Prevalence of Microbial Isolates Cultured from Endometrial Swab Samples Collected from United Kingdom Thoroughbred Mares from 2014 to 2020
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
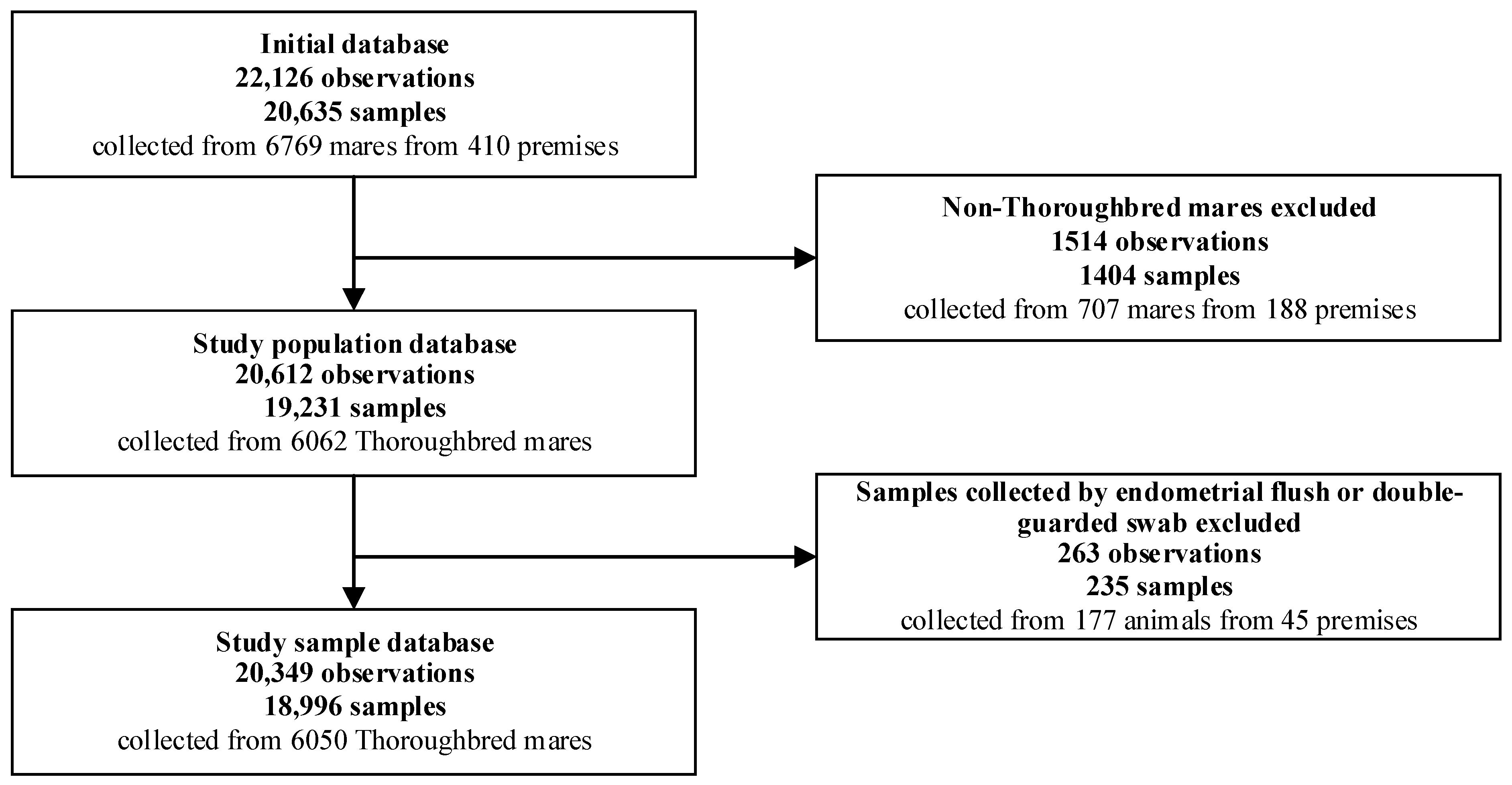
2.1. Source Population, Study Population and Study Sample
2.2. Sample Collection and Laboratory Analyses
2.2.1. Microbial Cultures
2.2.2. Cytology
