Prevalence and Diversity of Haemotropic Mycoplasma Species in Cats and Their Ectoparasites (Fleas and Ticks)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Domestic Cats Blood Collection
2.2. Ectoparasite Sample Collection
2.3. DNA Extraction and PCR Amplification
2.4. Statistical Analysis
2.5. Clinical Infection Cases
3. Results
Clinical Infection Cases
4. Discussion
Clinical Infection Cases
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Razin, S.; Yogev, D.; Naot, Y. Molecular Biology and Pathogenicity of Mycoplasmas. Microbiol. Mol. Biol. Rev. 1998, 62, 1094–1156. [Google Scholar] [CrossRef]
- Barker, E.; Tasker, S. Haemoplasmas: Lessons Learnt from Cats. N. Z. Vet. J. 2013, 61, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Tasker, S.; Hofmann-Lehmann, R.; Belák, S.; Frymus, T.; Addie, D.D.; Pennisi, M.G.; Boucraut-Baralon, C.; Egberink, H.; Hartmann, K.; Hosie, M.J.; et al. Haemoplasmosis in Cats: European Guidelines from the ABCD on Prevention and Management. J. Feline Med. Surg. 2018, 20, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Sykes, J.E.; Drazenovich, N.L.; Ball, L.M.; Leutenegger, C.M. Use of Conventional and Real-Time Polymerase Chain Reaction to Determine the Epidemiology of Hemoplasma Infections in Anemic and Nonanemic Cats. J. Vet. Intern. Med. 2007, 21, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Díaz, V.L.; Silvestre-Ferreira, A.C.; Vilhena, H.; Pastor, J.; Francino, O.; Altet, L. Prevalence and Co-Infection of Haemotropic Mycoplasmas in Portuguese Cats by Real-Time Polymerase Chain Reaction. J. Feline Med. Surg. 2013, 15, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Walker Vergara, R.; Morera Galleguillos, F.; Gómez Jaramillo, M.; Pereira Almosny, N.R.; Arauna Martínez, P.; Grob Behne, P.; Acosta-Jamett, G.; Müller, A. Prevalence, Risk Factor Analysis, and Hematological Findings of Hemoplasma Infection in Domestic Cats from Valdivia, Southern Chile. Comp. Immunol. Microbiol. Infect. Dis. 2016, 46, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, M.; Hartmann, K. Vector-Borne Diseases in Cats in Germany. Tierarztl Prax Ausg K Kleintiere Heimtiere 2017, 45, 329–335. [Google Scholar] [PubMed]
- Lappin, M.R.; Tasker, S.; Roura, X. Role of Vector-Borne Pathogens in the Development of Fever in Cats: 1. Flea-Associated Diseases. J. Feline Med. Surg. 2020, 22, 31–39. [Google Scholar] [CrossRef]
- Messick, J.B. Hemotrophic Mycoplasmas (Hemoplasmas): A Review and New Insights into Pathogenic Potential. Vet. Clin. Pathol. 2004, 33, 2–13. [Google Scholar] [CrossRef]
- Santos, A.P.D.; Conrado, F.D.O.; Messick, J.B.; Biondo, A.W.; Oliveira, S.T.D.; Guimaraes, A.M.S.; Nascimento, N.C.D.; Pedralli, V.; Lasta, C.S.; González, F.H.D. Hemoplasma prevalence and hematological abnormalities associated with infection in three different cat populations from Southern Brazil. Rev. Bras. Parasitol. Veterinária 2014, 23, 428–434. [Google Scholar] [CrossRef]
