Simple Summary
The gray wolf (Canis lupus) is the largest representative of the family Canidae widespread in Eurasia and North America. Sarcocystis and Trichinella parasites were previously reported in the muscles of gray wolves. Apicomplexan Sarcocystis forms sarcocysts in the muscles of intermediate hosts and develops sporocysts in the intestines of definite hosts. Members of the genus Trichinella are cosmopolitan hazardous nematodes. The species composition of these parasites in gray wolves from Lithuania has not been studied so far. We examined muscle samples from 15 gray wolves, and species of parasites were confirmed using DNA analysis methods. Microscopically, Trichinella larvae were observed in 12 animals, and sarcocysts formed by Sarcocystis spp. were noticed in four. Trichinella britovi was also identified in the examined wolves. Current data show that zoonotic T. britovi is the dominant Trichinella species in gray wolves from nearby countries. In the case of Sarcocystis, two animals harbored S. svanai, and another two individuals were infected by S. svanai and S. arctica. Future studies are needed to assess the pathogenesis of the identified Sarcocystis spp.
Abstract
Apicomplexan Sarcocystis and Trichinella nematodes are food-borne parasites whose life cycle is carried-out in various wildlife and domestic animals. The gray wolf (Canis lupus) is an apex predator acting as an ecosystem engineer. This study aimed to identify the species of Sarcocystis and Trichinella found in the muscles of gray wolves in Lithuania. During the 2017–2022 period, diaphragm, heart, and hind leg samples of 15 animals were examined. Microscopical analysis showed the presence of two types of Sarcocystis parasites in 26.7% of the analyzed muscle samples. Based on the sequencing of five loci, nuclear 18S rDNA, 28S rDNA, ITS1, mitochondrial cox1, and apicoplast rpoB, S. arctica, and S. svanai were identified. The current work presents the first report of S. svanai in gray wolf. Phylogenetically, S. svanai clustered together with S. lutrae, infecting various carnivorans, and S. arctica was most closely related to S. felis from domestic cats. Trichinella spp. were found in 12 gray wolves (80%). For the first time, Trichinella species were molecularly identified in gray wolves from Lithuania. Trichinella britovi was confirmed in all of the isolated Trichinella larvae using a multiplex PCR. Gray wolves in Lithuania may serve as a major source of zoonotic pathogens due to the presence of these parasites.
Keywords:
Sarcocystis; Trichinella; gray wolf; molecular identification; host-specificity; phylogeny 1. Introduction
Within the European Union (EU) (Large Carnivore Initiative for Europe 2022), the gray wolf (Canis lupus) population is estimated to be around 19,000 animals across the 27 EU Member States. In 2016, a population of 14,300 gray wolves was assessed [1]. Likewise, the gray wolf population grows in Lithuania. A 2021 report from the LIFE project shows that are about 504 wolves in the country. The gray wolf is a protected species in Europe according to the Council Directive 92/43/EEC on the Conservation of Natural Habitats and of Wild Fauna and Flora [1].
The gray wolf has the most extensive distributional range of any terrestrial mammal, encompassing North America, Europe, and Asia [2,3]. The animal can be found in many different places, such as deserts, grasslands, mountains, taiga, temperate forests, and arctic tundra [2,3,4]. This carnivore is the largest extant member of the family Canidae and is considered a habitat generalist, highly territorial, mobile, and has large individual territories [2,3,4,5]. Canis lupus is an apex predator species that indicates environmental health and plays a prominent role in any ecosystem they inhabit as ecosystem engineers [2,3,6]. The gray wolf primarily preys on red deer (Cervus elaphus), roe deer (Capreolus capreolus), elk (Cervus canadensis), American bison (Bison bison), wild boar (Sus scrofa), and other ungulates [2,3,4,7]. They also hunt small animals like beavers, rodents, and hares [2,8]. Canis lupus serves as a host for various parasites, including nematodes such as Ancylostoma spp. [9,10,11,12], Capillaria/Eucoleus spp. [9,11,12], Trichinella spp. [10,13], Trichuris spp. [9,11,12], Toxocara spp. [9,11,12], Uncinaria spp. [9,10,11,12], cestodes of Echinococcus spp. [7,10], Taenia spp. [9,10,11,12] and trematodes, as Alaria alata [9,10,11,12] and numerous unicellular organisms such as Sarcocystis spp. [14,15].
Zoonotic Trichinella spp. and Sarcocystis spp. can be found in the muscle tissue of gray wolves [10,13,14,15,16]. Sarcocystis (Apicomplexa: Sarcocystidae) and Trichinella (Nematoda: Trichinellidae) are worldwide-distributed parasites that infect mammals, birds, and reptiles [10,13,14,15,16,17].
Apicomplexan parasites of the genus Sarcocystis have an obligatory two-host life cycle based on a nutritional predator–prey relationship [15,16]. Asexual stages (merogony) develop only in the intermediate host (IH) (prey). During the stages of merogony and nuclear division, a motile merozoite forms [18]. Through the process of endodyogeny, banana-shaped zoites called bradyzoites are produced, which are located in the medullas of sarcocysts [19]. The IH acquires infection by ingesting food or water contaminated with excreted sporocysts. The sexual stages (gametogony) and sporulation of oocysts in the intestine evolve only in the definitive host (DH) (predator or scavenger) [18,19]. The DH becomes infected by consuming tissues harboring intracellular tissue cysts called sarcocysts [15,19]. Sarcocystis is a common genus of parasite in the Apicomplexa phylum, with over 200 known species [15]. Gray wolves usually act as DHs for numerous Sarcocystis spp. by producing sporocysts in their intestines [7,15,20]. However, this carnivore can also become an IH for Sarcocystis species. To date, only S. arctica has been described in the tongue muscles of the Alaskan wolf (Canis lupus) in 2016 [14]. Notably, it has been considered that S. arctica and S. caninum, described in the muscles of domestic dog (Canis familiaris), are the same species of Sarcocystis, and S. caninum is assumed to be a junior synonym of S. arctica [21,22].
Two Sarcocystis species, S. arctica and S. lutrae, have been identified in the muscles of Lithuanian carnivorans. Both of these species were detected in the hind leg muscles of the red fox (Vulpes vulpes) [23]. In addition, S. lutrae has been identified in the muscles of various mustelids, including the American mink (Neovison vison), the beech marten (Martes foina), the Eurasian badger (Meles meles), the Eurasian otter (Lutra lutra), and the European polecat (Mustela putorius) [24]. Until now, gray wolves have not been investigated as IHs for Sarcocystis parasites in Lithuania.
Trichinella nematodes have an exclusive life cycle, which contains two generations of parasites in the same host [25]. These parasites are released from larvae in the stomach after eating infected meat. The Trichinella larvae enter the intestinal lining, mature into adult stage, and then the adult males and females mate. Adult female worms release newborn larvae that can travel through the blood and lymphatic vessels in the body. Once the newborn larvae reach the striated muscle, they actively penetrate the muscle cells. The larvae mature inside infected host muscles (forming nurse cells) [26]. Trichinella parasites are circulated in two cycles maintained in nature, in domestic animals, for instance, in swine (Sus scrofa domesticus), horse (Equus caballus), and in sylvatic ones, for example, in the wild boar, the gray wolf, and the red fox [10,17,26,27,28]. In 2001, Trichinella species were confirmed in wolves using genetic methods in Estonia, Russia, and Spain [29,30,31]. Since then, these parasites have been extensively studied throughout Europe using multiplex PCRs [32]. In Lithuania, parasitological Trichinella spp. studies were conducted on wolves; however, species were not distinguished using molecular analysis methods [33].
The present study aimed to search for and identify Sarcocystis and Trichinella species in the muscles of gray wolves from Lithuania.
2. Materials and Methods
2.1. Sample Collection
Although gray wolves are protected throughout the EU by the Habitats Directive and the Bern Convention, limited hunting is permitted as long as it does not affect the conservation status of the population in Lithuania. The number of gray wolves hunted each season is set by the order of the Minister of Environment in Lithuania, and these mammals are hunted from October 15th to April 1st. In cooperation with local hunters, samples of muscle tissue (diaphragm, heart, and muscles of hind legs) were taken from 15 gray wolves and delivered to the Laboratory of Molecular Ecology, Nature Research Centre, Vilnius, Lithuania, for detailed morphological and molecular analysis of Sarcocystis spp. and Trichinella spp. No gray wolves were killed for the purpose of the present study. No permit was needed for the investigations in the current study, as stated by the requirements of the Minister of Environment in Lithuania. Samples were obtained between 2017 and 2022 from central and southern Lithuania (Figure 1). The muscle samples were stored frozen (at –20 °C) until further analysis.
