baltrūnaitė, L.; Czarnomska, S.D.; Leonard, J.A.; Randi, E.; Nowak, C.; et al. Wolf population genetics in Europe: A systematic review, meta-analysis and suggestions for conservation and management. Biol. Rev. 2017, 92, 1601–1629. [Google Scholar] [CrossRef] [PubMed]
- Lesniak, I.; Heckmann, I.; Franz, M.; Greenwood, A.D.; Heitlinger, E.; Hofer, H.; Krone, O. Recolonizing gray wolves increase parasite infection risk in their prey. Ecol. Evol. 2018, 8, 2160–2170. [Google Scholar] [CrossRef]
- Newsome, T.M.; Boitani, L.; Chapron, G.; Ciucci, P.; Dickman, C.R.; Dellinger, J.A.; López-Bao, J.V.; Peterson, R.O.; Shores, C.R.; Wirsing, A.J.; et al. Food habits of the world’s grey wolves. Mamm. Rev. 2016, 46, 255–269. [Google Scholar] [CrossRef]
- Craig, H.L.; Craig, P.S. Helminth parasites of wolves (Canis lupus): A species list and an analysis of published prevalence studies in Nearctic and Palaearctic populations. J. Helminthol. 2005, 79, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Bagrade, G.; Kirjušina, M.; Vismanis, K.; Ozoliņš, J. Helminth parasites of the wolf Canis lupus from Latvia. J. Helminthol. 2009, 83, 63–68. [Google Scholar] [CrossRef]
- Muñoz, S.; Ramos, P.L.; Carretón, E.; Diosdado, A.; González-Miguel, J.; Simón, F.; Morchón, R. Intestinal helminths in Iberian wolves (Canis lupus signatus) from Northwest Spain. Parasitol. 2018, 6, 106–111. [Google Scholar] [CrossRef]
- Bindke, J.D.; Springer, A.; Janecek-Erfurth, E.; Böer, M.; Strube, C. Helminth infections of wild European gray wolves (Canis lupus Linnaeus, 1758) in Lower Saxony, Germany, and comparison to captive wolves. Parasitol. Res. 2019, 118, 701–706. [Google Scholar] [CrossRef]
- Ricchiuti, L.; Petrini, A.; Interisano, M.; Ruberto, A.; Salucci, S.; Marino, L.; Del Riccio, A.; Cocco, A.; Badagliacca, P.; Pozio, E. First report of Trichinella pseudospiralis in a wolf (Canis lupus italicus). Int. J. Parasitol. Parasites Wildl. 2021, 15, 195–198. [Google Scholar] [CrossRef]
- Calero-Bernal, R.; Cerqueira-Cézar, C.K.; Verma, S.K.; Mowery, J.; Carmena, D.; Beckmen, K.; Dubey, J.P. Sarcocystis arctica (Apicomplexa: Sarcocystidae): Ultrastructural description and its new host record, the Alaskan wolf (Canis lupus). Parasitol. Res. 2016, 115, 2893–2897. [Google Scholar] [CrossRef]
- Dubey, J.P.; Calero-Bernal, R.; Rosenthal, B.M.; Speer, C.A.; Fayer, R. Sarcocystosis of Animals and Humans, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Rosenthal, B.M. Zoonotic Sarcocystis. Res. Vet. Sci. 2021, 136, 151–157. [Google Scholar] [CrossRef]
- Pozio, E. Adaptation of Trichinella spp. for survival in cold climates. Food Waterborne Parasitol. 2016, 4, 4–12. [Google Scholar] [CrossRef]
- Mehlhorn, H.; Heydorn, A.O. The sarcosporidia (Protozoa, Sporozoa): Life cycle and fine structure. Adv. Parasitol. 1978, 16, 43–91. [Google Scholar] [CrossRef] [PubMed]
- Fayer, R.; Esposito, D.H.; Dubey, J.P. Human infections with Sarcocystis species. Clin. Microbiol. Rev. 2015, 28, 295–311. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.A.; Evans, L. Prevalence of Sarcocystis spp. in two subspecies of caribou (Rangifer tarandus) in Newfoundland and Labrador, and foxes (Vulpes vulpes), wolves (Canis lupus), and husky dogs (Canis familiaris) as potential definitive hosts. J. Parasitol. 2006, 92, 662–663. [Google Scholar] [CrossRef]