- Museux, K.; Boretti, F.S.; Willi, B.; Riond, B.; Hoelzle, K.; Hoelzle, L.E.; Wittenbrink, M.M.; Tasker, S.; Wengi, N.; Reusch, C.E.; et al. In Vivo Transmission Studies of ’ Candidatus Mycoplasma Turicensis’ in the Domestic Cat. Vet. Res. 2009, 40, 45. [Google Scholar] [CrossRef]
- Abdullah, S.; Helps, C.; Tasker, S.; Newbury, H.; Wall, R. Pathogens in Fleas Collected from Cats and Dogs: Distribution and Prevalence in the UK. Parasit. Vectors 2019, 12, 71. [Google Scholar] [CrossRef]
- Alleman, A.R.; Pate, M.G.; Harvey, J.W.; Gaskin, J.M.; Barbet, A.F. Western Immunoblot Analysis of the Antigens of Haemobartonella Felis with Sera from Experimentally Infected Cats. J. Clin. Microbiol. 1999, 37, 1474–1479. [Google Scholar] [CrossRef]
- Foley, J.E.; Harrus, S.; Poland, A.; Chomel, B.; Pedersen, N.C. Molecular, clinical, and pathologic comparison of two distinct strains of Haemobartonella felis in domestic cats. Am. J. Vet. Res. 1998, 59, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhao, S.; Wei, C.; Liu, J.; Chen, J.; Xing, L.; Yan, H.; Zhang, Y.; Bai, R.; Zheng, Z. A Diagnostic Test of Real-Time PCR Detection in the Diagnosis of Clinical Bloodstream Infection. Ann. Palliat. Med. 2022, 11, 3224–3230. [Google Scholar] [CrossRef] [PubMed]
- Ter, S.K.; Rattanavong, S.; Roberts, T.; Sengduangphachanh, A.; Sihalath, S.; Panapruksachat, S.; Vongsouvath, M.; Newton, P.N.; Simpson, A.J.H.; Robinson, M.T. Molecular detection of pathogens in negative blood cultures in the Lao people’s democratic republic. Am. J. Trop. Med. Hyg. 2021, 104, 1582. [Google Scholar] [CrossRef]
- Baker, A.S. Mites and Ticks of Domestic Animals…—“Google” Mokslinčius. 1999. Available online: https://scholar.google.lt/scholar?hl=lt&as_sdt=0%2C5&q=Baker+AS.+1999.+Mites+and+ticks+of+domestic+animals.+An+identification+guide+and+information+source.+TSO%2C+London.&btnG= (accessed on 29 August 2023).
- Brinck-Lindroth, G.; Smit, F. The Fleas (Siphonaptera) of Fennoscandia and Denmark; Brill: Leiden, The Netherlands, 2007. [Google Scholar]
- Rijpkema, S.; Golubić, D.; Molkenboer, M.; Verbeek-De Kruif, N.; Schellekens, J. Identification of Four Genomic Groups of Borrelia Burgdorferi Sensu Lato in Ixodes Ricinus Ticks Collected in a Lyme Borreliosis Endemic Region of Northern Croatia. Exp. Appl. Acarol. 1996, 20, 23–30. [Google Scholar] [CrossRef]
- Varanat, M.; Maggi, R.; Linder, K.; Breitschwerdt, E. Molecular Prevalence of Bartonella, Babesia, and Hemotropic Mycoplasma sp. in Dogs with Splenic Disease. J. Vet. Intern. Med. 2011, 25, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Tasker, S. Haemotropic Mycoplasmas: What’s Their Real Significance in Cats? J. Feline Med. Surg. 2010, 12, 369–381. [Google Scholar] [CrossRef]
