Ehrlichia canis Vaccine Development: Challenges and Advances
Simple Summary
Abstract
1. Introduction
2. Canine Monocytic Ehrlichiosis
3. Challenges for Developing Vaccine for E. canis
4. History of Vaccine Production for E. canis
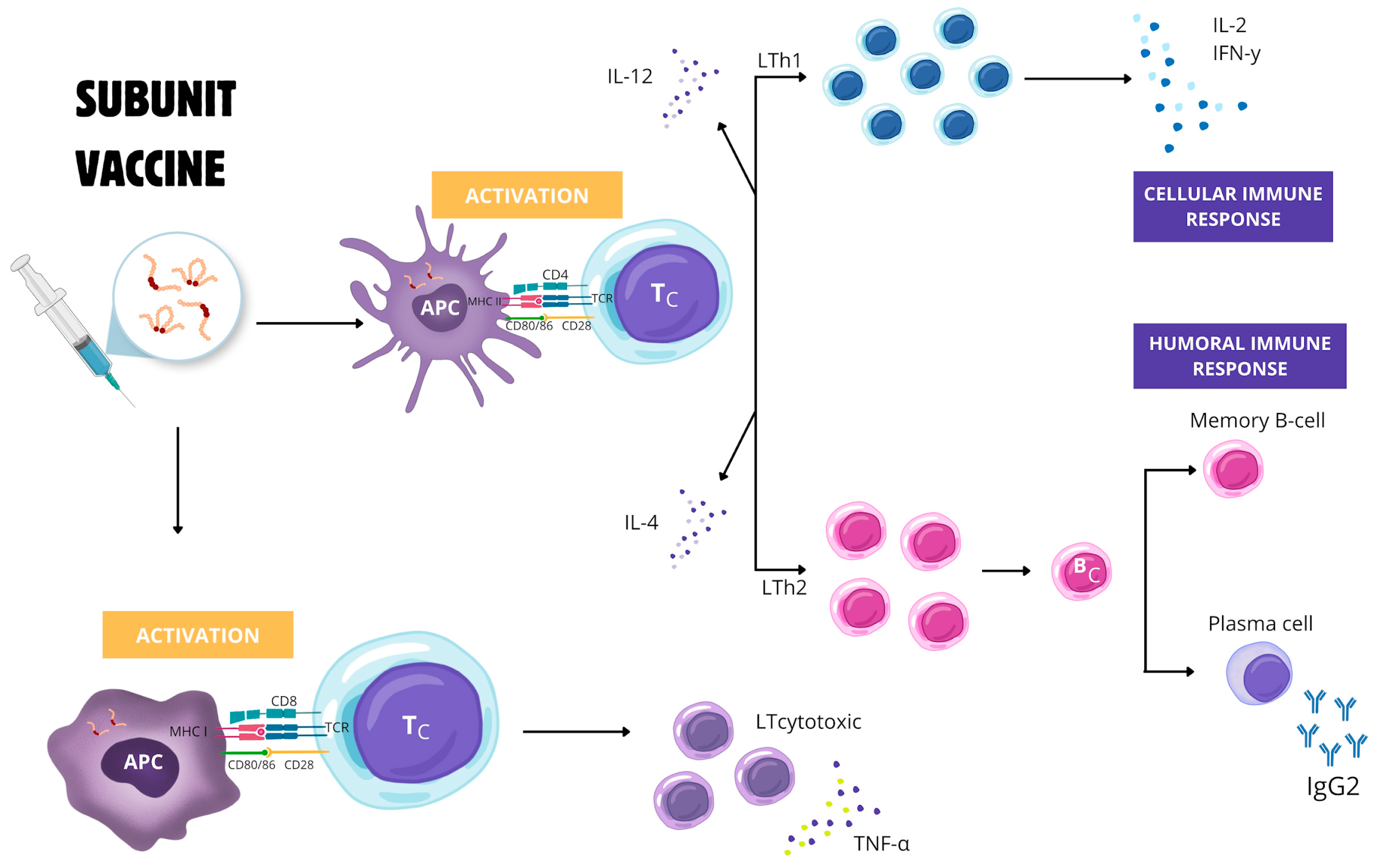
5. Prospects for Vaccine Development Against E. canis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dumler, J.S.; Barbet, A.F.; Bekker, C.P.J.; Dasch, G.A.; Palmer, G.H.; Ray, S.C.; Rikihisa, Y.; Rurangirwa, F.R. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: Unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and HGE agent as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. Evol. Microbiol. 2001, 51, 2145–2165. [Google Scholar] [CrossRef] [PubMed]
- Braga, Í.A.; Santos, L.G.F.D.; Ramos, D.G.D.S.; Melo, A.L.T.; Mestre, G.L.D.C.; Aguiar, D.M. Detection of Ehrlichia canis in domestic cats in the central-western region of Brazil. Braz. J. Microbiol. 2014, 45, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Bouza-Mora, L.; Dolz, G.; Solórzano-Morales, A.; Romero-Zuñiga, J.J.; Salazar-Sánchez, L.; Labruna, M.B.; Aguiar, D.M. Novel genotype of Ehrlichia canis detected in samples of human blood bank donors in Costa Rica. Ticks Tick Borne Dis. 2017, 81, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Braga, Í.A.; Taques, I.I.G.G.; Grontoski, E.C.; Dias, I.S.O.; Pereira, N.A.; Ramos, D.G.S.; Aguiar, D.M. Exposure of domestic cats to distinct Ehrlichia canis TRP genotypes. Vet. Sci. 2021, 8, 310. [Google Scholar] [CrossRef]
- Salje, J. Cells within cells: Rickettsiales and the obligate intracellular bacterial lifestyle. Nat. Rev. Microbiol. 2021, 19, 375–390. [Google Scholar] [CrossRef]
- van Schaik, E.J.; Fratzke, A.P.; Gregory, A.E.; Dumaine, J.E.; Samuel, J.E. Vaccine development: Obligate intracellular bacteria new tools, old pathogens: The current state of vaccines against obligate intracellular bacteria. Front. Cell. Infect. Microbiol. 2024, 14, 1282183. [Google Scholar] [CrossRef]
- McBride, J.W.; Walker, D.H. Molecular and cellular pathobiology of Ehrlichia infection: Targets for new therapeutics and immunomodulation strategies. Expert Rev. Mol. Med. 2011, 13, e3. [Google Scholar] [CrossRef]