- Dubey, J.P.; Sykes, J.E.; Shelton, G.D.; Sharp, N.; Verma, S.K.; Calero-Bernal, R.; Viviano, J.; Sundar, N.; Khan, A.; Grigg, M.E. Sarcocystis caninum and Sarcocystis svanai n. spp. (Apicomplexa: Sarcocystidae) associated with severe myositis and hepatitis in the domestic dog (Canis familiaris). J. Eukaryot. Microbiol. 2015, 62, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Liang, Y.; Hu, J.; Huang, Z.; Zhang, Y. First isolation of Sarcocystis caninum sarcocysts from two domestic dogs (Canis familiaris) from China. Parasitol. Res. 2018, 117, 3613–3618. [Google Scholar] [CrossRef] [PubMed]
- Kirillova, V.; Prakas, P.; Calero-Bernal, R.; Gavarāne, I.; Fernández-García, J.L.; Martínez-González, M.; Rudaitytė-Lukošienė, E.; Martínez-Estéllez, M.Á.H.; Butkauskas, D.; Kirjušina, M. Identification and genetic characterization of Sarcocystis arctica and Sarcocystis lutrae in red foxes (Vulpes vulpes) from Baltic States and Spain. Parasit. Vectors. 2018, 11, 1–9. [Google Scholar] [CrossRef]
- Prakas, P.; Strazdaitė-Žielienė, Ž.; Rudaitytė-Lukošienė, E.; Servienė, E.; Butkauskas, D. Molecular identification of Sarcocystis lutrae (Apicomplexa: Sarcocystidae) in muscles of five species of the family Mustelidae. Parasitol. Res. 2018, 117, 1989–1993. [Google Scholar] [CrossRef]
- Pozio, E.; Rossi, P. Guidelines for the identification and development of sampling methods and design of suitable protocols for monitoring of Trichinella infection in indicator species. Ann. Ist. Super. Sanita. 2008, 44, 200–204. [Google Scholar]
- Pozio, E. New patterns of Trichinella infection. Vet. Parasitol. 2001, 98, 133–148. [Google Scholar] [CrossRef]
- Pozio, E. The broad spectrum of Trichinella hosts: From cold- to warm-blooded animals. Vet. Parasitol. 2005, 132, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Järvis, T.; Miller, I.; Pozio, E. Epidemiological studies on animal and human trichinellosis in Estonia. Parasite 2001, 8, S86–S87. [Google Scholar] [CrossRef] [PubMed]
- Pozio, E.; Casulli, A.; Bologov, V.V.; Marucci, G.; La Rosa, G. Hunting practices increase the prevalence of Trichinella infection in wolves from European Russia. J. Parasitol. 2001, 87, 1498–1501. [Google Scholar] [CrossRef] [PubMed]
- Segovia, J.M.; Torres, J.; Miquel, J.; Llaneza, L.; Feliu, C. Helminths in the wolf, Canis lupus, from North-Western Spain. J. Helminthol. 2001, 75, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Pozio, E.; La Rosa, G. PCR-derived methods for the identification of Trichinella parasites from animal and human samples. Methods Mol. Biol. 2003, 216, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Malakauskas, A.; Paulauskas, V.; Järvis, T.; Keidans, P.; Eddi, C.; Kapel, C.M.O. Molecular epidemiology of Trichinella spp. in three Baltic countries: Lithuania, Latvia, and Estonia. Parasitol. Res. 2007, 100, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Mayer-Scholl, A.; Pozio, E.; Gayda, J.; Thaben, N.; Bahn, P.; Nöckler, K. Magnetic stirrer method for the detection of Trichinella larvae in muscle samples. J. Vis. Exp. 2017, 3, 55354. [Google Scholar] [CrossRef]
- European Union Reference Laboratory for Parasites; Istituto Superiore di Sanita. Identification of Trichinella Muscle Stage Larvae at the Species Level by Multiplex PCR. Available online: https://www.iss.it/documents/20126/0/Instruction-PT-03-rev-5+%281%29.pdf/ (accessed on 21 January 2024).