- Sivajothi, S.; Sudhakara Reddy, B.; Swetha, K. Clinical, haemato-biochemical and electrocardiographic studies in persian breed cats with haemobartonellosis. Explor. Anim. Med. Res. 2023, 13, 131–135. [Google Scholar] [CrossRef]
- Ravicini, S.; Pastor, J.; Hawley, J.; Brewer, M.; Castro-López, J.; Beall, M.; Lappin, M.R. Prevalence of Selected Infectious Disease Agents in Stray Cats in Catalonia, Spain. J. Feline Med. Surg. Open Rep. 2016, 2, 205511691663410. [Google Scholar] [CrossRef]
- Marcondes, M.; Hirata, K.Y.; Vides, J.P.; Sobrinho, L.S.V.; Azevedo, J.S.; Vieira, T.S.W.J.; Vieira, R.F.C. Infection by Mycoplasma spp., Feline Immunodeficiency Virus and Feline Leukemia Virus in Cats from an Area Endemic for Visceral Leishmaniasis. Parasit. Vectors 2018, 11, 131. [Google Scholar] [CrossRef]
- Rosenqvist, M.B.; Meilstrup, A.-K.H.; Larsen, J.; Olsen, J.E.; Jensen, A.L.; Thomsen, L.E. Prevalence of Feline Haemoplasma in Cats in Denmark. Acta Vet. Scand. 2016, 58, 78. [Google Scholar] [CrossRef] [PubMed]
- Mylonakis, M.E.; Schreeg, M.; Chatzis, M.K.; Pearce, J.; Marr, H.S.; Saridomichelakis, M.N.; Birkenheuer, A.J. Molecular Detection of Vector-Borne Pathogens in Greek Cats. Ticks Tick Borne Dis. 2018, 9, 171–175. [Google Scholar] [CrossRef]
- Berzina, I.; Capligina, V.; Namina, A.; Visocka, A.; Ranka, R. Haemotropic Mycoplasma Species in Pet Cats in Latvia: A Study, Phylogenetic Analysis and Clinical Case Report. J. Feline Med. Surg. Open Rep. 2021, 7, 205511692110280. [Google Scholar] [CrossRef]
- Silaghi, C.; Knaus, M.; Rapti, D.; Kusi, I.; Shukullari, E.; Hamel, D.; Pfister, K.; Rehbein, S. Survey of Toxoplasma Gondii and Neospora Caninum, Haemotropic Mycoplasmas and Other Arthropod-Borne Pathogens in Cats from Albania. Parasit. Vectors 2014, 7, 62. [Google Scholar] [CrossRef]
- Schäfer, I.; Peukert, A.; Kerner, K.; Müller, E. Vector-Borne Pathogens in Stray Cats in Eastern Germany (Thuringia). Animals 2023, 13, 2574. [Google Scholar] [CrossRef] [PubMed]
- Bauer, N.; Balzer, H.-J.; Thüre, S.; Moritz, A. Prevalence of Feline Haemotropic Mycoplasmas in Convenience Samples of Cats in Germany. J. Feline Med. Surg. 2008, 10, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Willi, B.; Boretti, F.S.; Meli, M.L.; Bernasconi, M.V.; Casati, S.; Hegglin, D.; Puorger, M.; Neimark, H.; Cattori, V.; Wengi, N.; et al. Real-Time PCR Investigation of Potential Vectors, Reservoirs, and Shedding Patterns of Feline Hemotropic Mycoplasmas. Appl. Environ. Microbiol. 2007, 73, 3798–3802. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.; Lappin, M.R. “Candidatus Mycoplasma Haemominutum” Infections in 21 Client-Owned Cats. J. Am. Anim. Hosp. Assoc. 2007, 43, 249–257. [Google Scholar] [CrossRef]