- Zhang, X.; Luo, T.; Keysary, A.; Baneth, G.; Miyashiro, S.; Strenger, C.; McBride, J.W. Genetic and antigenic diversities of major immunoreactive proteins in globally distributed Ehrlichia canis strains. Clin. Vaccine Immunol. 2008, 15, 1080–1088. [Google Scholar] [CrossRef]
- Ali, A.; Ahmad, S.; Wadood, A.; Rehman, A.U.; Zahid, H.; Khan, M.Q.; Nawab, J.; Rahman, Z.U.; Alouffi, A.S. Modeling novel putative drugs and vaccine candidates against tick-borne pathogens: A subtractive proteomics approach. Vet. Sci. 2020, 7, 129. [Google Scholar] [CrossRef]
- Khan, M.A.; Amin, A.; Farid, A.; Ullah, A.; Waris, A.; Shinwari, K.; Khan, H. Recent advances in genomics-based approaches for the development of intracellular bacterial pathogen vaccines. Pharmaceutics 2022, 15, 152. [Google Scholar] [CrossRef]
- Sabzi, S.; Shahbazi, S.; Noori Goodarzi, N.; Haririzadeh Jouriani, F.; Habibi, M.; Bolourchi, N.; Badmasti, F. Genome-wide subtraction analysis and reverse vaccinology to detect novel drug targets and potential vaccine candidates against Ehrlichia chaffeensis. Appl. Biochem. Biotechnol. 2023, 195, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Patel, J.G.; Zhang, X.; Walker, D.H.; McBride, J.W. Ehrlichia chaffeensis and E. canis hypothetical protein immunoanalysis reveals small secreted immunodominant proteins and conformation-dependent antibody epitopes. NPJ Vaccines 2020, 5, 85. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Patel, J.G.; Zhang, X.; Walker, D.H.; McBride, J.W. Immunoreactive protein repertoires of Ehrlichia chaffeensis and E. canis reveal the dominance of hypothetical proteins and conformation-dependent antibody epitopes. Infect. Immun. 2021, 89, e00224-21. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Patel, J.G.; Zhang, X.; McBride, J.W. Antibody reactive immunomes of Ehrlichia chaffeensis and E. canis are diverse and defined by conformational antigenic determinants. Front. Cell. Infect. Microbiol. 2024, 13, 1321291. [Google Scholar] [CrossRef] [PubMed]
- Diniz, P.P.V.P.; Aguiar, D.M. Ehrlichiosis and Anaplasmosis: An Update. Vet. Clin. N. Am. Small Anim. Pract. 2022, 52, 1225–1266. [Google Scholar] [CrossRef]
- Chaber, A.L.; Easther, R.; Cumming, B.; Irving, R.; Keyburn, A.L.; Smart, C.; O’Handley, R.; Lignereux, L. Ehrlichia canis rapid spread and possible enzooty in northern South Australia and distribution of its vector Rhipicephalus linnaei. Aust. Vet. J. 2022, 100, 533–538. [Google Scholar] [CrossRef]