- Prakas, P.; Butkauskas, D.; Švažas, S.; Stanevičius, V. Morphological and genetic characterisation of Sarcocystis halieti from the great cormorant (Phalacrocorax carbo). Parasitol. Res. 2018, 117, 3663–3667. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Reiczigel, J. Confidence intervals for the binomial parameter: Some new considerations. Stat. Med. 2003, 22, 611–621. [Google Scholar] [CrossRef]
- Reiczigel, J.; Marozzi, M.; Fábián, I.; Rózsa, L. Biostatistics for parasitologists—A primer to quantitative parasitology. Trends Parasitol. 2019, 35, 277–281. [Google Scholar] [CrossRef]
- Juozaitytė-Ngugu, E.; Švažas, S.; Šneideris, D.; Rudaitytė-Lukošienė, E.; Butkauskas, D.; Prakas, P. The role of birds of the family Corvidae in transmitting Sarcocystis protozoan parasites. Animals 2021, 11, 3258. [Google Scholar] [CrossRef]
- Máca, O.; González-Solís, D. Sarcocystis cristata sp. nov. (Apicomplexa, Sarcocystidae) in the imported great blue turaco Corythaeola cristata (Aves, Musophagidae). Parasit. Vectors. 2021, 14, 1–7. [Google Scholar] [CrossRef]
- Dubey, J.P.; Chapman, J.L.; Rosenthal, B.M.; Mense, M.; Schueler, R.L. Clinical Sarcocystis neurona, Sarcocystis canis, Toxoplasma gondii, and Neospora caninum infections in dogs. Vet. Parasitol. 2006, 137, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Calero-Bernal, R.; Mauroo, N.F.; Hui, S.W.; Kuiken, T.; van de Bildt, M.W.; de Jong, A.W.; Osterhaus, A.D.; Sims, L.; Gendron-Fitzpatrick, A.; Carmena, D.; et al. Acute fatal sarcocystosis hepatitis in an Indo-Pacific bottlenose dolphin (Tursiops aduncus) in Hong Kong. Vet. Parasitol. 2017, 15, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Speer, C.A. Sarcocystis canis n. sp. (Apicomplexa: Sarcocystidae), the etiologic agent of generalized coccidiosis in dogs. J. Parasitol. 1991, 77, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.; Mense, M.; Dubey, J.P. Clinical muscular sarcocystosis in a dog. J. Parasitol. 2005, 91, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Cooley, A.J.; Barr, B.; Rejmanek, D. Sarcocystis neurona encephalitis in a dog. Vet. Pathol. 2007, 44, 956–961. [Google Scholar] [CrossRef]
- Sykes, J.E.; Dubey, J.P.; Lindsay, L.L.; Prato, P.; Lappin, M.R.; Guo, L.T.; Mizisin, A.P.; Shelton, G.D. Severe myositis associated with Sarcocystis spp. infection in 2 dogs. J. Vet. Intern. Med. 2011, 25, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Howe, D.K.; Furr, M.; Grigg, M.E.; Saville, W.J.; Marsh, A.E.; Reed, S.M. An update on Sarcocystis neurona infections in animals and equine protozoal myeloencephalitis (EPM). Vet. Parasitol. 2015, 209, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Hagner, K.; Jokinen, T.S.; Lavikainen, A.; Sukura, A. Acute fulminant necrotizing myopathy in a dog caused by co-infection with ultrastructural Sarcocystis caninum and Sarcocystis svanai-like apicomplexan protozoa. Vet. Parasitol. 2018, 252, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Berrocal, A.; López, A. Pulmonary sarcocystosis in a puppy with canine distemper in Costa Rica. J. Vet. Diagn. Invest. 2003, 15, 292–294. [Google Scholar] [CrossRef] [PubMed]
- Gottstein, B.; Pozio, E.; Nöckler, K. Epidemiology, diagnosis, treatment, and control of trichinellosis. Clin. Microbiol. Rev. 2009, 22, 127–145. [Google Scholar] [CrossRef]
- Kirjušina, M.; Deksne, G.; Marucci, G.; Bakasejevs, E.; Jahundoviča, I.; Daukšte, A.; Zdankovska, A.; Bērziņa, Z.; Esīte, Z.; Bella, A.; et al. A 38-year study on Trichinella spp. in wild boar (Sus scrofa) of Latvia shows a stable incidence with an increased parasite biomass in the last decade. Parasite. Vectors 2015, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Schellenberg, R.S.; Tan, B.J.; Irvine, J.D.; Stockdale, D.R.; Gajadhar, A.A.; Serhir, B.; Botha, J.; Armstong, C.A.; Shirley, A.W.; Blondeau, J.M.; et al. An outbreak of trichinellosis due to consumption of bear meat infected with Trichinella nativa in 2 northern Saskatchewan communities. J. Infect. Dis. 2003, 188, 835–843. [Google Scholar] [CrossRef]