- Assarasakorn, S.; Veir, J.K.; Hawley, J.R.; Brewer, M.M.; Morris, A.K.; Hill, A.E.; Lappin, M.R. Prevalence of Bartonella Species, Hemoplasmas, and Rickettsia Felis DNA in Blood and Fleas of Cats in Bangkok, Thailand. Res. Vet. Sci. 2012, 93, 1213–1216. [Google Scholar] [CrossRef]
- Hornok, S.; Meli, M.L.; Perreten, A.; Farkas, R.; Willi, B.; Beugnet, F.; Lutz, H.; Hofmann-Lehmann, R. Molecular Investigation of Hard Ticks (Acari: Ixodidae) and Fleas (Siphonaptera: Pulicidae) as Potential Vectors of Rickettsial and Mycoplasmal Agents. Vet. Microbiol. 2010, 140, 98–104. [Google Scholar] [CrossRef]
- Duplan, F.; Davies, S.; Filler, S.; Abdullah, S.; Keyte, S.; Newbury, H.; Helps, C.R.; Wall, R.; Tasker, S. Anaplasma Phagocytophilum, Bartonella spp., Haemoplasma Species and Hepatozoon spp. in Ticks Infesting Cats: A Large-Scale Survey. Parasit. Vectors 2018, 11, 201. [Google Scholar] [CrossRef]
- Shaw, S.E.; Kenny, M.J.; Tasker, S.; Birtles, R.J. Pathogen Carriage by the Cat Flea Ctenocephalides Felis (Bouché) in the United Kingdom. Vet. Microbiol. 2004, 102, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Lappin, M.R.; Griffin, B.; Brunt, J.; Riley, A.; Burney, D.; Hawley, J.; Brewer, M.M.; Jensen, W.A. Prevalence of Bartonella Species, Haemoplasma Species, Ehrlichia Species, Anaplasma Phagocytophilum, and Neorickettsia Risticii DNA in the Blood of Cats and Their Fleas in the United States. J. Feline Med. Surg. 2006, 8, 85–90. [Google Scholar] [CrossRef]
- Woods, J.E.; Brewer, M.M.; Hawley, J.R.; Wisnewski, N.; Lappin, M.R. Evaluation of experimental transmission of Candidatus Mycoplasma haemominutum and Mycoplasma haemofelis by Ctenocephalides felis to cats. Am. J. Vet. Res. 2005, 66, 1008–1012. [Google Scholar] [CrossRef] [PubMed]
- Woods, J.E.; Wisnewski, N.; Lappin, M.R. Attempted transmission of Candidatus Mycoplasma haemominutum and Mycoplasma haemofelis by feeding cats infected Ctenocephalides felis. Am. J. Vet. Res. 2006, 67, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Lappin, M.R. Feline haemoplasmas are not transmitted by Ctenocephalides felis. In Proceedings of the 9th Symposium of the CVBD World Forum, Lisbon, Portugal, 22–25 March 2014; pp. 44–46. [Google Scholar]
- Azrizal-Wahid, N.; Sofian-Azirun, M.; Low, V.L. Flea-borne pathogens in the cat flea Ctenocephalides felis and their association with mtDNA diversity of the flea host. Comp. Immunol. Microbiol. Infect. Dis. 2021, 75, 101621. [Google Scholar] [CrossRef]
- Lappin, M.R. Update on Flea and Tick Associated Diseases of Cats. Vet. Parasitol. 2018, 254, 26–29. [Google Scholar] [CrossRef]
- Aklilu, E.; Shaharulnizim, N.; Francis, J.; Biomed, S.A.-T. Molecular Investigation of Mycoplasma Haemofelis in Stray Cats in Kota Bharu, Kelantan. Trop Biomed. 2016, 33, 608–612. [Google Scholar] [PubMed]