- Stich, R.W.; Schaefer, J.J.; Bremer, W.G.; Needham, G.R.; Jittapalapong, S. Host surveys, ixodid tick biology and transmission scenarios as related to the tick-borne pathogen, Ehrlichia canis. Vet. Parasitol. 2008, 158, 256–273. [Google Scholar] [CrossRef]
- Bremer, W.G.; Schaefer, J.J.; Wagner, E.R.; Ewing, S.A.; Rikihisa, Y.; Needham, G.R.; Jittapalapong, S.; Moore, D.L.; Stich, R.W. Transstadial and intrastadial experimental transmission of Ehrlichia canis by male Rhipicephalus sanguineus. Vet. Parasitol. 2005, 131, 95–105. [Google Scholar] [CrossRef]
- Saito, T.B.; Walker, D.H. Ehrlichioses: An Important One Health Opportunity. Vet. Sci. 2016, 3, 20. [Google Scholar] [CrossRef]
- Maggi, R.G.; Krämer, F. A review on the occurrence of companion vector-borne diseases in pet animals in Latin America. Parasit. Vectors 2019, 12, 145. [Google Scholar] [CrossRef]
- Miró, G.; Wright, I.; Michael, H.; Burton, W.; Hegarty, E.; Rodón, J.; Buch, J.; Pantchev, N.; von Samson Himmelstjerna, G. Seropositivity of main vector-borne pathogens in dogs across Europe. Parasit. Vectors 2022, 15, 189. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.; Cerqueira, A.; Castro, T.; Ferreira, E.; Neves, F.; Barbosa, A.; Macieira, D.; Almosny, N. Genetic diversity of Ehrlichia canis strains from naturally infected dogs in Rio de Janeiro, Brazil. Braz. Jn. Vet. Parasitol. 2014, 23, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Judy, L.; David, K.; Peter, K.; Dhaval, S. Canine ehrlichiosis seropositivity and associated factors in Kenya and Tanzania: A retrospective study. BMC Vet. Res. 2023, 19, 175. [Google Scholar] [CrossRef] [PubMed]
- Alcón-Chino, M.E.; De-Simone, S.G. Advancements in Understanding and Diagnosing Canine Ehrlichiosis: A Comprehensive Review. Preprints 2024. [Google Scholar] [CrossRef]
- Szabó, M.P.J.; Mangold, A.J.; João, C.F.; Bechara, G.H.; Guglielmone, A.A. Biological and DNA evidence of two dissimilar populations of the Rhipicephalus sanguineus tick group (Acari: Ixodidae) in South America. Vet. Parasitol. 2005, 130, 131–140. [Google Scholar] [CrossRef]
- Moraes-Filho, J.; Marcili, A.; Nieri-Bastos, F.A.; Richtzenhain, L.J.; Labruna, M.B. Genetic analysis of ticks belonging to the Rhipicephalus sanguineus group in Latin America. Acta Trop. 2011, 117, 51–55. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; Latrofa, M.S.; Annoscia, G.; Giannelli, A.; Parisi, A. Morphological and genetic diversity of Rhipicephalus sanguineus sensu lato from the New and Old Worlds. Parasit. Vectors 2013, 6, 213. [Google Scholar] [CrossRef]