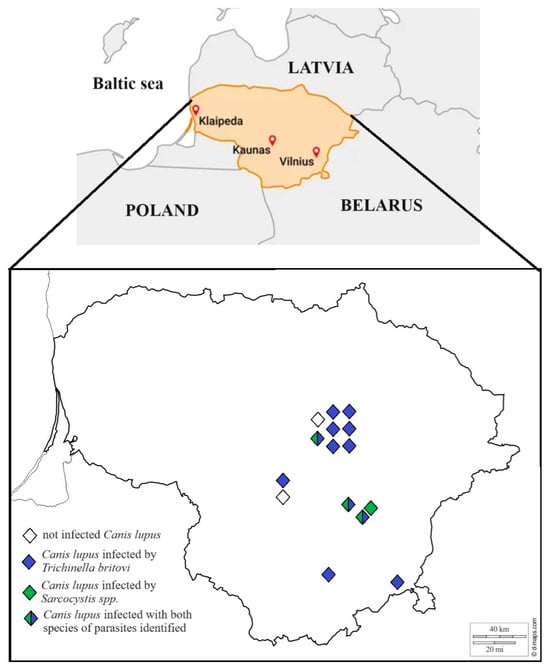
Figure 1.
Sarcocystis spp. and Trichinella sp. in the gray wolf in Lithuania. The filled diamond shape represents positive individuals, and the empty diamond shape represents negative individuals.
2.2. Morphological Examination for the Presence of Sarcocystis spp. and Trichinella spp.
The presence of Sarcocystis spp. and the infection intensity of sarcocysts were evaluated in methylene blue-stained muscle samples. For this aim, 28 oat-sized pieces of muscle were cut off and stained with a water (1:500) and methylene blue solution. Later, muscle samples were lightened with 1.5% acetic acid solution, and pressed in a glass compressor consisting of 28 cross-sections. Subsequently, the morphological characterization of the sarcocysts and bradyzoites was conducted using freshly squashed muscle samples. Sarcocysts were removed with two preparation needles, measured using a computerized image analysis system, and put in a tube.
To detect Trichinella, each muscle sample was digested separately using a modified magnetic stirrer procedure, as described previously [34]. Notably, each organ (the diaphragm, heart, and muscles of the hind legs) was tested by artificial digestion separately. Then, 25% hydrochloric acid (16 ± 0.5 mL) was added to 1.5 L of tap water that was preheated to 46–48 °C in a 2 L glass beaker. In addition, 10 ± 0.2 g of pepsin was added to the acidic solution. In addition, 50 g of muscle tissue (the diaphragm, the heart, or the hind legs muscle) from one wolf was chopped up in a grinder. The digestive fluids were mixed for 30 min. This method is recognized by the European Food Safety Authority as the most effective method for detecting Trichinella spp. The infection intensity was estimated by counting lpg (the number of larvae per gram of sample). Microscopic examination was conducted as previously described by EURLP [35].
For the detection and characterization of sarcocysts, a Nikon ECLIPSE 8oi light microscope (Nikon Corp., Tokyo, Japan) was used, while morphological examination of Trichinella spp. was performed with the help of a Kern OZL-463 stereo microscope (Kern, Germany).
Sarcocystis spp. excised from fresh muscle samples of gray wolves and Trichinella spp. larvae collected from digested samples were preserved individually, in separate tubes containing 96% ethyl alcohol, and preserved at –20 °C for the molecular examination.
2.3. Molecular Analysis of Sarcocystis spp. and Trichinella spp.
DNA extraction of Sarcocystis sarcocysts was carried out with the GeneJET Genomic DNA Purification Kit (Thermo Fisher Scientific Baltics, Vilnius, Lithuania) in accordance with the manufacturer’s recommendations. For each individual infected with Trichinella nematodes, 10 larvae were analyzed by molecular tests. DNA extraction of Trichinella spp. was carried out according to the methodology of Pozio et al., 2003 [32]. Each Trichinella larvae was washed in PBS, placed with 5 μL of PBS, and added 2 μL Tris-HCl, pH 7.6. Then, the sample was heated at 90 °C for 10 min and cooled on ice for 10–15 min. Then, 9 μL of proteinase K solution was added (final concentration 100 μg/mL). The sample was incubated at 48 °C for 3 h and then the process of heating at 90 °C for 10 min repeated. In the end, samples of DNA were stored at –20 °C until use. The genomic DNA was extracted from single Trichinella larvae separately.
Sarcocystis species were characterized at five loci, 18S ribosomal DNA (rDNA), 28S rDNA, ITS1 (internal transcribed spacer 1 region), cox1 (mitochondrial gene encoding subunit 1 of cytochrome c oxidase), and rpoB (RNA polymerase B gene of the apicoplast genome). The nearly complete 18S rDNA sequences, partial 28S rDNA sequences, complete ITS1 sequences, partial cox1 sequences, and partial rpoB sequences were amplified using primers previously mentioned by Prakas et al., 2018 [36]. Each PCR mixture consisted of 25 μL containing 12.5 μL of Dream Taq PCR Master Mix (Thermo Fisher Scientific, Vilnius, Lithuania), 0.5 µM of both forward and reverse primers, 4-μL template DNA, and nuclease-free water. The PCR cycling conditions started with 5 min at 95 °C, followed by 40 cycles of 45 s at 94 °C, 60 s at 50–60 °C depending on the primer pair, and 80 s at 72 °C, and ended with 7 min at 72 °C. PCR products were evaluated using a 1% agarose gel, visualized via UV light after staining with 0.05 μg/mL ethidium bromide, and 5 µL of each PCR product was purified with alkaline phosphatase FastAP and exonuclease ExoI (Thermo Fisher Scientific Baltics, Vilnius, Lithuania) to remove unincorporated nucleotides and primers. Purified PCR samples were sequenced using a Big-Dye®Terminator v3.1 Cycle Sequencing Kit (Thermo Fisher Scientific, Vilnius, Lithuania) and a 3500 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions. The identical forward and reverse primers used for the PCRs were used for both orientations of sequencing.
To calculate genetic similarity and choose Sarcocystis species for phylogenetic analysis, the DNA sequences from this study were compared with those of the Sarcocystidae family using Nucleotide BLAST [37]. For the phylogenetic study, sequences were aligned with the help of the MUSCLE algorithm implemented in MEGA7 [38]. The following software was used for the selection of nucleotide substitution models and the construction of phylogenetic trees based on the Maximum likelihood method. Taking into account the calculated lowest Bayesian Information Criterion values, T92+G+I was selected for 28S rDNA and rpob, T92+G was chosen for ITS1, GTR+G+I was set for cox1 and K2+G+I was selected for 18S rDNA [39]. The bootstrap method with 1000 replications was used to test the robustness of the phylogeny.
Trichinella species were identified using the multiplex PCR technique as described previously [32,35]. The primer pairs used for species identification amplify the ES5 (expansion segment 5) and ITS1 (internal transcribed spacer 1) genetic regions (Supplementary File, Table S1) of the genus Trichinella, which encode ribosomal components [35]. PCR was performed following the conditions outlined in Supplementary File, Table S2. Electrophoresis was performed on a 2% agarose gel with ethidium bromide. Five µL of each obtained PCR sample and GeneRuler Low-Range DNA Ladder molecular mass marker (Thermo Fisher Scientific Baltics, Lithuania) were injected into the well. Electrophoresis was performed for 50 min using a 90 V electric current on a gel soaked in a 1× TAE buffer. After the procedure, the PCR products were visualized under UV light. The identification of Trichinella species was further confirmed by Sanger sequencing. For this purpose, amplified species-specific PCR products, generated using DNA of larvae isolated from each infected animal (n = 12), were excised from agarose gel using GeneJET Gel Extraction Kit (Thermo Fisher Scientific Baltics, Vilnius, Lithuania) and subjected to sequencing. Purified PCR products were sequenced bidirectionally as described above. The obtained sequences were compared with those of Trichinella spp. using Nucleotide BLAST [37].
2.4. Data Analysis
The prevalence of Trichinella spp. and mean lpg were calculated for examined muscle tissues in gray wolves individually. Bootstrap two-sample t-tests [39] based on 2000 replications were used to compare mean lpg values established in diaphragm and limb muscles. p < 0.05 was considered statistically significant. Statistical tests were carried out using the Quantitative Parasitology 3.0 software [40].
3. Results
3.1. Prevalence and Morphology of Sarcocysts of Sarcocystis spp.
Based on the methylene blue-staining, sarcocysts of Sarcocystis spp. were detected in 26.7% (4/15) of the gray wolf (Table 1). One animal (isolate ClLt10) had 58 and 45 sarcocysts in one gram of diaphragm and limb muscles, respectively. Other infected gray wolves harbored sarcocysts only in diaphragms (isolates ClLt3; ClLt8; and ClLt14). The average parasite load was 16.8 ± 27.6 sarcocysts/g of diaphragm (Table 1). Parasites were not noticed in the heart muscles.

Table 1.
Presence, intensity, and molecular species identification of studied parasites in muscle samples of gray wolves from Lithuania.
In fresh samples, sarcocysts were detected in four animals. The sarcocysts found in two samples (isolates ClLt3 and ClLt8) were microscopic, ribbon-shaped, 950–1806 × 33–74 μm in size, with a thin (0.5–1.0 μm), apparently smooth cyst wall (Figure 2a,b). Bradyzoites were banana-shaped, 5.7–9.4 × 1.2–2.7 μm in size (Figure 2c). The DNA sequence analysis showed that these sarcocysts belong to S. svanai (Table 1).