- Pennisi, M.G.; Hartmann, K.; Addie, D.D.; Lutz, H.; Gruffydd-Jones, T.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Horzinek, M.C.; Hosie, M.J.; et al. Blood Transfusion in Cats. J. Feline Med. Surg. 2015, 17, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, M.G.; Persichetti, M.F.; Serrano, L.; Altet, L.; Reale, S.; Gulotta, L.; Solano-Gallego, L. Ticks and Associated Pathogens Collected from Cats in Sicily and Calabria (Italy). Parasit. Vectors 2015, 8, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Cordaux, R.; Paces-Fessy, M.; Raimond, M.; Michel-Salzat, A.; Zimmer, M.; Bouchon, D. Molecular Characterization and Evolution of Arthropod-Pathogenic Rickettsiella Bacteria. Appl. Environ. Microbiol. 2007, 73, 5045–5047. [Google Scholar] [CrossRef]
- Leclerque, A.; Kleespies, R.G. A Rickettsiella Bacterium from the Hard Tick, Ixodes Woodi: Molecular Taxonomy Combining Multilocus Sequence Typing (MLST) with Significance Testing. PLoS ONE 2012, 7, e38062. [Google Scholar] [CrossRef]
- Price, D.R.G.; Bartley, K.; Blake, D.P.; Karp-Tatham, E.; Nunn, F.; Burgess, S.T.G.; Nisbet, A.J. A Rickettsiella Endosymbiont Is a Potential Source of Essential B-Vitamins for the Poultry Red Mite, Dermanyssus Gallinae. Front. Microbiol. 2021, 12, 695346. [Google Scholar] [CrossRef]
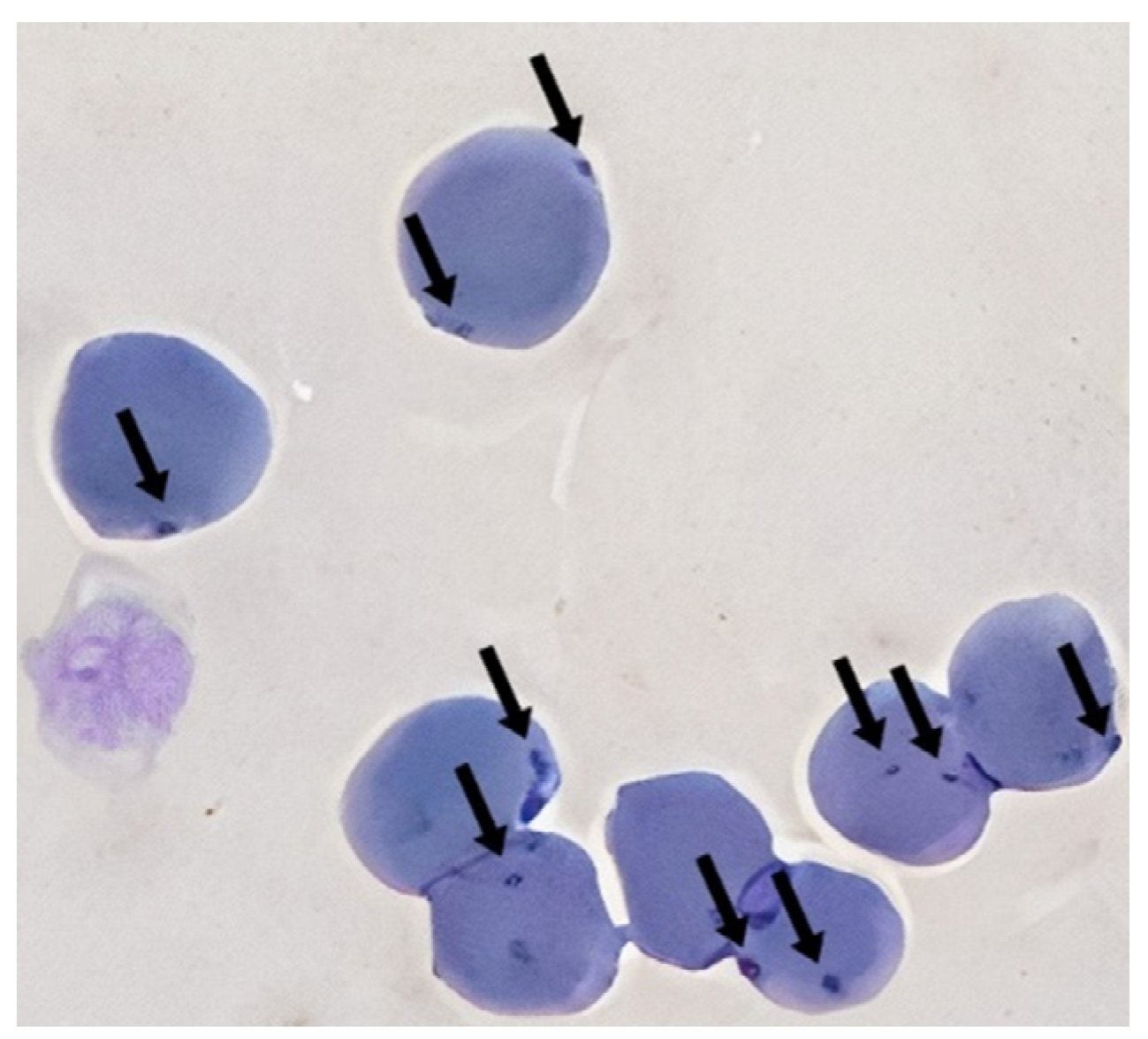

| Characteristics of Tested Cats | N | Mycoplasma-Positive Cats | Odds Ratio | 95% CI | p |
|---|---|---|---|---|---|
| shelter cats | 18 | 4 | 3.98 | 1.25–12.74 | p = 0.01 |
| owned cats | 523 | 35 | 1 | ||
| male | 287 | 22 | 1.16 | 0.6–2.23 | p = 0.66 * |
| female | 254 | 17 | 1 | ||
| young kittens < 1 year old | 42 | 0 | - | - | - |
| adults > 1 year old | 499 | 39 | - | ||
| healthy | 249 | 17 | 0.9 | 0.47–1.73 | p = 0.75 * |
| unhealthy | 292 | 22 | 1 |
| Ticks | Sex | N |
|---|---|---|
| I. ricinus | ♂ | 24 |
| ♀ | 242 | |
| D. reticulatus | ♂ | 21 |
| ♀ | 34 | |
| Total | 321 | |
| Cats | No of Mycoplasma Positive/No of Tested Fleas (%) |
|---|---|
| 1 | 4/34 (11.8%) |
| 2 | 2/12 (16.7%) |
| Fleas | Sex | N | Mycoplasma-Positive Fleas | Odds Ratio | 95% CI | χ2 | p | MIR (%) |
|---|---|---|---|---|---|---|---|---|
| Ct. felis | ♂ | 27 | 2 | 2.12 1 | 0.37–12.23 | 0.736 | p = 0.39 * | 4.379 |
| ♀ | 110 | 4 | ||||||
| Ct. canis | ♂ | 4 | 0 | - | - | - | - | - |
| ♀ | 11 | 0 | ||||||
| N. fasciatus | ♂ | 0 | 0 | - | - | - | - | - |
| ♀ | 1 | 0 | ||||||
| Total | 153 | 6 | ||||||
| Mycoplasma spp. | M. haemofelis | ‘Ca. M. haematominutum’ | |
|---|---|---|---|
| shelter cats | 4 | 0 | 4 |
| owned cats | 35 | 6 | 22 |
| Total in cats | 39 | 6 | 26 |
| Ct. felis fleas | 6 | 6 | 0 |
| GenBank Accession Numbers | Nucleotide Positions | Geographic Origin | |||
|---|---|---|---|---|---|
| 40 | 114 | 206 | 543 | ||