- Latrofa, M.S.; Dantas-Torres, F.; Annoscia, G.; Cantacess, C.; Otranto, D. Comparative analyses of mitochondrial and nuclear genetic markers for the molecular identification of Rhipicephalus spp. Infect. Genet. Evol. 2013, 20, 422–427. [Google Scholar] [CrossRef]
- Hekimoğlu, O. An update on the phylogeny and biogeographical history of Rhipicephalus sanguineus complex. Turk. J. Zool. 2024, 48, 21–35. [Google Scholar] [CrossRef]
- Moraes-Filho, J.; Krawczak, F.S.; Costa, F.B.; Soares, J.F.; Labruna, M.B. Comparative Evaluation of the Vector Competence of Four South American Populations of the Rhipicephalus sanguineus Group for the Bacterium Ehrlichia canis, the Agent of Canine Monocytic Ehrlichiosis. PLoS ONE 2015, 10, e0139386. [Google Scholar] [CrossRef]
- Sanches, G.S.; Villar, M.; Couto, J.; Ferrolho, J.; Fernández de Mera, I.G.; André, M.R.; Barros-Battesti, D.M.; Machado, R.Z.; Bechara, G.H.; Mateos-Hernández, L.; et al. Comparative Proteomic Analysis of Rhipicephalus sanguineus sensu lato (Acari: Ixodidae) Tropical and Temperate Lineages: Uncovering Differences During Ehrlichia canis Infection. Front. Cell. Infect. Microbiol. 2021, 10, 611113. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.B.; Machado, R.Z.; Aquino, L.P.; Alessi, A.C.; Costa, M.T. Experimental acute canine monocytic ehrlichiosis: Clinicopathological and immunopathological findings. Vet. Parasitol. 2004, 119, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Mylonakis, M.E.; Koutinas, A.F.; Breitschwerdt, E.B.; Hegarty, B.C.; Billinis, C.D.; Leontides, L.S.; Kontos, V.S. Chronic canine ehrlichiosis (Ehrlichia canis): A retrospective study of 19 natural cases. J. Am. Anim. Hosp. Assoc. 2004, 40, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, D.M.; Zhang, X.; Melo, A.L.; Pacheco, T.A.; Meneses, A.M.; Zanutto, M.S.; Horta, M.C.; Santarém, V.A.; Camargo, L.M.; McBride, J.W.; et al. Genetic diversity of Ehrlichia canis in Brazil. Vet. Microbiol. 2013, 164, 315–321. [Google Scholar] [CrossRef]
- Aguiar, D.M.; Melo, A.L. Divergence of the TRP36 protein (gp36) in Ehrlichia canis strains found in Brazil. Ticks Tick Borne Dis. 2015, 6, 103–105. [Google Scholar] [CrossRef]