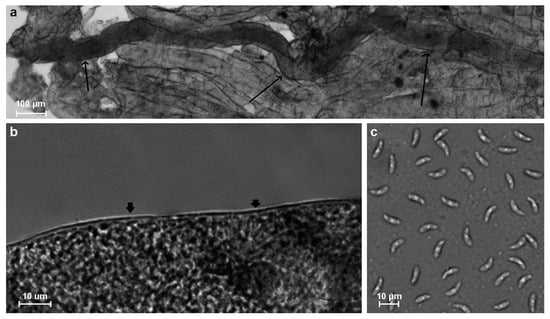
Figure 2.
Morphology of Sarcocystis svanai from muscle tissue of a gray wolf. Light micrographs. Fresh preparations (a–c). A portion of the ribbon-shaped sarcocyst (shown by arrows) (a), thin cyst wall (arrows) (b), lancet-shaped bradyzoites (c).
In the other two samples (ClLt10 and ClLt14), sarcocysts with smooth cyst walls were found, along the remnants of sarcocysts. In particular, the cyst wall had disappeared, leaving only the cyst-shaped bradyzoite nodules that were visible in the sarcocyst remnants (Figure 3). A whole cut piece of muscle was used for the DNA extraction of Sarcocystis sp. Further molecular investigations revealed that these remnants of sarcocysts belong to S. arctica (Table 1).
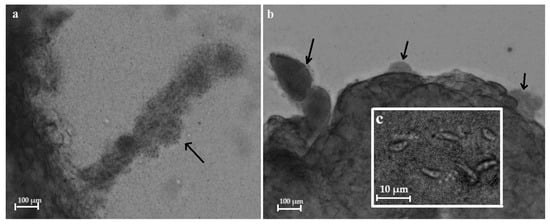
Figure 3.
Sarcocyst remnants from muscle tissue of the gray wolf (shown by arrows). Light micrographs (a–c). The elongated shape of sarcocyst-like bradyzoite nodules from limb muscle (a), the nodules of bradyzoites from the diaphragm (b), magnified image of lancet-shaped bradyzoites visible in the nodules of bradyzoites (c).
3.2. Genetic Characterisation and Phylogeny of S. arctica and S. svanai
The PCRs and sequencing were successful for all six isolates in the five genetic loci examined, except for two S. svanai isolates in ITS1. From the current study, the generated 1781 bp 18S rDNA, 1500 bp 28S rDNA and 958 bp ITS1 sequences of S. svanai, 1753 bp 18S rDNA, 1461 bp 28S rDNA and 697 bp ITS1 sequences of S. arctica, 1053 bp cox1, and 762 bp rpob sequences of S. svanai and S. arctica are available in NCBI GenBank under the accession numbers OR921254–OR921265, OR935783–OR935786, and OR939976–OR939987. The obtained sequences of S. arctica were 100% identical in all five loci examined, whereas S. svanai differed by one single nucleotide polymorphism (SNP) in ITS1. The alignment of our sequences displayed indels (insertions/deletion) within 18S rDNA, 28S rDNA, and ITS1, while rpob and cox1 sequences differed only by substitutions. A particularly large variation in length was observed when comparing the ITS1 sequences of two Sarcocystis species identified. These two Sarcocystis species showed very high similarity within 18S rDNA and cox1 (99.4–99.5%), a slightly lower similarity within 28S rDNA and rpob (97.9–98.5%), and even differences of 19.0% within ITS1 (Table 2). Comparing intraspecific and interspecific differences estimated, it has been noted that S. arctica and S. svanai cannot be identified by the cox1 fragment examined, whereas ITS1, rpob, and 28S rDNA are most suitable for the discrimination of these species.

Table 2.
The genetic comparison of Sarcocystis species identified in gray wolf from Lithuania.
In this work, obtained 18S rDNA sequences of S. arctica were 100% identical to S. arctica (KF601301, KY947306-7, KX022100-3, KX156838, MF596217-37, and MZ329343) and S. caninum (MH469238), 99.4% similar to S. fulicae (MG273671), S. halieti (JQ733511, MF946587, MH130211, MZ329386, MZ329390), S. lari (MF946588), S. turdusi (JF975681), and S. wobeseri (EU502869), using birds as their IHs and DHs [41,42]. Based on 28S rDNA, S. arctica from the Lithuanian gray wolf were 100% identical to S. caninum (MH469239), demonstrated 99.9–100% similarity to S. arctica (KF601312, KY609323, KY947308-9, KX022104-7, MF596240-60), 99.4–99.5% similarity to S. felis (OR436907–OR436910) from the domestic cat, and 98.5% similarity to S. lari (JQ733509, MF946611). The cox1 sequences of S. arctica shared 99.8–100% similarity compared to other isolates of this species (KF601318-21, KY609324, KY947304-5, KX022112-5, KX156839, MF596286-306, MZ332967); displayed 99.7–99.8% similarity to Sarcocystis sp. clone 1 (MW962266-9) from the black bear (Ursus americanus); 99.7% similarity to S. caninum (MH469240); 99.3–99.4% similarity to S. lutrae (KF601326, KM657808, MF596284-5, MG273661-70, MG372106-7, MT036250, MT036254, ON805825) circulating between predatory mammals of the families Mustelidae, Canidae and Procyonidae as IHs and birds as DHs [41,42]; and 97.3–99.4% similarity to multi-host adapted S. canis (KX721495-7) [43,44]. The rpob sequences of S. arctica from Lithuanian gray wolf showed 99.8–100% similarity to S. arctica (MF596311-21), 99.8% similarity to S. caninum (MH469242), 98.4% similarity to several Sarcocystis spp. (MF596307, MH138322, MH138325-6, LR884241), circulating between birds in their life cycle. Based on ITS1, the present study’s generated sequences of S. arctica were 100% identical to S. arctica (KF601306, KF601308, KY947310-1, KX022108-11, KX156837, MF596262-82, MZ333536, OK481372-6), had a 99.5–100% similarity to S. caninum (JX993923, MH469241), and an 88.0–96.3% similarity to S. felis (AY190081-2, MN508375-9, OQ676522).
The 18S rDNA sequences of S. svanai from gray wolves in Lithuania disclosed 99.9–100% similarity to S. svanai (KM362428, KY292483-7), followed by up to 99.8% similarity to Sarcocystis spp., which use bird–bird hosts in their life cycle. Based on 28S rDNA, S. svanai showed the greatest 98.3–98.5% similarity to S. arctica (KF601312, KY609323, KY947308-9, KX022104-7, MF596240-60), 98.4% similarity to S. caninum (MH469239), and 98.1–98.3% similarity to S. lutrae (KM657771-2, MF596238-9, MG272276-85, MG372104-5, MT036249, ON796572). At cox1, S. svanai was indistinguishable (100% identical) from S. lutrae (KF601326, KM657808, MF596284-5, MG273661-70, MG372106-7, MT036250, MT036254, ON805825) and S. lari (MF596283-4), and also showed high 99.8% similarity to some Sarcocystis spp. That employ birds as their hosts (MF946583, MH138308-9, MH138312, MH138314, MZ332968-9). Based on rpob, S. svanai from Lithuanian gray wolf were 100% identical to S. svanai (KC191640), displayed 98.8% similarity to S. lutrae (MF596309-10) and 98.4% similarity to S. campestris (GQ851963) from Richardson’s ground squirrel (Spermophilus richardsonii). The ITS1 sequences of S. svanai demonstrated 75.0% similarity to Sarcocystis sp. CRC-836 (HQ184185) from the sperm whale (Physeter macrocephalus), 72.8–74.9% similarity to S. lutrae (KM657773-805, MF596261, MG272296-305, MG372108-9, OK481377, ON806939) 74.1% similarity to S. kalvikus (GU200661) from the wolverine (Gulo gulo), and 73.9–74.1% similarity to Sarcocystis sp. (MH918015, MW264422) from the subantarctic fur seal (Arctocephalus tropicalis).
Two Sarcocystis species were identified in Lithuanian gray wolves clustered in phylogenetic trees together with other isolates of the same species (Figure 4). Our phylogenetic examination confirmed that S. arctica cannot be genetically differentiated from S. caninum in the five genetic loci studied. The phylogenetic analysis showed that S. arctica and S. svanai were placed together with Sarcocystis species using mammals of the order Carnivora as their IH (S. canis, S. caninum, S. felis and S. lutrae) and to species employing birds as their IHs and DHs (such as S. cornixi, S. halieti, S. lari, S. turdusi, S. wobeseri). Based on rpob, the 28S rDNA, ITS1, S. svanai was a sister species to S. lutrae. In the phylogenetic trees, generated using 28S rDNA and ITS1 sequences, S. arctica was most closely related to S. felis.