- Ozeretskovskaya, N.N.; Mikhailova, L.G.; Sabgaida, T.P.; Dovgalev, A.S. New trends and clinical patterns of human trichinellosis in Russia at the beginning of the XXI century. Vet. Parasitol. 2005, 132, 167–171. [Google Scholar] [CrossRef]
- Møller, L.N.; Petersen, E.; Kapel, C.M.; Melbye, M.; Koch, A. Outbreak of trichinellosis associated with consumption of game meat in West Greenland. Vet. Parasitol. 2005, 132, 131–136. [Google Scholar] [CrossRef]
- Dworkin, M.S.; Gamble, H.R.; Zarlenga, D.S.; Tennican, P.O. Outbreak of trichinellosis associated with eating cougar jerky. J. Infect. Dis. 1996, 174, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Kociecka, W. Trichinellosis: Human disease, diagnosis and treatment. Vet. Parasitol. 2000, 93, 365–383. [Google Scholar] [CrossRef]
- Diaz, J.H.; Warren, R.J.; Oster, M.J. The disease ecology, epidemiology, clinical manifestations, and management of trichinellosis linked to consumption of wild animal meat. Wilderness Environ. Med. 2020, 31, 235–244. [Google Scholar] [CrossRef]
- Bruschi, F.; Murrell, K.D. New aspects of human trichinellosis: The impact of new Trichinella species. Postgrad. Med. J. 2002, 78, 15–22. [Google Scholar] [CrossRef]
- Pavic, S.; Andric, A.; Sofronic-Milosavljevic, L.J.; Gnjatovic, M.; Mitić, I.; Vasilev, S.; Sparic, R.; Pavic, A. Trichinella britovi outbreak: Epidemiological, clinical, and biological features. Med. Mal. Infect. 2020, 50, 520–524. [Google Scholar] [CrossRef]
- Kapel, C.M.O. Host diversity and biological characteristics of the Trichinella genotypes and their effect on transmission. Vet. Parasitol. 2000, 93, 263–278. [Google Scholar] [CrossRef]
- Scioscia, N.P.; Olmos, L.; Gorosábel, A.; Bernad, L.; Pedrana, J.; Hecker, Y.P.; Gual, I.; Gos, M.L.; Denegri, G.M.; Moore, D.P.; et al. Pampas fox (Lycalopex gymnocercus) new intermediate host of Sarcocystis svanai (Apicomplexa: Sarcocystidae). Parasitol. Int. 2017, 66, 214–218. [Google Scholar] [CrossRef]
- Gjerde, B.; Schulze, J. Muscular sarcocystosis in two arctic foxes (Vulpes lagopus) due to Sarcocystis arctica n. sp.: Sarcocyst morphology, molecular characteristics and phylogeny. Parasitol. Res. 2014, 113, 811–821. [Google Scholar] [CrossRef]
- Cerqueira-Cézar, C.K.; Thompson, P.C.; Verma, S.K.; Mowery, J.; Calero-Bernal, R.; Antunes Murata, F.H.; Sinnett, D.R.; Van Hemert, C.; Rosenthal, B.M.; Dubey, J.P. Morphological and molecular characterization of Sarcocystis arctica-like sarcocysts from the Arctic fox (Vulpes lagopus) from Alaska, USA. Parasitol. Res. 2017, 116, 1871–1878. [Google Scholar] [CrossRef]
- Pavlásek, I.; Máca, O. Morphological and molecular identification of Sarcocystis arctica sarcocysts in three red foxes (Vulpes vulpes) from the Czech Republic. Parasitol. Int. 2017, 66, 603–605. [Google Scholar] [CrossRef]
- Gjerde, B.; Josefsen, T.D. Molecular characterisation of Sarcocystis lutrae n. sp. and Toxoplasma gondii from the musculature of two Eurasian otters (Lutra lutra) in Norway. Parasitol. Res. 2015, 114, 873–886. [Google Scholar] [CrossRef]
- Lepore, T.; Bartley, P.M.; Chianini, F.; Macrae, A.I.; Innes, E.A.; Katzer, F. Molecular detection of Sarcocystis lutrae in the European badger (Meles meles) in Scotland. Parasitol. 2017, 144, 1426–1432. [Google Scholar] [CrossRef] [PubMed]
- Máca, O. Molecular identification of Sarcocystis lutrae in the European otter (Lutra lutra) and the European badger (Meles meles) from the Czech Republic. Parasitol. Res. 2018, 117, 943–945. [Google Scholar] [CrossRef] [PubMed]
- Máca, O. Molecular identification of Sarcocystis lutrae (Apicomplexa: Sarcocystidae) from the raccoon dog, Nyctereutes procyonoides, and the common raccoon, Procyon lotor, in the Czech Republic. Parasit. Vectors. 2020, 13, 231. [Google Scholar] [CrossRef] [PubMed]
- Prakas, P.; Butkauskas, D.; Rudaitytė, E.; Kutkienė, L.; Sruoga, A.; Pūraitė, I. Morphological and molecular characterization of Sarcocystis taeniata and Sarcocystis pilosa n. sp. from the sika deer (Cervus nippon) in Lithuania. Parasitol. Res. 2016, 115, 3021–3032. [Google Scholar] [CrossRef] [PubMed]