- Hsieh, Y.C.; Lee, C.C.; Tsang, C.L.; Chung, Y.T. Detection and characterization of four novel genotypes of Ehrlichia canis from dogs. Vet. Microbiol. 2010, 146, 70–75. [Google Scholar] [CrossRef]
- Navarrete, M.G.; Hodžić, A.; Corona-González, B.; Cordeiro, M.D.; da Silva, C.B.; Báez, L.C.; Cabezas-Cruz, A. Novo genogrupo de Ehrlichia canis em cães com ehrlichiose canina em Cuba. Parasit. Vectors 2022, 15, 295. [Google Scholar] [CrossRef]
- Zweygarth, E.; Cabezas-Cruz, A.; Josemans, A.I.; Oosthuizen, M.C.; Matjila, P.T.; Lis, K.; Broniszewska, M.; Schöl, H.; Ferrolho, J.; Grubhoffer, L.M.; et al. In vitro culture and structural differences in the major immunoreactive protein gp36 of geographically distant Ehrlichia canis isolates. Ticks Tick Borne Dis. 2014, 5, 423–431. [Google Scholar] [CrossRef]
- Aktas, M.; Özübek, S. Genetic diversity of Ehrlichia canis in dogs from Turkey inferred by TRP36 sequence analysis and phylogeny. Comp. Immunol. Microbiol. Infect. Dis. 2019, 64, 20–24. [Google Scholar] [CrossRef]
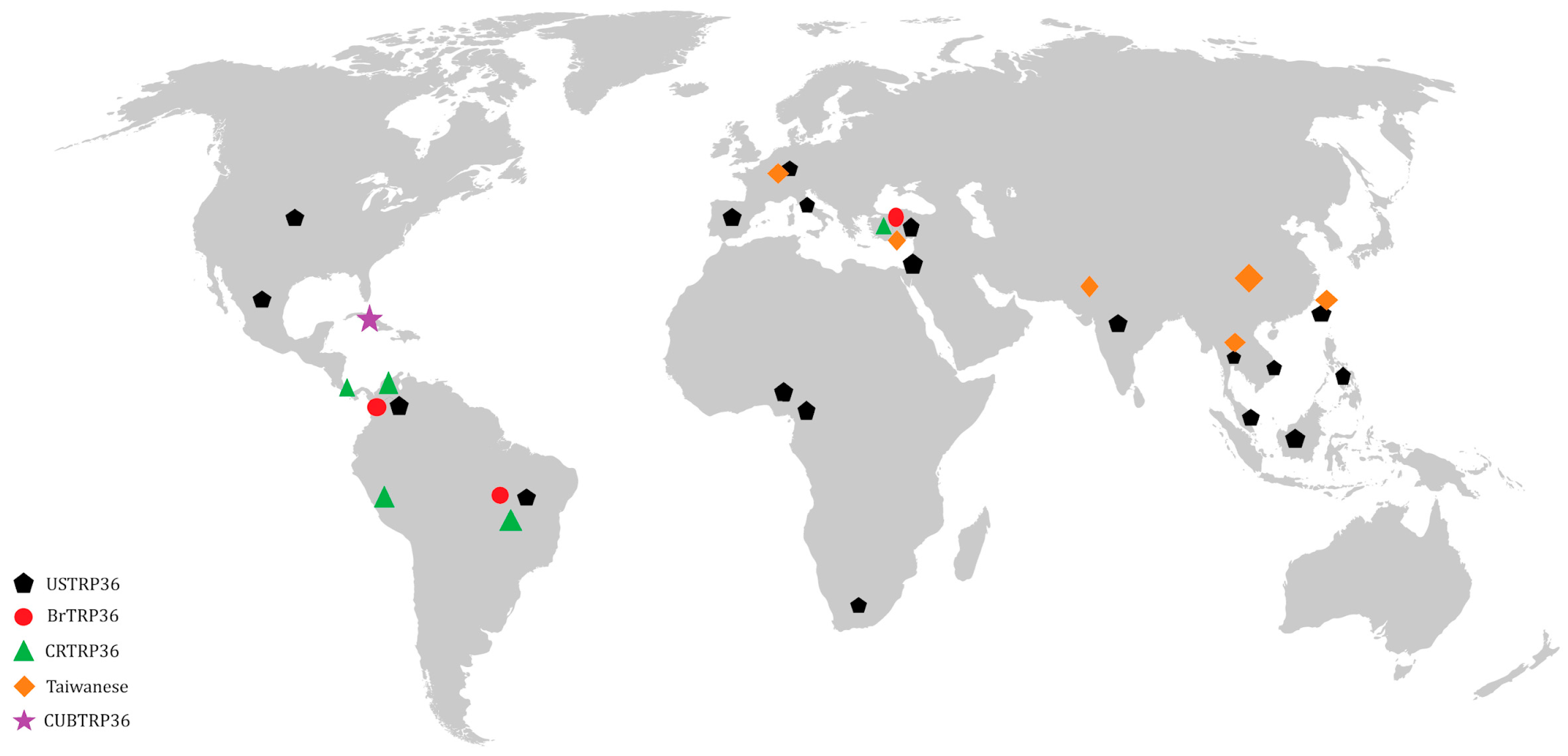
- Taques, I.I.G.G.; Campos, A.N.S.; Kavasaki, M.L.; de Almeida, S.L.H.; de Aguiar, D.M. Geographic Distribution of Ehrlichia canis TRP Genotypes in Brazil. Vet. Sci. 2020, 7, 165. [Google Scholar] [CrossRef]
- Borges, K.I.N.; Pereira, N.d.A.; Aguiar, D.M.; Taques, I.I.G.G.; Alves-Ribeiro, B.S.; Ramos, D.G.S.; Braga, Í.A. Costa Rican Genotype of Ehrlichia canis: A Current Concern. Vet. Sci. 2023, 10, 316. [Google Scholar] [CrossRef] [PubMed]
- Arroyave, E.; Rodas-González, J.D.; Zhang, X.; Labruna, M.B.; González, M.S.; Fernández-Silva, J.A.; McBride, J.W. Ehrlichia canis TRP36 diversity in naturally infected-dogs from an urban area of Colombia. Ticks Tick Borne Dis. 2020, 11, 101367. [Google Scholar] [CrossRef] [PubMed]
- Taques, I.I.G.G.; Koiyama, M.F.G.; Campos, A.N.S.; Costa, J.S.; Hongyu, K.; Aguiar, D.M. Canonical correlative analyses among an enzyme-linked immunosorbent assay using synthetic peptides, an indirect fluorescent antibody test, and hematologic measurements in dogs infected with Ehrlichia canis. Vet. Clin. Pathol. 2020, 49, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Zorzo, C.; Pereira, N.A.; Hongyu, K.; Aguiar, D.M. Correlation between canine biochemical analytes and TRP36 ELISA seropositivity for Ehrlichia canis in Brazil. Vet. Clin. Pathol. 2023, 52, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Lorente, C.; Sainz, A.; Tesouro, M.A. Immunophenotype of dogs with subclinical ehrlichiosis. Ann. N. Y. Acad. Sci. 2008, 1149, 114–117. [Google Scholar] [CrossRef]