2.3. Data Analyses
2.3.1. Outcomes and Explanatory Variables
2.3.2. Prevalence of Endometrial Isolates
Effects of Day-to-Day Variation and Mare- and Premises Characteristics on the Prevalence of Endometrial Isolates
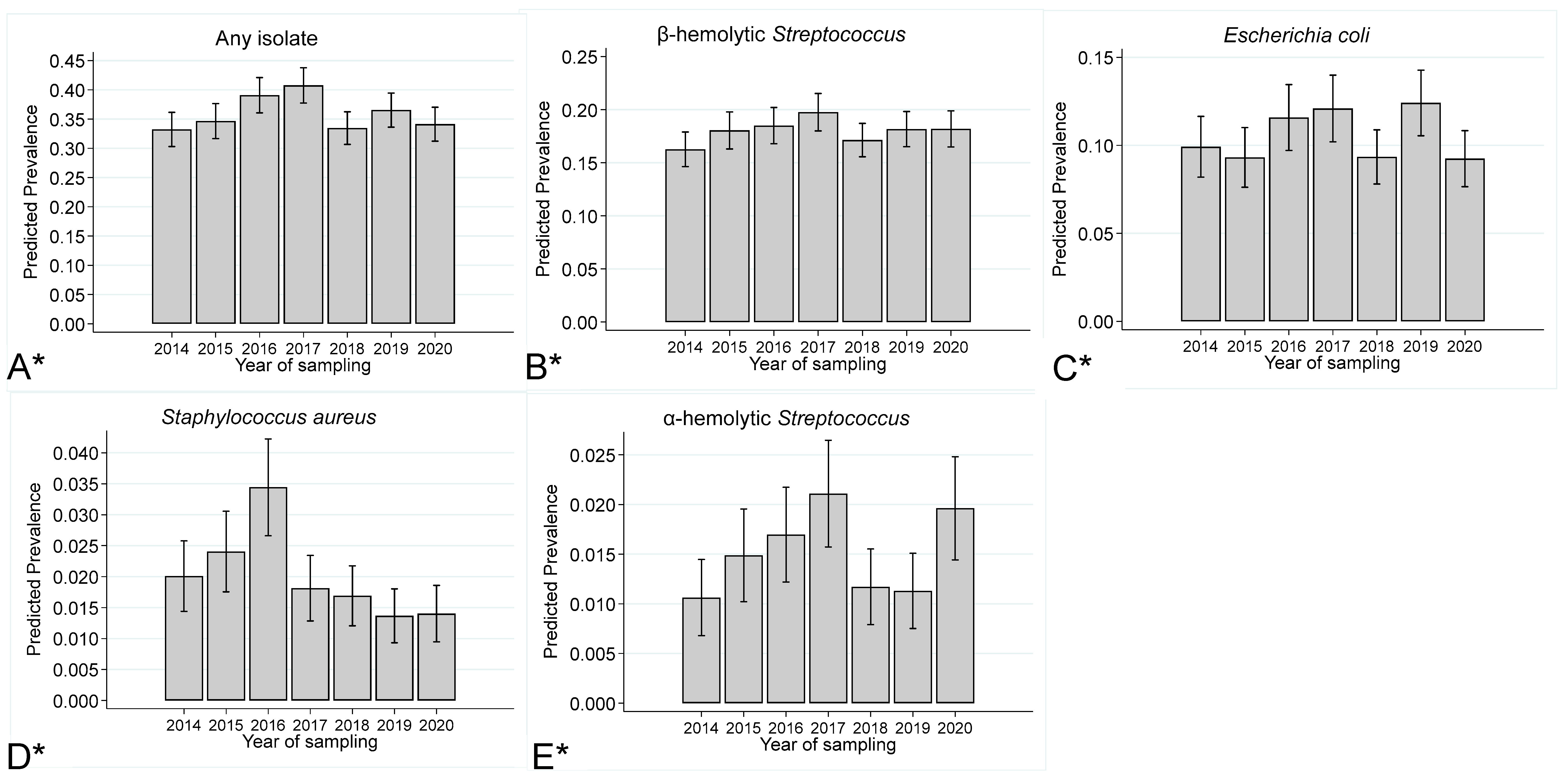
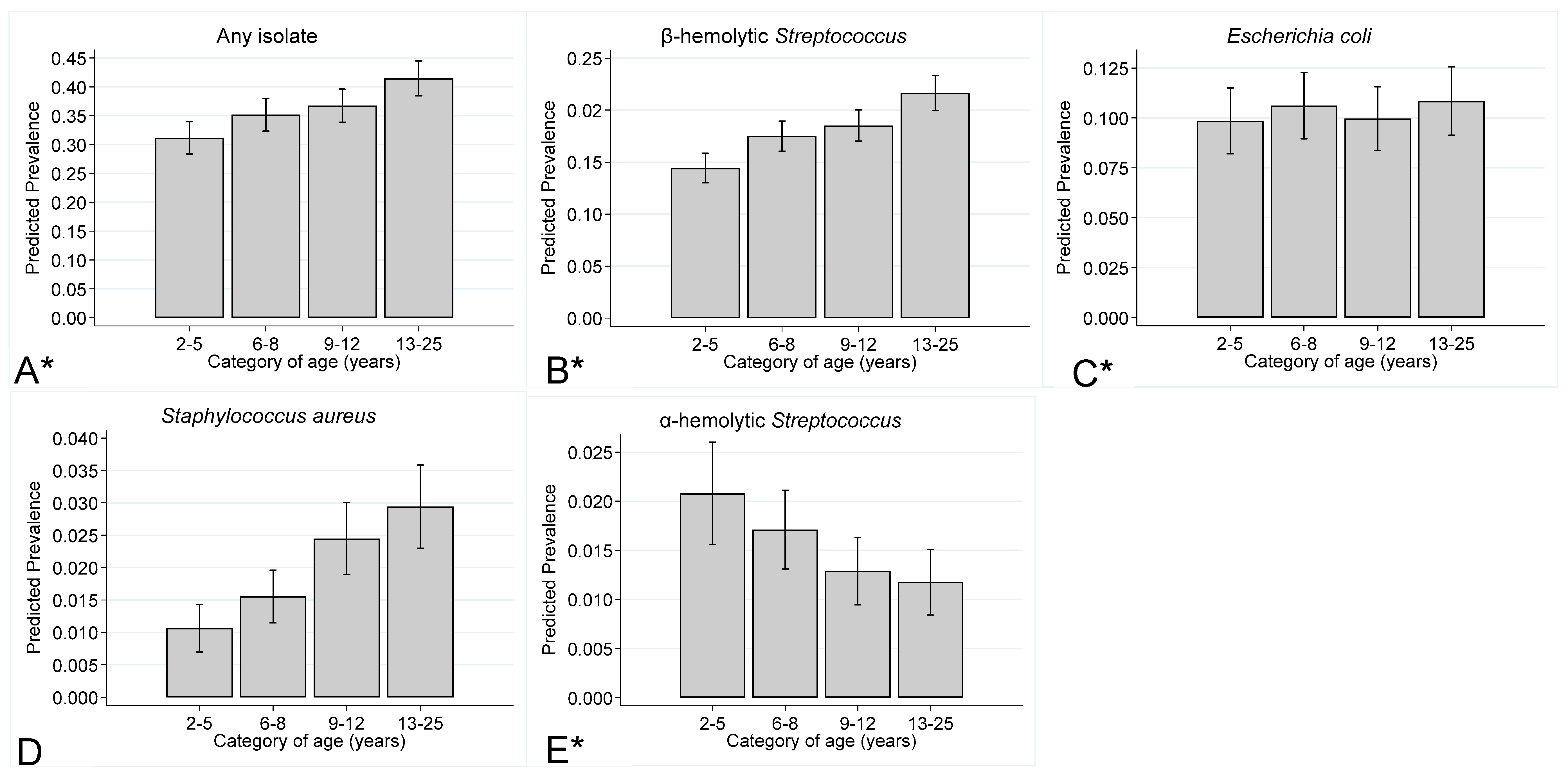
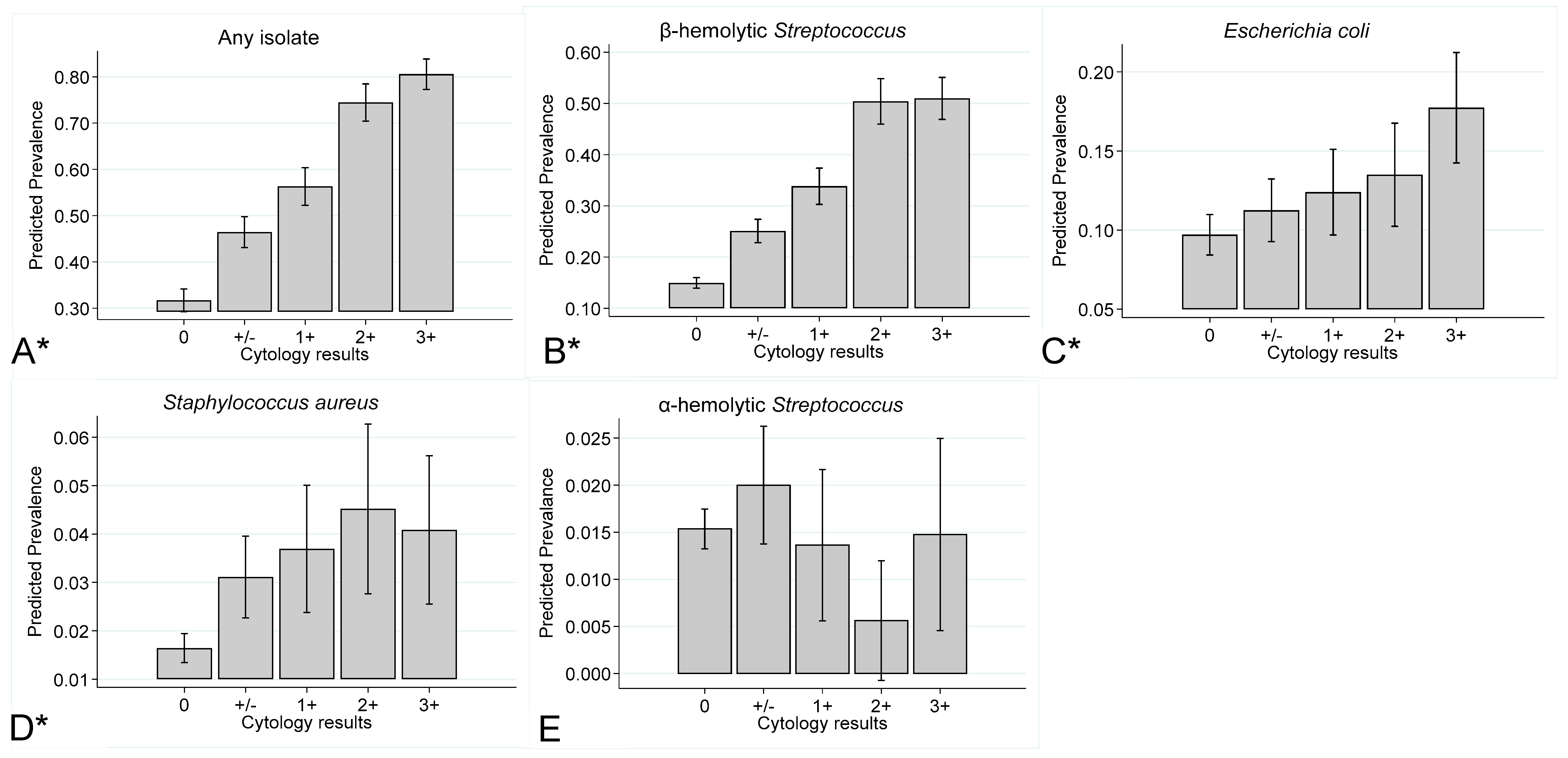
Prevalence of Endometrial Isolates by Year of Submission, Age of the Mare and Inflammatory Response