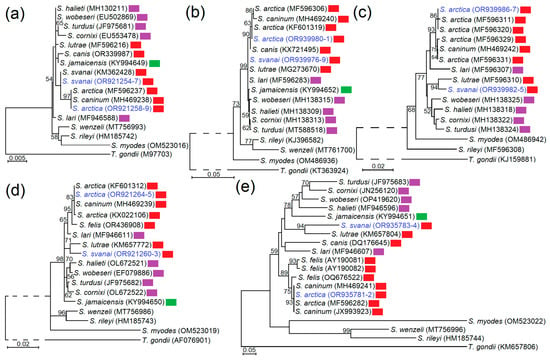
Figure 4.
The phylogenetic placement of S. arctica and S. svanai isolated from gray wolves in Lithuania on the basis of 18S rDNA (a), cox1 (b), rpob (c), 28S rDNA (d), and ITS1 (e). Phylogenetic trees were constructed using Maximum Likelihood method, scaled according to branch length and rooted on Toxoplasma gondii. The dashed line points out that its length does not correspond to the evolutionary distance. Figures next to branches display bootstrap support values. Sequences obtained in the present work are highlighted in indigo. Red rectangles show Sarcocystis species using members of order Carnivora as their intermediate hosts. Sarcocystis species employing birds in their life cycle are indicated with purple rectangles and Sarcocystis species cycling between rodents and birds are indicated with green rectangles.
3.3. Microscopical and Molecular Examination of Trichinella spp.
Out of the 15 tested gray wolves, Trichinella spp. larvae (Figure 5a) were detected in 12 animals (80.0%). Two wolves (No. 1 and No. 2) were excluded from the statistical analysis of lpg, since it was not possible to check the diaphragms of these wolves and the intensity of Trichinella spp. infection in the hind legs of these animals was relatively high (Table 1). The intensity of Trichinella infection varied between 0.2 and 17 lpg in the diaphragm and 0.7 and 21 lpg in the muscles of the hind legs. The mean larval burden was not significantly different between two muscle samples ( = 5.79 ± 5.6 in the diaphragm and = 5.63 ± 7.3 in the limb, p = 0.9790). No Trichinella parasites were detected in the heart muscle of gray wolves in present study.
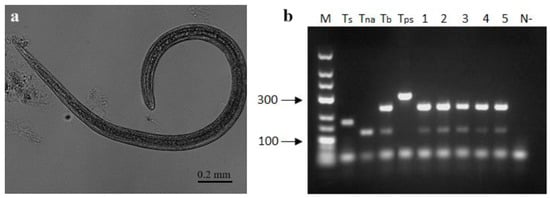
Figure 5.
Morphological and molecular examination of Trichinella spp. in gray wolf. (a) Trichinella spp. larvae found by the method of artificial digestion in the diaphragm. (b) Agarose gel electrophoresis (2%) of multiplex PCR of Trichinella britovi in 5 larvae. M—“GeneRuler Low Range DNA Ladder” molecular weight marker, sizes are in base pairs, positive controls of: Ts—T. spiralis, Tna—T. nativa, Tb—T. britovi, Tps—T. pseudospiralis, 1–5—individual larvae from one sample, and N—Negative control.
Trichinella spp. larvae from 220 isolates were successfully identified at the species level by multiplex PCR. All analyzed muscle samples contained Trichinella britovi (Figure 5b), and no instances of species co-infection were observed. The PCR results were then confirmed with sequencing data. Twelve 253 bp ITS1 sequences obtained from larvae isolated from all 12 infected gray wolves were 100% identical and were submitted to GenBank under accession number PP153335. These sequences from the Lithuanian gray wolf were 100% identical to sequences of some isolates of T. britovi (OK483203, OK483205-7, OK483214-5, KU374878-9, KU374883-4), 98.1–99.6% similarity to sequences of other isolates of T. britovi (OK483202, OK483204, OK483208-13, OK483216, KU374867, KU374875, KU374877, KU374881, KU374885), 97.3–98.4% similarity to those of Trichinella murrelli, 96.8% similarity to those of Trichinella nativa (KP307962-6), and 95.7–96.4% similarity to those of Trichinella sp. T6 (KP307967-71).
4. Discussion
4.1. Pathogenic Impact of Parasites Identified in Gray Wolf
The gray wolf is a “keystone species” that plays a vital role in maintaining the health, structure, and balance of ecosystems [2,3,4,5,8]. Gray wolves may spread more than 10 viral, bacterial, and mycotic diseases and more than 70 species of helminths and protists [9,10,11,12]. In the present study, sarcocysts of S. svanai were identified for the first time in gray wolves. Furthermore, Trichinella britovi and Sarcocystis arctica were for the first time confirmed in gray wolves in Lithuania.
Some Sarcocystis spp. may be pathogenic for IH [15]. To date, at least three pathogenic Sarcocystis species have been reported in canids, S. caninum/S. arctica, Sarcocystis canis-like, and S. neurona [15,23,43,45,46,47,48]. Sarcocysts morphologically similar to S. caninum/S. arctica have been reported in the muscles of two dogs from the USA [48] and in a dog from Canada [47] which suffered from severe myositis. In addition, more severe symptoms such as ataxia, stiff gait or inability to walk, generalized pain, anorexia, diarrhea, fever, and panting were retrieved in four dogs from the USA caused by S. caninum/S. arctica [49]. Later, of the eight reported cases of muscular sarcocystosis in dogs, five were related to clinical signs [15]. A fatal S. caninum/S. arctica and S. svanai coinfection revealed severe monophasic multifocal myodegeneration with severe pyogranulomatous inflammation in a dog reported from Finland [50]. Infections with highly pathogenic and multi-host-adapted S. canis-like and S. neurona have also been reported in dogs [47,51]. Thus, comprehensive investigations into the pathogenesis of S. arctica and S. svanai in canids are needed.
Trichinella nematodes cause a serious, and sometimes fatal, human disease called trichinellosis, which is a food-borne zoonotic disease with worldwide distribution [25,26,27,28]. Frequently, humans become infected with these parasites by eating raw or undercooked meat from infected animal products. In general, domestic swine and related products continue to be the most significant source of human Trichinella infection [26]. However, cases of trichinosis in humans have been recorded when the main source was game meat, such as wild boar [52], brown bear (Ursus arctos) [53,54], badger [54], walrus (Odobenus rosmarus) [55], and cougar (Puma concolor) [56]. Human symptoms of parasitic infections vary depending on the type of parasite, the level of infection, and the host’s immune response [57]. The life cycle of Trichinella in humans or animals follows three stages: the enteral phase (intestinal disorders), the parenteral phase (allergic reactions, myalgia, and fever), and the encysting phase (recovery) [58]. Trichinella larvae can survive in the muscles of their hosts for years, depending on the adaptations of the species. Even though parasites of this genus cause various symptoms in humans, Trichinella larvae do not appear to be pathogenic to other hosts (wild, domestic, or synanthropic animals) unless in large numbers in muscle [59]. While T. spiralis is known to be the most pathogenic species of Trichinella in humans, T. britovi is the second species of greatest concern. About 80% of people infected with T. britovi had myalgia, weakness, and arthralgia, about 70% experienced headaches, fever, and edema, and 20% had gastrointestinal disorders [60]. Also, T. britovi is one of the greatest concerns because it has some resistance to low temperatures and can survive in the host muscle for up to 6 months at a temperature of −20 °C [61].
4.2. Host Specificity of Sarcocystis Species from Canids
In this study, we identified S. svanai in gray wolves for the first time. Previously, this Sarcocystis species was detected in the muscles of two domestic dogs from the USA [21] and in the muscles of 19 Pampas foxes (Lycalopex gymnocercus) from Argentina [62]. Based on histopathological analysis, S. svanai was potentially also identified in one dog from Finland [50], whereas S. arctica was described in the muscles of two Arctic foxes (Vulpes lagopus) from Norway in 2014 [63]. Subsequently, this species was recorded in the muscles of one Alaskan wolf (Canis lupus) in 2016 [14], nine Arctic foxes from Alaska in 2017 [64], three red foxes from the Czech Republic in 2017 [65], and ten, two, and three red foxes from Latvia, Lithuania, and Spain in 2018 [23]. To date, at least four Sarcocystis spp., S. arctica/caninum, S. canis-like, S. lutrae, and S. svanai have been described in predatory mammals of the family Canidae [15,21,23,62,63,64,65]. Furthermore, dogs may serve as an aberrant dead-end host for highly pathogenic S. neurona [49]. Sarcocystis vulpis, found in the muscles of the red fox, is considered to be a species of inquirendae [15]. Some authors do not list S. corsaci, found in the corsac fox (Alopex corsac), as a valid species due to a lack of molecular data on this parasite [63]. Most of the Sarcocystis species are generally host-specific for their IHs [15]. The host specificity of S. arctica and S. svanai found in this study is restricted to the family Canidae. Meanwhile, S. lutrae has been identified in the muscles of three Carnivora families, Canidae, Mustelidae, and Procyonidae [23,24,66,67,68,69]. Asexual stages of S. canis have been identified in seven different mammalian families (Canidae, Chinchillidae, Delphinidae, Equidae, Otariidae, Phocidae, and Ursidae) [43,45,49]. In summary, further comprehensive investigations of the Sarcocystis spp. specificity for their IHs are required.