- Moks, E.; Jõgisalu, I.; Saarma, U.; Talvik, H.; Järvis, T.; Valdmann, H. Helminthologic survey of the wolf (Canis lupus) in Estonia, with an emphasis on Echinococcus granulosus. J. Wildl. Dis. 2006, 42, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Bakasejevs, E.; Daukšte, A.; Zolovs, M.; Zdanovska, A. Investigation of Trichinella in wildlife in Latgale region (Latvia). Acta. Biol. Daugavp. 2012, 12, 1–5. [Google Scholar]
- Deksne, G.; Segliņa, Z.; Jahundoviča, I.; Esīte, Z.; Bakasejevs, E.; Bagrade, G.; Keidane, D.; Interisano, M.; Marucci, G.; Tonanzi, D.; et al. High prevalence of Trichinella spp. in sylvatic carnivore mammals of Latvia. Vet. Parasitol. 2016, 231, 118–123. [Google Scholar] [CrossRef]
- Teodorović, V.; Vasilev, D.; Ćirović, D.; Marković, M.; Ćosić, N.; Djurić, S.; Djurković-Djaković, O. The wolf (Canis lupus) as an indicator species for the sylvatic Trichinella cycle in the Central Balkans. J. Wildl. Dis. 2014, 50, 911–915. [Google Scholar] [CrossRef]
- Airas, N.; Saari, S.; Mikkonen, T.; Virtala, A.M.; Pellikka, J.; Oksanen, A.; Isomursu, M.; Kilpelä, S.S.; Lim, C.W.; Sukura, A. Sylvatic Trichinella spp. infection in Finland. J. Parasitol. 2010, 96, 67–76. [Google Scholar] [CrossRef]
- Beck, R.; Beck, A.; Kusak, J.; Mihaljević, Ž.; Lučinger, S.; Živičnjak, T.; Huber, D.; Gudan, A.; Marinculić, A. Trichinellosis in wolves from Croatia. Vet. Parasitol. 2009, 159, 308–311. [Google Scholar] [CrossRef]
- Bień, J.; Moskwa, B.; Goździk, K.; Cybulska, A.; Kornacka, A.; Welc, M.; Popiołek, M.; Cabaj, W. The occurrence of nematodes of the genus Trichinella in wolves (Canis lupus) from the Bieszczady Mountains and Augustowska Forest in Poland. Vet. Parasitol. 2016, 231, 115–117. [Google Scholar] [CrossRef]
- Blaga, R.; Gherman, C.; Cozma, V.; Zocevic, A.; Pozio, E.; Boireau, P. Trichinella species circulating among wild and domestic animals in Romania. Vet. Parasitol. 2009, 159, 218–221. [Google Scholar] [CrossRef]
- Zarnke, R.L.; Worley, D.E.; Ver Hoef, J.M.; McNay, M.E. Trichinella sp. in wolves from interior Alaska. J. Wildl. Dis. 1999, 35, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Badagliacca, P.; Di Sabatino, D.; Salucci, S.; Romeo, G.; Cipriani, M.; Sulli, N.; Dall’Acqua, F.; Ruggieri, M.; Calistri, P.; Morelli, D. The role of the wolf in endemic sylvatic Trichinella britovi infection in the Abruzzi region of Central Italy. Vet. Parasitol. 2016, 231, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Carrasco, C.; Moroni, B.; García-Garrigós, A.; Robetto, S.; Carella, E.; Zoppi, S.; Tizzani, P.; Gonzálvez, M.; Orusa, R.; Rossi, L. Wolf Is Back: A Novel Sensitive Sentinel Rejoins the Trichinella Cycle in the Western Alps. Vet. Sci. 2023, 10, 206. [Google Scholar] [CrossRef]
- Sharma, R.; Thompson, P.C.; Hoberg, E.P.; Scandrett, W.B.; Konecsni, K.; Harms, N.J.; Kukka, P.M.; Jung, T.S.; Elkin, B.; Mulders, R.; et al. Hiding in plain sight: Discovery and phylogeography of a cryptic species of Trichinella (Nematoda: Trichinellidae) in wolverine (Gulo gulo). Int. J. Parasitol. 2020, 50, 277–287. [Google Scholar] [CrossRef]
- Gómez-Morales, M.A.; Ludovisi, A.; Amati, M.; Cherchi, S.; Tonanzi, D.; Pozio, E. Differentiation of Trichinella species (Trichinella spiralis/Trichinella britovi versus Trichinella pseudospiralis) using western blot. Parasite. Vectors 2018, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pozio, E.; Rinaldi, L.; Marucci, G.; Musella, V.; Galati, F.; Cringoli, G.; Boireau, P.; La Rosa, G. Hosts and habitats of Trichinella spiralis and Trichinella britovi in Europe. Int. J. Parasitol. 2009, 39, 71–79. [Google Scholar] [CrossRef]
- Senutaitė, J.; Grikienienė, J. Prevalence of Trichinella in muscles of some domestic and wild mammals in Lithuania and their impact on the organism. Acta Zool. Litu. 2001, 11, 395–404. [Google Scholar] [CrossRef]
- Trichineliozės Pamiršti Negalima. Available online: https://nmvrvi.lt/trichineliozes-pamirsti-negalima/ (accessed on 5 December 2023).