3. Results
3.1. Study Sample
3.2. Prevalence of Endometrial Isolates
3.3. Effects of Day-to-Day Variation and Mare and Premises Characteristics on the Prevalence of Endometrial Isolates
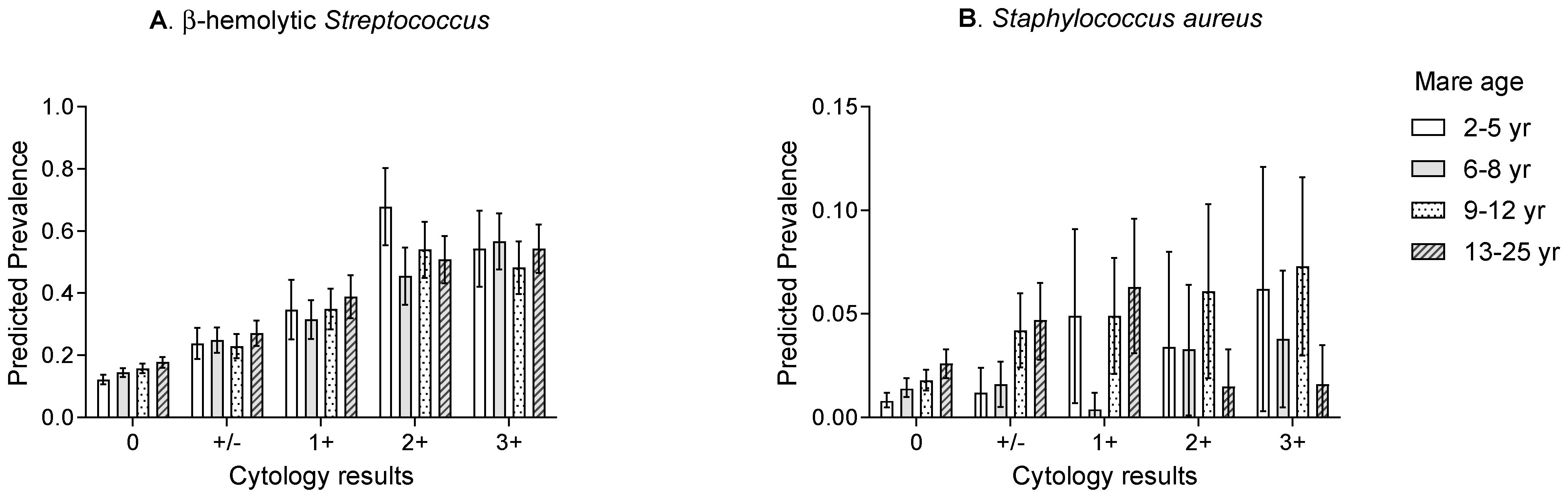
3.4. Prevalence of Endometrial Isolates stratified by Year of Sampling, Mare Age, and Inflammatory Response
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morris, L.H.A.; McCue, P.M.; Aurich, C. Equine endometritis: A review of challenges and new approaches. Reproduction 2020, 16, 95–110. [Google Scholar] [CrossRef]
- Horserace Betting Levy Board. International Codes of Practice. 2023. Available online: https://codes.hblb.org.uk/2023-Codes-of-Practice-full-document.pdf (accessed on 1 June 2023).