4.3. Morphological and Molecular Characteristics of Identified Sarcocystis Species
In the present study, two Sarcocystis species, S. arctica and S. svanai may be identified by clearly different sarcocyst wall appearances. It has been shown that the sarcocyst wall of S. arctica has short knob-like or dome-shaped protrusions, approximately 1–1.5 μm wide and 0.5–1 μm long [23,63,64,65], while the cyst of S. svanai is thin-walled (Figure 2b) [21]. However, in the present study, S. arctica sarcocysts were not detected; only cyst remnants and bradyzoites were visible (Figure 3). Freezing of gray wolf muscles may have adversely affected the sarcocyst structure of S. arctica. Similar observation issues have also been noticed in other studies on Sarcocystis spp. [15,62]. The freshness of muscle samples is therefore very important for the morphological analysis of sarcocysts and identification of Sarcocystis species.
In this study, we have, for the first time, genetically characterized S. svanai in 28S rDNA, ITS1 and cox1, as previously only 18S rDNA and rpoB sequences of this species were available [21,62]. 18S rDNA and rpoB sequences of S. svanai from gray wolf were 100% identical to S. svanai from domestic dog [21] and based on 18S rDNA our sequences differed by one SNP (A/C) compared to S. svanai from Pampas fox (Lycalopex gymnocercus) [62]. Phylogenetic results showed that S. svanai clustered together with S. lutrae (Figure 4), while S. arctica was most closely related to S. felis. The noticed phylogenetic grouping is in agreement with morphological similarities of sarcocysts of species analyzed. Sarcocysts of S. svanai and S. lutrae are characterized by transmission electron microscopy as having 1a sarcocyst wall type [21,23,62], while sarcocysts of S. arctica/S. caninum and S. felis had similar 9c and 9a cyst wall types, respectively [15,63].
Based on the compiled data, S. arctica showed intraspecific variation within four genetic loci, 28S rRNA, ITS1, cox1 and rpob (Table 2). Previously, it was suggested that two genetic lineages of S. arctica distinguished by cox1 are diverging along the latitudinal cline [23]. Such an assumption was proposed, since only cox1 haplotype A was identified in gray wolf and arctic fox in Alaska, whereas the haplotype B was found in domestic dog in China and in red fox in Spain, and finally both haplotypes were present in red fox from the Czech Republic and from the Baltic States [23,65]. Here we also determined haplotype A for S. arctica isolated from the gray wolf in Lithuania.
Among the five examined loci, the identified Sarcocystis species had the highest variation within ITS1, followed by 28S rDNA and rpob (Table 2). However, at 28S rDNA and rpob interspecific similarity compared to most related species were still high, exceeding 98%. The genetic variability was very low in 18S rDNA and cox1. These genes were not suitable for accurately differentiating the studied Sarcocystis species (Table 2, Figure 4). The results of our study complement previous investigations showing little value of 18S rDNA and cox1 in discrimination of Sarcocystis spp. employing Carnivora as their IH [23,63,64,65]. Notably, these two genes are mostly used for the identification of numerous Sarcocystis species with ungulates as their IHs [70]. In summary, due to the small genetic variability of Sarcocystis species parasitizing carnivorous mammals in 18S rDNA, 28S rDNA, cox1 and rpoB, other genes need to be found for further detailed genetic characterization of these Sarcocystis species.
4.4. Prevalence and Species Composition of Trichinella in Gray Wolf
The identified high percentage of positivity of Trichinella spp. infection in gray wolf from Lithuania (80.0%) corresponds to high infection rates established in this host in Latvia (69.7–100%), Estonia (50–79.4%), and Russia (97.3%) [10,29,71,72,73]. Lower values of prevalence of Trichinella parasites in gray wolf had previously been documented in the Central Balkans (46.5%) [74], Finland (33.33–39.2%) [75], Croatia (31%) [76], Poland (54.5%) [77], Romania (31–40%) [78], Alaska (28–36%) [79], Italy (27.1%) [80], and the Western part of the Italian Alps (11.53%) [81]. In the current study, no significant differences in lpg were observed in the diaphragm (0.2–17; = 5.79 ± 5.6) and limb (0.7–21; = 5.63 ± 7.3) muscles. Similarly, relatively high lpg values varying between 0.009 and 27 lpg, 0.1 and 41.8 lpg, and 0.01 and 44.9 lpg were estimated in infected gray wolves from Poland [77], Latvia [73], and Estonia [29], respectively. The high infection prevalence and intensity of Trichinella spp. have been attributed to inappropriate hunting practices, such as not burying the carcasses of hunted animals or the use of meat for animals baiting [30]. In accordance with the considerations set out above and as established by EU legislation, it is also important that systematic testing and monitoring for Trichinella be carried out in all slaughtered pigs, wild boar and horses. Appropriate rodent control campaigns are also necessary. Using control procedures and protocols is important for ensuring the safety of food for consumers and monitoring the health of wild animals. The safety of meat should always be a top priority, regardless of its intended use. Additionally, improving hunter training (good slaughtering practices and proper hunter handling) is essential.
Currently, 10 Trichinella species (T. spiralis, T. nativa, T. britovi, T. murrelli, T. nelsoni, T. patagoniensis, T. chanchalensis, T. pseudospiralis, T. papuae, and T. zimbabwensis) and three genotypes (Trichinella T6, Trichinella T8, and Trichinella T9) are known worldwide [82]. Four species of the genus Trichinella, T. britovi, T. nativa, T. pseudospiralis, and T. spiralis, are found in Europe [83]. In the current study, T. britovi was identified in all 220 isolated larvae. Among other Trichinella species, T. britovi has the widest geographical distribution. High infection rates of T. britovi were reported in different carnivore families, Canidae, Felidae, and Ursidae, in various European countries [84]. Notably, Trichinella species in Lithuanian gray wolves have not been previously investigated by molecular methods. However, based on the examination of other wild canids sampled in 2000–2002 in the Baltic States, a high prevalence of T. britovi was recorded in red foxes (89.8%) and raccoon dogs (91.3%) from Lithuania and in raccoon dogs (91.3%) from Latvia [33]. In 2016, T. britovi was the most common species among studied wild predators in Latvia, accounting 94% [73]. A single T. nativa (1.1% infection) and two different mixed infections of T. nativa/T. britovi (4.4%) and T. spiralis/T. britovi (0.5%) were also detected in this study. Based on this study, T. britovi was found to be 100% common among gray wolves [73]. Previous similar surveys in Poland also showed that T. britovi was the main species in wolves [77]. Trichinella britovi is likely the most common species among gray wolves and other wild predators in neighboring countries [29,30,33,73,77]. The high prevalence of T. britovi infection may indicate that gray wolf may be an important contributor to the sylvatic cycle maintenance of this hazardous nematode. One hypothesis is that wild boar may serve as a natural reservoir of Trichinella infection for carnivorous [52]. For comparison purposes, in 2001 the distribution of Trichinella spp. among wild boars in Lithuania was 1.3% [85]. In 2019, the National Food and Veterinary Risk Assessment Institute of Lithuania found that 0.5% (43 out of 9200) of wild boars were infected with Trichinella larvae [86]. There is a lack of data on Trichinella infection rates in wild carnivores and omnivores in Lithuania to document the impact of wild boar on the spread of this disease. The reality is that the infection of wild boar with Trichinella spp. may be higher than the calculated rates. Another reason for the high percentage of Trichinella infection in predators studied could be the fact that the gray wolf population, which is rapidly growing as mentioned in the introduction, scavenges and cannibalizes more often. Also, our study raises the idea that humans influence the high percentage of Trichinella infection due to improper hunting practices as it has been shown in Russia [30].
5. Conclusions
In the present study, a new host record, i.e., gray wolf was provided for S. svanai. Furthermore, it was demonstrated that gray wolves from Lithuania are also infected with S. arctica. It was revealed that the muscles of a single gray wolf could be infected with two Sarcocystis species. Among five loci studied, ITS1, 28S rDNA and rpob were most valuable for the genetic identification, and phylogeny of Sarcocystis species detected. Moreover, T. britovi was genetically confirmed in all isolated Trichinella larvae in the muscles of gray wolf for the first time in Lithuania. In wildlife, carnivore species such as the gray wolf may be an important reservoir of Sarcocystis spp. and zoonotic Trichinella spp. in Lithuania.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vetsci11020085/s1, Table S1: The primer pairs used for Trichinella species identification; Table S2: PCR cycling conditions for Trichinella species identification.
Author Contributions
Conceptualization, E.J.-N., P.P., E.M. and D.B.; methodology, P.P., E.J.-N. and D.B.; software, P.P. and E.M.; validation, D.B., M.K. and J.S.; formal analysis, E.J.-N., P.P., E.M., R.V. and D.B.; investigation, E.J.-N., P.P., E.M., R.V. and J.S.; resources, D.B., M.K. and R.V.; data curation, E.J.-N., P.P. and J.S.; writing—original draft preparation, E.J.-N., P.P., E.M. and D.B.; writing—review and editing, E.J.-N., P.P., E.M., M.K., R.V., J.S. and D.B.; visualization, E.J.-N., P.P., E.M. and R.V.; supervision, P.P. and D.B. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Open Access research infrastructure of the Nature Research Centre under the Lithuanian open access network initiative.