- Wikel, S.K. Tick modulation of host immunity: An important factor in pathogen transmission. Int. J. Parasitol. 1999, 29, 851–859. [Google Scholar] [CrossRef]
- Rikihisa, Y. Molecular events involved in cellular invasion by Ehrlichia chaffeensis and Anaplasma phagocytophilum. Vet. Parasitol. 2010, 167, 155–166. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Sinha, M.; Luxon, B.A.; Yu, X.J. Survival strategy of obligately intracellular Ehrlichia chaffeensis: Novel modulation of immune response and host cell cycles. Infect. Immun. 2004, 72, 498–507. [Google Scholar] [CrossRef]
- Tajima, T.; Wada, M. Inhibitory effect of interferon gamma on frequency of Ehrlichia canis-infected cells in vitro. Vet. Immunol. Immunopathol. 2013, 156, 200–204. [Google Scholar] [CrossRef]
- McBride, J.W.; Corstvet, R.E.; Gaunt, S.D.; Boudreaux, C.; Guedry, T.; Walker, D.H. Kinetics of Antibody Response to Ehrlichia canis Immunoreactive Proteins. Infect. Immun. 2003, 71, 2516–2524. [Google Scholar] [CrossRef]
- Allerton, F.; Prior, C.; Bagcigil, A.F.; Broens, E.; Callens, B.; Damborg, P.; Dewulf, J.; Filippitzi, M.E.; Carmo, L.P.; Gómez-Raja, J.; et al. Overview and evaluation of existing guidelines for rational antimicrobial use in small-animal veterinary practice in Europe. Antibiotics 2021, 10, 409. [Google Scholar] [CrossRef] [PubMed]
- Rosário, C.J.R.M.; Aguiar, D.M.; Lima, C.A.A.; Coutinho, D.F.; Pereira, J.G.; Melo, F.A.; Rocha, C.Q. Association of polar fraction of Ageratum conyzoides from the Brazilian Amazon with doxycycline against infection of macrophages with Ehrlichia canis. S. Afr. J. Bot. 2023, 155, 90–97. [Google Scholar] [CrossRef]
- Chanda, S.; Rakholiya, K. Combination therapy: Synergism between natural plant extracts and antibiotics against infectious diseases. Science Against Microbial Pathogens: Communicating Current Research and Technological Advances. 2011, pp. 520–529. Available online: https://www.researchgate.net/publication/268064090_Combination_therapy_Synergism_between_natural_plant_extracts_and_antibiotics_against_infectious_diseases (accessed on 2 December 2024).