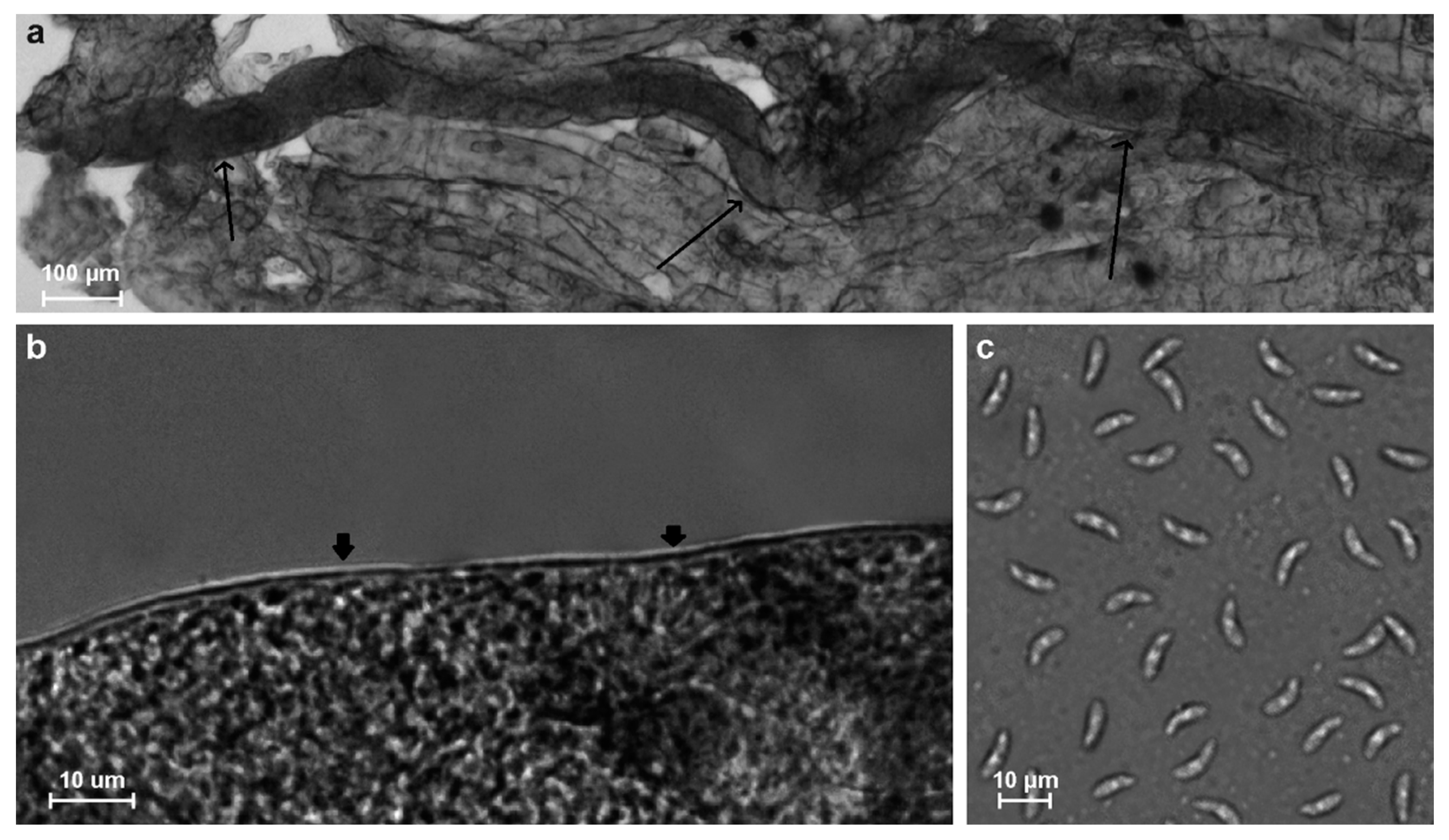
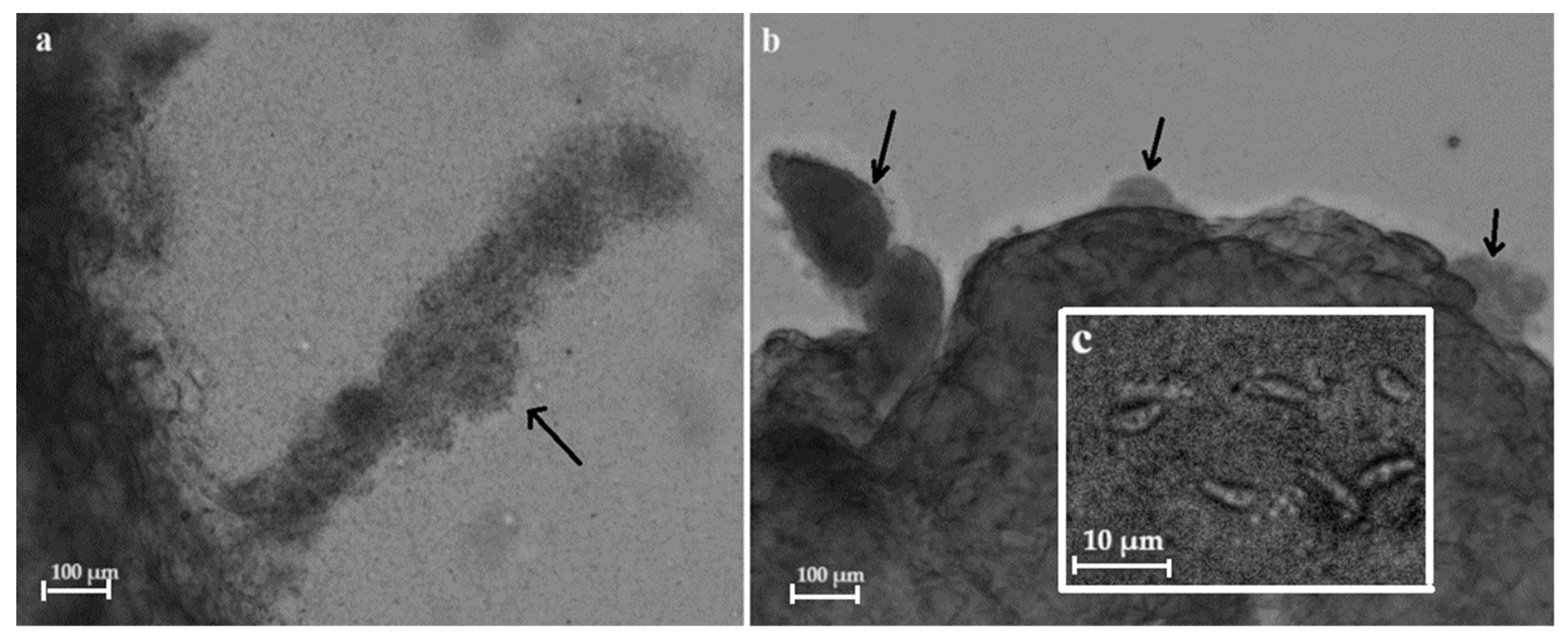
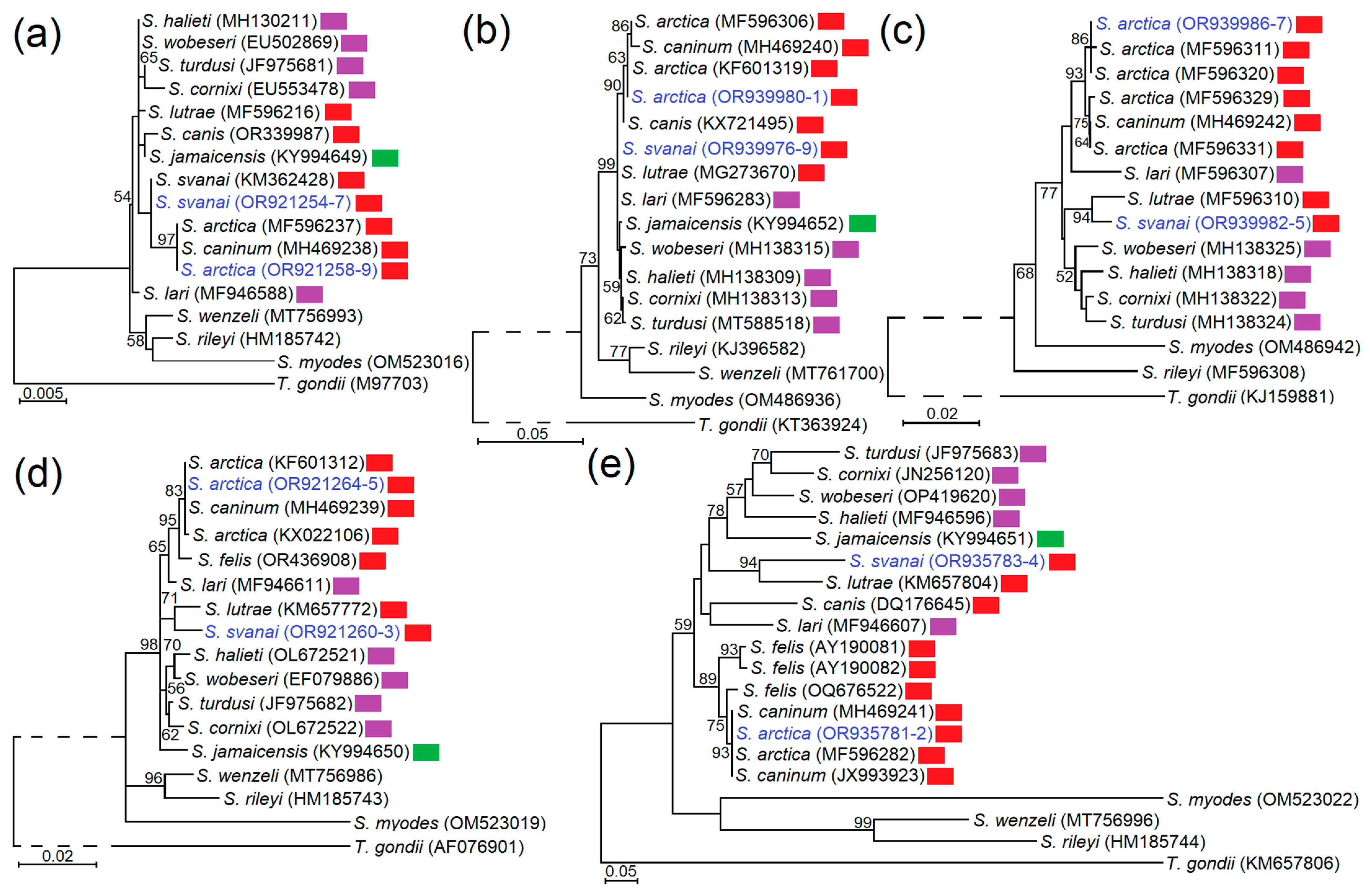

| No. | lpg | Trichinella Genotype | Sarcocystis spp. | Sarcocystis Species | ||
|---|---|---|---|---|---|---|
| Diaphragm | Limb | Diaphragm | Limb | |||
| 1 | No data | 46 | T3 | - | - | |
| 2 | No data | 16 | T3 | - | - | |
| 3 | 12.04 | 2.26 | T3 | 7 | - | S. svanai |
| 4 | - | - | - | - | - | |
| 5 | 2.24 | 0.96 | T3 | - | - | |
| 6 | - | - | - | - | - | |
| 7 | 0.42 | 17.58 | T3 | - | - | |
| 8 | 0.2 | 3.58 | T3 | 1 | - | S. svanai |
| 9 | 6.7 | 1.96 | T3 | - | - | |
| 10 | 0.64 | 0.7 | T3 | 58 | 45 | S. svanai and S. arctica |
| 11 | 3.32 | 4 | T3 | - | - | |
| 12 | 6.24 | 2.5 | T3 | - | - | |
| 13 | 9.06 | 1.74 | T3 | - | - | |
| 14 | - | - | - | 1 | - | S. svanai and S. arctica |
| 15 | 17 | 21 | T3 | - | - | |