Institutional Review Board Statement
The research of current study was conducted under the approval guidelines of the Ethics Committee of Nature Research Centre (no. GGT-1). Gray wolves are protected throughout the EU by the Habitats Directive and the Bern Convention. The approval (2014-09-15, No. D1-699) of the conservation plan for the wolf (Canis lupus) was declared by the Ministry of Environment of the Republic of Lithuania. Each season, the number of gray wolves hunted is set by the order of the Minister of Environment in Lithuania (6 February 2018 no. D1-86; 19 October 2018 no. D1-897; 14 October 2019 no. D1-608; 14 October 2020 no. D1-629; 13 October 2021 no. D1-582; 11 October 2022 no. D1-328). The limited hunting is permitted as long as it does not affect the conservation status of the population in Lithuania.
Informed Consent Statement
Not applicable.
Data Availability Statement
The sequences of S. arctica, S. svanai, and Trichinella britovi generated in the current research were submitted to the NCBI GenBank database. The 18S rDNA, 28S rDNA, ITS1, cox1 and rpoB sequences of two Sarcocystis species are available under accession numbers OR921254–OR921259, OR921260–OR921265, OR935783–OR935786 and OR939976–OR939981, OR939982–OR939987. Twelve identical ITS1 sequences of Trichinella britovi were submitted to GenBank under accession number PP153335.
Acknowledgments
The authors are grateful to Donatas Mažiulis, Marijonas Mackevičius and Juozas Zdanevičius for providing gray wolf samples for this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Boitani, L.P.; Kaczensky, F.; Alvares, H.; Andrén, V.; Balys, J.; Blanco, G.; Chapron, S.; Chiriac, D.; Cirovic, N.; Drouet-Houguet, C.; et al. Assessment of the Conservation Status of the Wolf (Canis Lupus) in Europe; Prepared for the Berne Convention on the Conservation of European Wildlife and Natural Habitats and the Council of Europe: Strasbourg, France, 2022; pp. 1–25. Available online: https://rm.coe.int/inf45e-2022-wolf-assessment-bern-convention-2791-5979-4182-1-2/1680a7fa47 (accessed on 20 November 2023).
- Nowak, R.M. A perspective on the taxonomy of wolves in North America. In Wolves in Canada and Alaska: Their Status, Biology, and Management; Carbyn, L.N., Ed.; Canadian Wildlife Service: Ottawa, ON, Canada, 1983; pp. 10–19. [Google Scholar]
- Paquet, P.C.; Carbyn, L.N. Gray wolf. In Wild Mammals of North America: Biology, Management, and Conservation, 2nd ed.; Feldhamer, A.G., Thompson, B.C., Joseph, A., Chapman, J.A., Eds.; John Hopkins University Press: Baltimore, MD, USA, 2003; pp. 482–510. [Google Scholar]
- Mech, L.D.; Boitani, L. Wolves: Behavior, Ecology, and Conservation; The University of Chicago Press: Chicago, IL, USA, 2003. [Google Scholar]
- Ciucci, P.; Reggioni, W.; Maiorano, L.; Boitani, L. Long-distance dispersal of a rescued wolf from the northern Apennines to the western Alps. J. Wildl. Manage 2009, 73, 1300–1306. [Google Scholar] [CrossRef]
- Hindrikson, M.; Remm, J.; Pilot, M.; Godinho, R.; Stronen, A.V.; Baltrūnaitė, L.; Czarnomska, S.D.; Leonard, J.A.; Randi, E.; Nowak, C.; et al. Wolf population genetics in Europe: A systematic review, meta-analysis and suggestions for conservation and management. Biol. Rev. 2017, 92, 1601–1629. [Google Scholar] [CrossRef] [PubMed]
- Lesniak, I.; Heckmann, I.; Franz, M.; Greenwood, A.D.; Heitlinger, E.; Hofer, H.; Krone, O. Recolonizing gray wolves increase parasite infection risk in their prey. Ecol. Evol. 2018, 8, 2160–2170. [Google Scholar] [CrossRef]
- Newsome, T.M.; Boitani, L.; Chapron, G.; Ciucci, P.; Dickman, C.R.; Dellinger, J.A.; López-Bao, J.V.; Peterson, R.O.; Shores, C.R.; Wirsing, A.J.; et al. Food habits of the world’s grey wolves. Mamm. Rev. 2016, 46, 255–269. [Google Scholar] [CrossRef]
- Craig, H.L.; Craig, P.S. Helminth parasites of wolves (Canis lupus): A species list and an analysis of published prevalence studies in Nearctic and Palaearctic populations. J. Helminthol. 2005, 79, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Bagrade, G.; Kirjušina, M.; Vismanis, K.; Ozoliņš, J. Helminth parasites of the wolf Canis lupus from Latvia. J. Helminthol. 2009, 83, 63–68. [Google Scholar] [CrossRef]
- Muñoz, S.; Ramos, P.L.; Carretón, E.; Diosdado, A.; González-Miguel, J.; Simón, F.; Morchón, R. Intestinal helminths in Iberian wolves (Canis lupus signatus) from Northwest Spain. Parasitol. 2018, 6, 106–111. [Google Scholar] [CrossRef]
- Bindke, J.D.; Springer, A.; Janecek-Erfurth, E.; Böer, M.; Strube, C. Helminth infections of wild European gray wolves (Canis lupus Linnaeus, 1758) in Lower Saxony, Germany, and comparison to captive wolves. Parasitol. Res. 2019, 118, 701–706. [Google Scholar] [CrossRef]
- Ricchiuti, L.; Petrini, A.; Interisano, M.; Ruberto, A.; Salucci, S.; Marino, L.; Del Riccio, A.; Cocco, A.; Badagliacca, P.; Pozio, E. First report of Trichinella pseudospiralis in a wolf (Canis lupus italicus). Int. J. Parasitol. Parasites Wildl. 2021, 15, 195–198. [Google Scholar] [CrossRef]
- Calero-Bernal, R.; Cerqueira-Cézar, C.K.; Verma, S.K.; Mowery, J.; Carmena, D.; Beckmen, K.; Dubey, J.P. Sarcocystis arctica (Apicomplexa: Sarcocystidae): Ultrastructural description and its new host record, the Alaskan wolf (Canis lupus). Parasitol. Res. 2016, 115, 2893–2897. [Google Scholar] [CrossRef]
- Dubey, J.P.; Calero-Bernal, R.; Rosenthal, B.M.; Speer, C.A.; Fayer, R. Sarcocystosis of Animals and Humans, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Rosenthal, B.M. Zoonotic Sarcocystis. Res. Vet. Sci. 2021, 136, 151–157. [Google Scholar] [CrossRef]
- Pozio, E. Adaptation of Trichinella spp. for survival in cold climates. Food Waterborne Parasitol. 2016, 4, 4–12. [Google Scholar] [CrossRef]
- Mehlhorn, H.; Heydorn, A.O. The sarcosporidia (Protozoa, Sporozoa): Life cycle and fine structure. Adv. Parasitol. 1978, 16, 43–91. [Google Scholar] [CrossRef] [PubMed]
- Fayer, R.; Esposito, D.H.; Dubey, J.P. Human infections with Sarcocystis species. Clin. Microbiol. Rev. 2015, 28, 295–311. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.A.; Evans, L. Prevalence of Sarcocystis spp. in two subspecies of caribou (Rangifer tarandus) in Newfoundland and Labrador, and foxes (Vulpes vulpes), wolves (Canis lupus), and husky dogs (Canis familiaris) as potential definitive hosts. J. Parasitol. 2006, 92, 662–663. [Google Scholar] [CrossRef]
- Dubey, J.P.; Sykes, J.E.; Shelton, G.D.; Sharp, N.; Verma, S.K.; Calero-Bernal, R.; Viviano, J.; Sundar, N.; Khan, A.; Grigg, M.E. Sarcocystis caninum and Sarcocystis svanai n. spp. (Apicomplexa: Sarcocystidae) associated with severe myositis and hepatitis in the domestic dog (Canis familiaris). J. Eukaryot. Microbiol. 2015, 62, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Liang, Y.; Hu, J.; Huang, Z.; Zhang, Y. First isolation of Sarcocystis caninum sarcocysts from two domestic dogs (Canis familiaris) from China. Parasitol. Res. 2018, 117, 3613–3618. [Google Scholar] [CrossRef] [PubMed]
- Kirillova, V.; Prakas, P.; Calero-Bernal, R.; Gavarāne, I.; Fernández-García, J.L.; Martínez-González, M.; Rudaitytė-Lukošienė, E.; Martínez-Estéllez, M.Á.H.; Butkauskas, D.; Kirjušina, M. Identification and genetic characterization of Sarcocystis arctica and Sarcocystis lutrae in red foxes (Vulpes vulpes) from Baltic States and Spain. Parasit. Vectors. 2018, 11, 1–9. [Google Scholar] [CrossRef]