- Schaefer, J.J.; Needham, G.R.; Bremer, W.G.; Rikihisa, Y.; Ewing, S.A.; Stich, R.W. Tick acquisition of Ehrlichia canis from dogs treated with doxycycline hyclate. Antimicrob. Agents Chemother. 2007, 51, 3394–3396. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mylonakis, M.E.; Harrus, S.; Breitschwerdt, E.B. An update on the treatment of canine monocytic ehrlichiosis (Ehrlichia canis). Vet. J. 2019, 246, 45–53. [Google Scholar] [CrossRef]
- Sunkara, P.; Chennuru, S.; Krovvidi, S.; Chitichoti, J. Cypermethrin and ivermectin resistance in field populations of Rhipicephalus sanguineus sensu lato (Latrielle, 1806) collected from dogs in south India. Vet. Res. Commun. 2022, 46, 67–78. [Google Scholar] [CrossRef]
- Sánchez Pérez, M.; Feira Arroyo, T.P.; Venegas Barrera, C.S.; Sosa-Gutiérrez, C.; Torres, J.; Marrom, K.A.; Gordillo Pérez, G. Predicting the Impact of Climate Change on the Distribution of Rhipicephalus sanguineus in the Americas. Sustainability 2023, 15, 4557. [Google Scholar] [CrossRef]
- Luzzi, M.C.; Carvalho, L.A.L.; Pinheiro, D.G.; Lima-Duarte, L.; Camargo, J.V.; Kishi, L.T.; Fernandes, C.C.; Machado, R.Z.; Soares, J.F.; André, M.R.; et al. Analysis on the prokaryotic microbiome in females and embryonic cell cultures of Rhipicephalus sanguineus tropical and temperate lineages from two specific localities in Brazil. Rev. Bras. Parasitol. Vet. 2021, 30, e005721. [Google Scholar] [CrossRef]
- Ferrolho, J.; Antunes, S.; Sanches, G.S.; Couto, J.; Évora, P.M.; Rosa, C.; André, M.R.; Machado, R.Z.; Bechara, G.H.; Domingos, A. Ferritin 1 silencing effect in Rhipicephalus sanguineus sensu lato (Acari: Ixodidae) during experimental infection with Ehrlichia canis. Ticks Tick Borne Dis. 2017, 8, 174–184. [Google Scholar] [CrossRef]
- McBride, J.W.; Yu, X.J.; Walker, D.H. Molecular cloning of the gene for a conserved major immunoreactive 28-kilodalton protein of Ehrlichia canis: A potential serodiagnostic antigen. Clin. Diagn. Lab. Immunol. 1999, 6, 392–399. [Google Scholar] [CrossRef]
- Nethery, K.A.; Doyle, C.K.; Zhang, X.; McBride, J.W. Ehrlichia canis gp200 contains dominant species-specific antibody epitopes in terminal acidic domains. Infect. Immun. 2007, 75, 4900–4908. [Google Scholar] [CrossRef]
- Doyle, C.K.; Cardenas, A.M.; Aguiar, D.M.; Labruna, M.B.; Ndip, L.M.; Yu, X.; Mcbride, J.W. Molecular Characterization of E. canis gp36 and E. chaffeensis gp47 Tandem Repeats among Isolates from Different Geographic Locations. Ann. N. Y. Acad. Sci. 2005, 1063, 433–435. [Google Scholar] [CrossRef] [PubMed]
- McBride, J.W.; Doyle, C.K.; Zhang, X.; Cardenas, A.M.; Popov, V.L.; Nethery, K.A.; Woods, M.E. Identification of a Glycosylated Ehrlichia canis 19-Kilodalton Major Immunoreactive Protein with a Species-Specific Serine-Rich Glycopeptide Epitope. Infect. Immun. 2007, 75, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Dunphy, P.S.; McBride, J.W. Ehrlichia chaffeensis Tandem Repeat Effector Targets Differentially Influence Infection. Front. Cell. Infect. Microbiol. 2017, 7, 178. [Google Scholar] [CrossRef] [PubMed]