| Felis catus AY150976, AY150985, EU839978, CP002808, EU442633, KR905465, MW633343; Panthera leo DQ825453; Felis catus OQ355649, OQ361729, OQ361730, OQ361731, OQ361732, OQ361733; Ctenocephalides felis OQ361734 (n = 6) | T | A | T | C | Australia, United Kingdom, Italy, United States of America, Brazil, Spain, Angola, Tanzania, Lithuania |
| Felis catus KR905462, AY150984; Felis silvestris DQ825441 | . | . | . | T | Brazil, United Kingdom, France |
| Prionailurus viverinus KU645930 | C | . | . | . | Thailand |
| Felis catus EU930823 | . | . | C | . | Brazil |
| Felis catus AF548631 | . | C | . | T | South Africa |
| GenBank Accession Numbers | Sequence Variants | Nucleotide Positions | Geographic Origin | ||||
|---|---|---|---|---|---|---|---|
| 78 | 222 | 333 | 337 | 456 | |||
| Felis catus EU128752, KF743738, KR905451, OQ361736, OQ361738, OQ361739, OQ361743, OQ361744, OQ361745, OQ361747, OQ361752, OQ361753, OQ361755, OQ361757, OQ361759, OQ361760 | I | T | T | C | A | C | Hungary, United States of America, Italy, Lithuania |
| Apodemus argenteus U88564; Felis catus OQ361740, OQ361748, OQ361749, OQ361750, OQ361756 | II | . | . | . | G | . | United States of America, Lithuania |
| Felis catus OQ361735, OQ361741, OQ361742, OQ361746, OQ361751, OQ361758 | III | G | . | . | . | T | Lithuania |
| Felis catus OQ361737 | IV | . | . | T | . | . | Lithuania |
| Panthera pardus saxicolor KU852586; Felis catus OQ361754 | V | . | . | . | . | T | Iran, Lithuania |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razgūnaitė, M.; Lipatova, I.; Paulauskas, A.; Snegiriovaitė, J.; Karvelienė, B.; Zamokas, G.; Laukutė, M.; Radzijevskaja, J. Prevalence and Diversity of Haemotropic Mycoplasma Species in Cats and Their Ectoparasites (Fleas and Ticks). Vet. Sci. 2024, 11, 81. https://doi.org/10.3390/vetsci11020081
Razgūnaitė M, Lipatova I, Paulauskas A, Snegiriovaitė J, Karvelienė B, Zamokas G, Laukutė M, Radzijevskaja J. Prevalence and Diversity of Haemotropic Mycoplasma Species in Cats and Their Ectoparasites (Fleas and Ticks). Veterinary Sciences. 2024; 11(2):81. https://doi.org/10.3390/vetsci11020081
Chicago/Turabian StyleRazgūnaitė, Miglė, Indrė Lipatova, Algimantas Paulauskas, Justina Snegiriovaitė, Birutė Karvelienė, Gintaras Zamokas, Monika Laukutė, and Jana Radzijevskaja. 2024. "Prevalence and Diversity of Haemotropic Mycoplasma Species in Cats and Their Ectoparasites (Fleas and Ticks)" Veterinary Sciences 11, no. 2: 81. https://doi.org/10.3390/vetsci11020081
APA StyleRazgūnaitė, M., Lipatova, I., Paulauskas, A., Snegiriovaitė, J., Karvelienė, B., Zamokas, G., Laukutė, M., & Radzijevskaja, J. (2024). Prevalence and Diversity of Haemotropic Mycoplasma Species in Cats and Their Ectoparasites (Fleas and Ticks). Veterinary Sciences, 11(2), 81. https://doi.org/10.3390/vetsci11020081