- Prakas, P.; Strazdaitė-Žielienė, Ž.; Rudaitytė-Lukošienė, E.; Servienė, E.; Butkauskas, D. Molecular identification of Sarcocystis lutrae (Apicomplexa: Sarcocystidae) in muscles of five species of the family Mustelidae. Parasitol. Res. 2018, 117, 1989–1993. [Google Scholar] [CrossRef]
- Pozio, E.; Rossi, P. Guidelines for the identification and development of sampling methods and design of suitable protocols for monitoring of Trichinella infection in indicator species. Ann. Ist. Super. Sanita. 2008, 44, 200–204. [Google Scholar]
- Pozio, E. New patterns of Trichinella infection. Vet. Parasitol. 2001, 98, 133–148. [Google Scholar] [CrossRef]
- Pozio, E. The broad spectrum of Trichinella hosts: From cold- to warm-blooded animals. Vet. Parasitol. 2005, 132, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Järvis, T.; Miller, I.; Pozio, E. Epidemiological studies on animal and human trichinellosis in Estonia. Parasite 2001, 8, S86–S87. [Google Scholar] [CrossRef] [PubMed]
- Pozio, E.; Casulli, A.; Bologov, V.V.; Marucci, G.; La Rosa, G. Hunting practices increase the prevalence of Trichinella infection in wolves from European Russia. J. Parasitol. 2001, 87, 1498–1501. [Google Scholar] [CrossRef] [PubMed]
- Segovia, J.M.; Torres, J.; Miquel, J.; Llaneza, L.; Feliu, C. Helminths in the wolf, Canis lupus, from North-Western Spain. J. Helminthol. 2001, 75, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Pozio, E.; La Rosa, G. PCR-derived methods for the identification of Trichinella parasites from animal and human samples. Methods Mol. Biol. 2003, 216, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Malakauskas, A.; Paulauskas, V.; Järvis, T.; Keidans, P.; Eddi, C.; Kapel, C.M.O. Molecular epidemiology of Trichinella spp. in three Baltic countries: Lithuania, Latvia, and Estonia. Parasitol. Res. 2007, 100, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Mayer-Scholl, A.; Pozio, E.; Gayda, J.; Thaben, N.; Bahn, P.; Nöckler, K. Magnetic stirrer method for the detection of Trichinella larvae in muscle samples. J. Vis. Exp. 2017, 3, 55354. [Google Scholar] [CrossRef]
- European Union Reference Laboratory for Parasites; Istituto Superiore di Sanita. Identification of Trichinella Muscle Stage Larvae at the Species Level by Multiplex PCR. Available online: https://www.iss.it/documents/20126/0/Instruction-PT-03-rev-5+%281%29.pdf/ (accessed on 21 January 2024).
- Prakas, P.; Butkauskas, D.; Švažas, S.; Stanevičius, V. Morphological and genetic characterisation of Sarcocystis halieti from the great cormorant (Phalacrocorax carbo). Parasitol. Res. 2018, 117, 3663–3667. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Reiczigel, J. Confidence intervals for the binomial parameter: Some new considerations. Stat. Med. 2003, 22, 611–621. [Google Scholar] [CrossRef]
- Reiczigel, J.; Marozzi, M.; Fábián, I.; Rózsa, L. Biostatistics for parasitologists—A primer to quantitative parasitology. Trends Parasitol. 2019, 35, 277–281. [Google Scholar] [CrossRef]
- Juozaitytė-Ngugu, E.; Švažas, S.; Šneideris, D.; Rudaitytė-Lukošienė, E.; Butkauskas, D.; Prakas, P. The role of birds of the family Corvidae in transmitting Sarcocystis protozoan parasites. Animals 2021, 11, 3258. [Google Scholar] [CrossRef]
- Máca, O.; González-Solís, D. Sarcocystis cristata sp. nov. (Apicomplexa, Sarcocystidae) in the imported great blue turaco Corythaeola cristata (Aves, Musophagidae). Parasit. Vectors. 2021, 14, 1–7. [Google Scholar] [CrossRef]
- Dubey, J.P.; Chapman, J.L.; Rosenthal, B.M.; Mense, M.; Schueler, R.L. Clinical Sarcocystis neurona, Sarcocystis canis, Toxoplasma gondii, and Neospora caninum infections in dogs. Vet. Parasitol. 2006, 137, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Calero-Bernal, R.; Mauroo, N.F.; Hui, S.W.; Kuiken, T.; van de Bildt, M.W.; de Jong, A.W.; Osterhaus, A.D.; Sims, L.; Gendron-Fitzpatrick, A.; Carmena, D.; et al. Acute fatal sarcocystosis hepatitis in an Indo-Pacific bottlenose dolphin (Tursiops aduncus) in Hong Kong. Vet. Parasitol. 2017, 15, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Speer, C.A. Sarcocystis canis n. sp. (Apicomplexa: Sarcocystidae), the etiologic agent of generalized coccidiosis in dogs. J. Parasitol. 1991, 77, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.; Mense, M.; Dubey, J.P. Clinical muscular sarcocystosis in a dog. J. Parasitol. 2005, 91, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Cooley, A.J.; Barr, B.; Rejmanek, D. Sarcocystis neurona encephalitis in a dog. Vet. Pathol. 2007, 44, 956–961. [Google Scholar] [CrossRef]
- Sykes, J.E.; Dubey, J.P.; Lindsay, L.L.; Prato, P.; Lappin, M.R.; Guo, L.T.; Mizisin, A.P.; Shelton, G.D. Severe myositis associated with Sarcocystis spp. infection in 2 dogs. J. Vet. Intern. Med. 2011, 25, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Howe, D.K.; Furr, M.; Grigg, M.E.; Saville, W.J.; Marsh, A.E.; Reed, S.M. An update on Sarcocystis neurona infections in animals and equine protozoal myeloencephalitis (EPM). Vet. Parasitol. 2015, 209, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Hagner, K.; Jokinen, T.S.; Lavikainen, A.; Sukura, A. Acute fulminant necrotizing myopathy in a dog caused by co-infection with ultrastructural Sarcocystis caninum and Sarcocystis svanai-like apicomplexan protozoa. Vet. Parasitol. 2018, 252, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Berrocal, A.; López, A. Pulmonary sarcocystosis in a puppy with canine distemper in Costa Rica. J. Vet. Diagn. Invest. 2003, 15, 292–294. [Google Scholar] [CrossRef] [PubMed]
- Gottstein, B.; Pozio, E.; Nöckler, K. Epidemiology, diagnosis, treatment, and control of trichinellosis. Clin. Microbiol. Rev. 2009, 22, 127–145. [Google Scholar] [CrossRef]
- Kirjušina, M.; Deksne, G.; Marucci, G.; Bakasejevs, E.; Jahundoviča, I.; Daukšte, A.; Zdankovska, A.; Bērziņa, Z.; Esīte, Z.; Bella, A.; et al. A 38-year study on Trichinella spp. in wild boar (Sus scrofa) of Latvia shows a stable incidence with an increased parasite biomass in the last decade. Parasite. Vectors 2015, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Schellenberg, R.S.; Tan, B.J.; Irvine, J.D.; Stockdale, D.R.; Gajadhar, A.A.; Serhir, B.; Botha, J.; Armstong, C.A.; Shirley, A.W.; Blondeau, J.M.; et al. An outbreak of trichinellosis due to consumption of bear meat infected with Trichinella nativa in 2 northern Saskatchewan communities. J. Infect. Dis. 2003, 188, 835–843. [Google Scholar] [CrossRef]
- Ozeretskovskaya, N.N.; Mikhailova, L.G.; Sabgaida, T.P.; Dovgalev, A.S. New trends and clinical patterns of human trichinellosis in Russia at the beginning of the XXI century. Vet. Parasitol. 2005, 132, 167–171. [Google Scholar] [CrossRef]
- Møller, L.N.; Petersen, E.; Kapel, C.M.; Melbye, M.; Koch, A. Outbreak of trichinellosis associated with consumption of game meat in West Greenland. Vet. Parasitol. 2005, 132, 131–136. [Google Scholar] [CrossRef]
- Dworkin, M.S.; Gamble, H.R.; Zarlenga, D.S.; Tennican, P.O. Outbreak of trichinellosis associated with eating cougar jerky. J. Infect. Dis. 1996, 174, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Kociecka, W. Trichinellosis: Human disease, diagnosis and treatment. Vet. Parasitol. 2000, 93, 365–383. [Google Scholar] [CrossRef]