- Arango-Sabogal, J.C.; Mouncey, R.; de Mestre, A.M.; Verheyen, K. Date of birth and purchase price as foals or yearlings are associated with Thoroughbred flat race performance in the United Kingdom and Ireland. Vet. Rec. Open 2022, 9, e43. [Google Scholar] [CrossRef]
- Weatherbys. Fact Book. Weatherbys Bloodstock Publications 2020. Available online: https://issuu.com/weatherbys/docs/fact_book_-_2021-02-27_-_fb_final_plan (accessed on 1 June 2023).
- Mouncey, R.; Arango-Sabogal, J.C.; de Mestre, A.M.; Verheyen, K.L. Descriptive study of medication usage and occurrence of disease and injury during gestation in Thoroughbred broodmares. J. Equine Vet. Sci. 2022, 118, e104104. [Google Scholar] [CrossRef]
- Rose, B.; Firth, M.; Morris, B.; Roach, J.; Wathes, D.; Verheyen, K.; de Mestre, A. Descriptive study of current therapeutic practices, clinical reproductive findings and incidence of pregnancy loss in intensively managed thoroughbred mares. Anim. Reprod. Sci. 2018, 188, 74–84. [Google Scholar] [CrossRef]
- Mitchell, A.R.; Diel de Amorium, M.; Thachil, A.J.; Altier, C.; Cheong, S.H. Uterine Bacterial Isolates from Mares and Their Resistance to Antimicrobials. J. Equine Vet. Sci. 2018, 66, 114. [Google Scholar] [CrossRef]
- Davis, H.A.; Stanton, M.B.; Thungrat, K.; Boothe, D.M. Uterine bacterial isolates from mares and their resistance to antimicrobials: 8296 cases (2003–2008). JAVMA 2013, 242, 977–983. [Google Scholar] [CrossRef]
- Ravaioli, V.; Raffini, E.; Tamburini, M.; Galletti, G.; Frasnelli, M. Infectious Endometritis in Mares: Microbiological Findings in Field Samples. J. Equine Vet. Sci. 2022, 112, 103913. [Google Scholar] [CrossRef]
- Davies Morel, M.C.; Lawlor, O.; Nash, D.M. Equine endometrial cytology and bacteriology: Effectiveness for predicting live foaling rates. Vet. J. 2013, 198, 206–211. [Google Scholar] [CrossRef]
- Riddle, W.; LeBlanc, M.; Stromberg, A. Relationships between uterine culture, cytology and pregnancy rates in a Thoroughbred practice. Theriogenology 2007, 68, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Bertrana, M.L.; Deleuze, S.; Pitti Rios, L.; Yeste, M.; Morales Farina, I.; Rivera Del Alamo, M.M. Microbial Prevalence and Antimicrobial Sensitivity in Equine Endometritis in Field Conditions. Animals 2021, 11, 1476. [Google Scholar] [CrossRef] [PubMed]
- Pisello, L.; Rampacci, E.; Stefanetti, V.; Beccati, F.; Hyatt, D.R.; Coletti, M.; Passamonti, F. Temporal efficacy of antimicrobials against aerobic bacteria isolated from equine endometritis: An Italian retrospective analysis (2010–2017). Vet. Rec. 2019, 185, 598. [Google Scholar] [CrossRef] [PubMed]
- Frontoso, R.; De Carlo, E.; Pasolini, M. Retrospective study of bacterial isolates and their antimicrobial susceptibilities in equine uteri during fertility problems. Res. Vet. Sci. 2008, 84, 1–6. [Google Scholar] [CrossRef]
- Albihn, A.; Baverud, V.; Magnusson, U. Uterine microbiology and anti-microbial susceptibility in isolated bacteria from mares with fertility problems. Acta Vet. Scand. 2003, 44, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Holyoak, G.R. The Equine Endometrial Microbiome: A Brief Review. Am. J. Biomed. Sci. Res. 2021, 11, 532–534. [Google Scholar] [CrossRef]
- Malaluang, P.; Wilen, E.; Lindahl, J.F.; Hansson, I.; Morrell, J.M. Antimicrobial resistance in vaginal bacteris in inseminated mares. Pathogens 2023, 12, 375. [Google Scholar] [CrossRef] [PubMed]
- Scott, C.J.; de Mestre, A.M.; Verheyen, K.L.; Arango-Sabogal, J.C. Bayesian accuracy estimates and fit for purpose thresholds of cytology and culture of endometrial swab samples for detecting endometritis in mares. Prev. Vet. Med. 2022, 209, 105783. [Google Scholar] [CrossRef] [PubMed]