| Genetic Loci | S. arctica/S. caninum a | S. svanai | Differences between S. arctica and S. svanai | ||
|---|---|---|---|---|---|
| Intraspecific Differences b | Interspecific Differences | Intraspecific Differences b | Interspecific Differences | ||
| 18S rRNA | 0 | ≥0.5 | 0.1 | ≥0.2 | 0.5 |
| 28S rRNA | 0–0.1 | ≥0.5 | 0 c | ≥1.5 | 1.5–1.7 |
| ITS1 | 0–0.5 | ≥3.7 | 0 c | ≥25.0 | 19.0 d |
| cox1 | 0–0.3 | ≥0.2 | 0 c | ≥0 | 0.6 |
| rpob | 0–0.2 | ≥1.6 | 0 | ≥1.2 | 2.0–2.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juozaitytė-Ngugu, E.; Maziliauskaitė, E.; Kirjušina, M.; Prakas, P.; Vaitkevičiūtė, R.; Stankevičiūtė, J.; Butkauskas, D. Identification of Sarcocystis and Trichinella Species in Muscles of Gray Wolf (Canis lupus) from Lithuania. Vet. Sci. 2024, 11, 85. https://doi.org/10.3390/vetsci11020085
Juozaitytė-Ngugu E, Maziliauskaitė E, Kirjušina M, Prakas P, Vaitkevičiūtė R, Stankevičiūtė J, Butkauskas D. Identification of Sarcocystis and Trichinella Species in Muscles of Gray Wolf (Canis lupus) from Lithuania. Veterinary Sciences. 2024; 11(2):85. https://doi.org/10.3390/vetsci11020085
Chicago/Turabian StyleJuozaitytė-Ngugu, Evelina, Evelina Maziliauskaitė, Muza Kirjušina, Petras Prakas, Rasa Vaitkevičiūtė, Jolanta Stankevičiūtė, and Dalius Butkauskas. 2024. "Identification of Sarcocystis and Trichinella Species in Muscles of Gray Wolf (Canis lupus) from Lithuania" Veterinary Sciences 11, no. 2: 85. https://doi.org/10.3390/vetsci11020085
APA StyleJuozaitytė-Ngugu, E., Maziliauskaitė, E., Kirjušina, M., Prakas, P., Vaitkevičiūtė, R., Stankevičiūtė, J., & Butkauskas, D. (2024). Identification of Sarcocystis and Trichinella Species in Muscles of Gray Wolf (Canis lupus) from Lithuania. Veterinary Sciences, 11(2), 85. https://doi.org/10.3390/vetsci11020085