- Diaz, J.H.; Warren, R.J.; Oster, M.J. The disease ecology, epidemiology, clinical manifestations, and management of trichinellosis linked to consumption of wild animal meat. Wilderness Environ. Med. 2020, 31, 235–244. [Google Scholar] [CrossRef]
- Bruschi, F.; Murrell, K.D. New aspects of human trichinellosis: The impact of new Trichinella species. Postgrad. Med. J. 2002, 78, 15–22. [Google Scholar] [CrossRef]
- Pavic, S.; Andric, A.; Sofronic-Milosavljevic, L.J.; Gnjatovic, M.; Mitić, I.; Vasilev, S.; Sparic, R.; Pavic, A. Trichinella britovi outbreak: Epidemiological, clinical, and biological features. Med. Mal. Infect. 2020, 50, 520–524. [Google Scholar] [CrossRef]
- Kapel, C.M.O. Host diversity and biological characteristics of the Trichinella genotypes and their effect on transmission. Vet. Parasitol. 2000, 93, 263–278. [Google Scholar] [CrossRef]
- Scioscia, N.P.; Olmos, L.; Gorosábel, A.; Bernad, L.; Pedrana, J.; Hecker, Y.P.; Gual, I.; Gos, M.L.; Denegri, G.M.; Moore, D.P.; et al. Pampas fox (Lycalopex gymnocercus) new intermediate host of Sarcocystis svanai (Apicomplexa: Sarcocystidae). Parasitol. Int. 2017, 66, 214–218. [Google Scholar] [CrossRef]
- Gjerde, B.; Schulze, J. Muscular sarcocystosis in two arctic foxes (Vulpes lagopus) due to Sarcocystis arctica n. sp.: Sarcocyst morphology, molecular characteristics and phylogeny. Parasitol. Res. 2014, 113, 811–821. [Google Scholar] [CrossRef]
- Cerqueira-Cézar, C.K.; Thompson, P.C.; Verma, S.K.; Mowery, J.; Calero-Bernal, R.; Antunes Murata, F.H.; Sinnett, D.R.; Van Hemert, C.; Rosenthal, B.M.; Dubey, J.P. Morphological and molecular characterization of Sarcocystis arctica-like sarcocysts from the Arctic fox (Vulpes lagopus) from Alaska, USA. Parasitol. Res. 2017, 116, 1871–1878. [Google Scholar] [CrossRef]
- Pavlásek, I.; Máca, O. Morphological and molecular identification of Sarcocystis arctica sarcocysts in three red foxes (Vulpes vulpes) from the Czech Republic. Parasitol. Int. 2017, 66, 603–605. [Google Scholar] [CrossRef]
- Gjerde, B.; Josefsen, T.D. Molecular characterisation of Sarcocystis lutrae n. sp. and Toxoplasma gondii from the musculature of two Eurasian otters (Lutra lutra) in Norway. Parasitol. Res. 2015, 114, 873–886. [Google Scholar] [CrossRef]
- Lepore, T.; Bartley, P.M.; Chianini, F.; Macrae, A.I.; Innes, E.A.; Katzer, F. Molecular detection of Sarcocystis lutrae in the European badger (Meles meles) in Scotland. Parasitol. 2017, 144, 1426–1432. [Google Scholar] [CrossRef] [PubMed]
- Máca, O. Molecular identification of Sarcocystis lutrae in the European otter (Lutra lutra) and the European badger (Meles meles) from the Czech Republic. Parasitol. Res. 2018, 117, 943–945. [Google Scholar] [CrossRef] [PubMed]
- Máca, O. Molecular identification of Sarcocystis lutrae (Apicomplexa: Sarcocystidae) from the raccoon dog, Nyctereutes procyonoides, and the common raccoon, Procyon lotor, in the Czech Republic. Parasit. Vectors. 2020, 13, 231. [Google Scholar] [CrossRef] [PubMed]
- Prakas, P.; Butkauskas, D.; Rudaitytė, E.; Kutkienė, L.; Sruoga, A.; Pūraitė, I. Morphological and molecular characterization of Sarcocystis taeniata and Sarcocystis pilosa n. sp. from the sika deer (Cervus nippon) in Lithuania. Parasitol. Res. 2016, 115, 3021–3032. [Google Scholar] [CrossRef] [PubMed]
- Moks, E.; Jõgisalu, I.; Saarma, U.; Talvik, H.; Järvis, T.; Valdmann, H. Helminthologic survey of the wolf (Canis lupus) in Estonia, with an emphasis on Echinococcus granulosus. J. Wildl. Dis. 2006, 42, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Bakasejevs, E.; Daukšte, A.; Zolovs, M.; Zdanovska, A. Investigation of Trichinella in wildlife in Latgale region (Latvia). Acta. Biol. Daugavp. 2012, 12, 1–5. [Google Scholar]
- Deksne, G.; Segliņa, Z.; Jahundoviča, I.; Esīte, Z.; Bakasejevs, E.; Bagrade, G.; Keidane, D.; Interisano, M.; Marucci, G.; Tonanzi, D.; et al. High prevalence of Trichinella spp. in sylvatic carnivore mammals of Latvia. Vet. Parasitol. 2016, 231, 118–123. [Google Scholar] [CrossRef]
- Teodorović, V.; Vasilev, D.; Ćirović, D.; Marković, M.; Ćosić, N.; Djurić, S.; Djurković-Djaković, O. The wolf (Canis lupus) as an indicator species for the sylvatic Trichinella cycle in the Central Balkans. J. Wildl. Dis. 2014, 50, 911–915. [Google Scholar] [CrossRef]
- Airas, N.; Saari, S.; Mikkonen, T.; Virtala, A.M.; Pellikka, J.; Oksanen, A.; Isomursu, M.; Kilpelä, S.S.; Lim, C.W.; Sukura, A. Sylvatic Trichinella spp. infection in Finland. J. Parasitol. 2010, 96, 67–76. [Google Scholar] [CrossRef]
- Beck, R.; Beck, A.; Kusak, J.; Mihaljević, Ž.; Lučinger, S.; Živičnjak, T.; Huber, D.; Gudan, A.; Marinculić, A. Trichinellosis in wolves from Croatia. Vet. Parasitol. 2009, 159, 308–311. [Google Scholar] [CrossRef]
- Bień, J.; Moskwa, B.; Goździk, K.; Cybulska, A.; Kornacka, A.; Welc, M.; Popiołek, M.; Cabaj, W. The occurrence of nematodes of the genus Trichinella in wolves (Canis lupus) from the Bieszczady Mountains and Augustowska Forest in Poland. Vet. Parasitol. 2016, 231, 115–117. [Google Scholar] [CrossRef]
- Blaga, R.; Gherman, C.; Cozma, V.; Zocevic, A.; Pozio, E.; Boireau, P. Trichinella species circulating among wild and domestic animals in Romania. Vet. Parasitol. 2009, 159, 218–221. [Google Scholar] [CrossRef]
- Zarnke, R.L.; Worley, D.E.; Ver Hoef, J.M.; McNay, M.E. Trichinella sp. in wolves from interior Alaska. J. Wildl. Dis. 1999, 35, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Badagliacca, P.; Di Sabatino, D.; Salucci, S.; Romeo, G.; Cipriani, M.; Sulli, N.; Dall’Acqua, F.; Ruggieri, M.; Calistri, P.; Morelli, D. The role of the wolf in endemic sylvatic Trichinella britovi infection in the Abruzzi region of Central Italy. Vet. Parasitol. 2016, 231, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Carrasco, C.; Moroni, B.; García-Garrigós, A.; Robetto, S.; Carella, E.; Zoppi, S.; Tizzani, P.; Gonzálvez, M.; Orusa, R.; Rossi, L. Wolf Is Back: A Novel Sensitive Sentinel Rejoins the Trichinella Cycle in the Western Alps. Vet. Sci. 2023, 10, 206. [Google Scholar] [CrossRef]
- Sharma, R.; Thompson, P.C.; Hoberg, E.P.; Scandrett, W.B.; Konecsni, K.; Harms, N.J.; Kukka, P.M.; Jung, T.S.; Elkin, B.; Mulders, R.; et al. Hiding in plain sight: Discovery and phylogeography of a cryptic species of Trichinella (Nematoda: Trichinellidae) in wolverine (Gulo gulo). Int. J. Parasitol. 2020, 50, 277–287. [Google Scholar] [CrossRef]
- Gómez-Morales, M.A.; Ludovisi, A.; Amati, M.; Cherchi, S.; Tonanzi, D.; Pozio, E. Differentiation of Trichinella species (Trichinella spiralis/Trichinella britovi versus Trichinella pseudospiralis) using western blot. Parasite. Vectors 2018, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pozio, E.; Rinaldi, L.; Marucci, G.; Musella, V.; Galati, F.; Cringoli, G.; Boireau, P.; La Rosa, G. Hosts and habitats of Trichinella spiralis and Trichinella britovi in Europe. Int. J. Parasitol. 2009, 39, 71–79. [Google Scholar] [CrossRef]
- Senutaitė, J.; Grikienienė, J. Prevalence of Trichinella in muscles of some domestic and wild mammals in Lithuania and their impact on the organism. Acta Zool. Litu. 2001, 11, 395–404. [Google Scholar] [CrossRef]
- Trichineliozės Pamiršti Negalima. Available online: https://nmvrvi.lt/trichineliozes-pamirsti-negalima/ (accessed on 5 December 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).