- Rathbone, P.; Arango-Sabogal, J.C.; de Mestre, A.M.; Scott, C.J. Antimicrobial resistance of endometrial bacterial isolates collected from UK Thoroughbred mares between 2014 and 2020. Vet. Rec. 2023, 19, e2591. [Google Scholar] [CrossRef] [PubMed]
- Dohoo, I.; Martin, W.; Stryhn, H. Introduction to clustered data. In Veterinary Epidemiologic Research, 2nd ed.; VER Inc.: Charlottetown, PE, Canada, 2009. [Google Scholar]
- Goldstein, H.; Browne, W.; Rasbash, J. Partitioning Variation in Multilevel Models. Underst. Stat. 2002, 1, 223–231. [Google Scholar] [CrossRef]
- Knol, M.J.; VanderWeele, T.J. Recommendations for presenting analyses of effect modification and interaction. Int. J. Epidemiol. 2012, 41, 514–520. [Google Scholar] [CrossRef]
- Malaluang, P.; Wilen, E.; Lindhal, J.; Hansson, I.; Morrell, J.M. Antimicrobial Resistance in Equine Reproduction. Animals 2021, 11, 3035. [Google Scholar] [CrossRef]
- LeBlanc, M.M.; Causey, R.C. Clinical and subclinical endometritis in the mare: Both threats to fertility. Reprod. Domest. Anim. 2009, 44, 10–22. [Google Scholar] [CrossRef]
- LeBlanc, M. The current status of antibiotic use in equine reproduction. Equine Vet. Educ. 2009, 21, 156–167. [Google Scholar] [CrossRef]
- Overbeck, W.; Witte, T.S.; Heuwieser, W. Comparison of three diagnostic methods to identify subclinical endometritis in mares. Theriogenology 2011, 75, 1311–1318. [Google Scholar] [CrossRef]
- Christoffersen, M.; Soderlind, M.; Rudefalk, S.R.; Pederson, H.G.; Allen, J.; Krekler, N. Risk factors associated with uterine fluid after breeding caused by Streptococcus zooepidemicus. Theriogenology 2015, 84, 1283–1290. [Google Scholar] [CrossRef]
- Buczkowska, J.; Kozdrowski, R.; Nowak, M.; Ras, A.; Staroniewicz, Z.; Siemieiuch, M.J. Comparison of the biopsy and cytobrush techniques for diagnosis of subclinical endometritis in mares. Reprod. Biol. Endocrinol. 2014, 12, 27. [Google Scholar] [CrossRef]
- Overbeck, W.; Jager, K.; Schoon, H.A.; Witte, T.S. Comparison of cytological and histological examinations in different locations of the equine uterus-an in vitro study. Theriogenology 2013, 79, 1262–1268. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.; Troedsson, M.; Pedersen, M. Diagnosis of endometritis in the mare based on bacteriological and cytological examinations of the endometrium: Comparison of results obtained by swabs and biopsies. J. Equine Vet. Sci. 2010, 30, 27–30. [Google Scholar] [CrossRef]
- Bindslev, M.M.; Villumsen, H.; Petersen, M.M.; Nielsen, J.M.; Bøgh, I.B.; Bojesen, A.M. Genetic diversity of S. equi ssp. Zooepidemicus and E. coli isolated from the reproductive tract of the mare. Reprod. Domest. Anim. 2008, 43, 110. [Google Scholar] [CrossRef]
- Katila, T. Post-mating inflammatory responses of the uterus. Reprod. Domest. Anim. 2012, 47, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Leeflang, M.M.G.; Rutjes, A.W.S.; Reitsma, J.B.; Hooft, L.; Bossuyt, P.M.M. Variation of a test’s sensitivity and specificity with disease prevalence. Can. Med. Aassociation J. 2013, 185, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.; Sandøe, P. Welfare in horse breeding. Vet. Rec. 2015, 176, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Raidal, S. Antimicrobial stewardship in equine practice. Aust. Vet. J. 2019, 97, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Zent, W.W.; Troedsson, M.H.T. Postbreeding uterine fluid accumulation in a normal population of Thoroughbred mares: A field study. In Proceedings of the AAEP Proceedings, Baltimore, MD, USA, 6–9 December 1998; Volume 44, pp. 64–65. Available online: https://www.ivis.org/sites/default/files/library/aaep/1998/Zent.pdf (accessed on 1 June 2023).
| Year of Sampling | Number of Samples Submitted | Number of Mares Sampled | Number of Samples with Microbial Growth | Percentage (95% CI) of Samples with Microbial Growth |
|---|---|---|---|---|
| 2014 | 2621 | 1413 | 707 | 27.0 (25.3–28.7) |
| 2015 | 2500 | 1365 | 725 | 29.0 (27.2–30.8) |
| 2016 | 2684 | 1447 | 886 | 33.0 (31.2–34.8) |
| 2017 | 2633 | 1444 | 919 | 34.9 (33.1–36.7) |
| 2018 | 2936 | 1709 | 817 | 27.8 (26.2–29.5) |
| 2019 | 2862 | 1593 | 901 | 31.5 (29.8–33.2) |
| 2020 | 2760 | 1518 | 830 | 30.1 (28.4–31.8) |
| Total | 18,996 | 6050 | 5785 | 30.5 (29.8–31.1) |
| Microorganism Isolated | Frequency | Percentage | 95% Confidence Interval |
|---|---|---|---|
| β-hemolytic Streptococcus | 3738 | 52.4 | (51.2–53.5) |
| Escherichia coli | 1842 | 25.8 | (24.8–26.8) |
| Staphylococcus aureus | 441 | 6.2 | (5.6–6.7) |
| Coagulase Negative Staphylococcus | 391 | 5.5 | (5.0–6.0) |
| α-hemolytic Streptococcus | 308 | 4.3 | (3.8–4.8) |
| Actinobacillus species | 133 | 1.9 | (1.6–2.2) |
| Enterococcus faecalis | 50 | 0.7 | (0.5–0.9) |
| Klebsiella pneumoniae | 43 | 0.6 | (0.4–0.8) |
| Acinetobacter species | 29 | 0.4 | (0.3–0.6) |
| Pseudomonas aeruginosa | 27 | 0.4 | (0.2–0.5) |
| Bacillus species | 27 | 0.4 | (0.2–0.5) |
| Enterobacter aerogenes | 22 | 0.3 | (0.2–0.4) |
| Enterococcus species | 16 | 0.2 | (0.1–0.3) |
| Proteus species | 14 | 0.2 | (0.1–0.3) |
| Pasteurella species | 10 | 0.2 | (0.0–0.2) |
| Enterobacter species | 6 | 0.1 | (0.0–0.2) |
| Pantoea agglomerans | 6 | 0.1 | (0.0–0.2) |
| Pseudomonas fluorescens | 5 | 0.1 | (0.0–0.2) |
| Corynebacterium species | 4 | 0.1 | (0.0–0.1) |
| Enterobacter agglomerans | 4 | 0.1 | (0.0–0.1) |
| Non-hemolytic Streptococcus | 4 | 0.1 | (0.0–0.1) |
| Mucor species | 3 | 0.0 | (0.0–0.1) |
| Aspergillus fumigatus | 2 | 0.0 | (0.0–0.1) |
| Kluyvera species | 2 | 0.0 | (0.0–0.1) |
| Pseudomonas stutzeri | 2 | 0.0 | (0.0–0.1) |
| Achromobacter species | 1 | 0.0 | (0.0–0.1) |
| Burkholderia cepacian | 1 | 0.0 | (0.0–0.1) |
| Enterobacter cloacae | 1 | 0.0 | (0.0–0.1) |
| Fungus | 1 | 0.0 | (0.0–0.1) |
| Klebsiella oxytoca | 1 | 0.0 | (0.0–0.1) |
| Pseudomonas putida | 1 | 0.0 | (0.0–0.1) |
| Pseudomonas species | 1 | 0.0 | (0.0–0.1) |
| Weeksella virosa | 1 | 0.0 | (0.0–0.1) |
| Total | 7138 | 100% | - |
| Cytology Result | Microbial Growth (%: 95% Confidence Interval) | Isolates (%: 95% Confidence Interval) | |||
|---|---|---|---|---|---|
| Number of Swabs | No | Yes | Monocultures (1 Isolate) | Mixed Cultures (>1 Isolate) | |
| 0 (No PMN) | 14,440 | 10,707 (74.1: 73.4–74.9) | 3733 (25.9: 25.1–26.6) | 2941 (78.8: 77.4–80.1) | 792 (21.2: 19.9–22.6) |
| +/− (≤0.5% PMN) | 1829 | 1110 (60.7: 58.4–62.9) | 719 (39.3: 37.1–41.6) | 562 (78.2: 75.0–81.0) | 157 (21.8: 19.0–25.1) |
| 1+ (>0.5–5% PMN) | 733 | 361 (49.3: 45.6–52.9) | 372 (50.8:47.1–54.4) | 295 (79.3: 74.9–83.1) | 77 (20.7: 16.9–25.1) |
| 2+ (>5–30% PMN) | 462 | 127 (27.5: 23.6–31.7) | 335 (72.5: 68.3–76.4) | 268 (80.0: 75.4–83.9) | 67 (20.0: 6.1–24.6) |
| 3+ (>30% PMN) | 474 | 104 (21.9: 18.4–25.9) | 370 (78.1: 74.1–81.6) | 305 (82.4: 78.2–86.0) | 65 (17.6: 14.0–21.8) |
| Total | 17,938 | 12,409 (69.2: 68.5–69.8) | 5529 (30.8: 30.1–31.5) | 4371 (79.1: 78.0–80.1) | 1158 (20.9: 19.9–22.0) |
| Isolate | Total | Monoculture (%: 95% Confidence Interval) | Mixed Culture (%: 95% Confidence Interval) |
|---|---|---|---|
| Any type of isolate | 5785 | 4577 (79.1: 78.0–80.1) | 1208 (20.9: 19.8–21.9) |
| β-hemolytic Streptococcus | 3738 | 2700 (72.2: 70.1–73.6) | 1038 (27.8: 26.3–29.2) |
| Escherichia coli | 1842 | 1192 (64.7: 62.5–66.9) | 650 (35.3: 33.1–37.5) |
| Staphylococcus aureus | 441 | 151 (34.2: 30.0–38.8) | 290 (65.8: 61.2–70.0) |
| α-hemolytic Streptococcus | 308 | 131 (42.5: 37.1–48.1) | 177 (57.5: 51.9–62.9) |
| Isolate | Marginal Predicted Probability (95% CI) | Variance Partitioning by Level | ||
|---|---|---|---|---|
| Farm | Mare | Sample/Isolate | ||
| Any type of isolate | 35.5 (33.0–37.9) | 0.26 | 0.57 | 0.17 |
| β-hemolytic Streptococcus | 17.9 (16.8–19.0) | 0.17 | 0.32 | 0.51 |
| Escherichia coli | 10.3 (9.0–11.6) | 0.29 | 0.61 | 0.10 |
| Staphylococcus aureus | 2.0 (1.6–2.3) | 0.22 | 0.75 | 0.03 |
| α-hemolytic Streptococcus | 1.5 (1.3–1.7) | 0.02 | 0.95 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mouncey, R.; Arango-Sabogal, J.C.; Rathbone, P.; Scott, C.J.; de Mestre, A.M. Prevalence of Microbial Isolates Cultured from Endometrial Swab Samples Collected from United Kingdom Thoroughbred Mares from 2014 to 2020. Vet. Sci. 2024, 11, 82. https://doi.org/10.3390/vetsci11020082
Mouncey R, Arango-Sabogal JC, Rathbone P, Scott CJ, de Mestre AM. Prevalence of Microbial Isolates Cultured from Endometrial Swab Samples Collected from United Kingdom Thoroughbred Mares from 2014 to 2020. Veterinary Sciences. 2024; 11(2):82. https://doi.org/10.3390/vetsci11020082
Chicago/Turabian StyleMouncey, Rebecca, Juan Carlos Arango-Sabogal, Polly Rathbone, Camilla J. Scott, and Amanda M. de Mestre. 2024. "Prevalence of Microbial Isolates Cultured from Endometrial Swab Samples Collected from United Kingdom Thoroughbred Mares from 2014 to 2020" Veterinary Sciences 11, no. 2: 82. https://doi.org/10.3390/vetsci11020082
APA StyleMouncey, R., Arango-Sabogal, J. C., Rathbone, P., Scott, C. J., & de Mestre, A. M. (2024). Prevalence of Microbial Isolates Cultured from Endometrial Swab Samples Collected from United Kingdom Thoroughbred Mares from 2014 to 2020. Veterinary Sciences, 11(2), 82. https://doi.org/10.3390/vetsci11020082