- Bui, D.C.; Luo, T.; McBride, J.W. Type 1 secretion system and effectors in Rickettsiales. Front. Cell. Infect. Microbiol. 2023, 13, 1175688. [Google Scholar] [CrossRef] [PubMed]
- Byerly, C.D.; Patterson, L.L.; McBride, J.W. Ehrlichia TRP effectors: Moonlighting, mimicry and infection. Pathog. Dis. 2021, 79, ftab026. [Google Scholar] [CrossRef]
- Doyle, C.K.; Nethery, K.A.; Popov, V.L.; McBride, J.W. Differentially expressed and secreted major immunoreactive protein orthologs of Ehrlichia canis and E. chaffeensis elicit early antibody responses to epitopes on glycosylated tandem repeats. Infect. Immun. 2006, 74, 711–720. [Google Scholar] [CrossRef]
- McBride, J.W.; Walker, D.H. Progress and obstacles in vaccine development for the ehrlichioses. Expert Rev. Vaccines 2010, 9, 1071–1082. [Google Scholar] [CrossRef]
- Jones, D.D.; Racine, R.; Wittmer, S.T.; Harston, L.; Papillion, A.M.; Dishaw, L.M.; Winslow, G.M. The omentum is a site of protective IgM production during intracellular bacterial infection. Infect. Immun. 2015, 83, 2139–2147. [Google Scholar] [CrossRef]
- Patel, J.G.; Luo, T.; Zhang, X.; McBride, J.W. Immuno- and expression analysis of Ehrlichia canis immunoreactive proteins. Front. Vet. Sci. 2024, 11, 1481934. [Google Scholar] [CrossRef]
- Immelman, A. Ehrlichia canis infection (Tropical canine pancytopaenia or canine rickettsiosis). J. S. Afr. Vet. 1973, 44, 241–245. [Google Scholar]
- Buhles, W.C., Jr.; Huxsoll, D.L.; Ristic, M. Tropical canine pancytopenia: Clinical, hematologic, and serologic response of dogs to Ehrlichia canis infection, tetracycline therapy, and challenge inoculation. J. Infect. Dis. 1974, 130, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Weisiger, R.M.; Ristic, M.; Huxsoll, D.L. Kinetics of antibody response to Ehrlichia canis assayed by the indirect fluorescent antibody method. Am. J. Vet. Res. 1975, 36, 689–694. [Google Scholar] [PubMed]
- Harrus, S.; Waner, T.; Bark, H.; Jongejan, F.; Cornelissen, A.W. Recent advances in determining the pathogenesis of canine monocytic ehrlichiosis. J. Clin. Microbiol. 1999, 37, 2745–2749. [Google Scholar] [CrossRef]
- Ristic, Y.; Holland, C.J. Canine Ehrlichiosis, in Rickettsial and Chlamydial Diseases of Domestic Animals; Woldenhiwet, Z., Ristic, M., Eds.; Pergamon Press: Oxford, UK, 1993. [Google Scholar]
- Breitschwerdt, E.B.; Hegarty, B.C.; Hancock, S.I. Doxycycline hyclate treatment of experimental canine ehrlichiosis followed by challenge inoculation with two Ehrlichia canis strains. Antimicrob. Agents Chemother. 1998, 42, 362–368. [Google Scholar] [CrossRef]
- Mahan, S.; Kelly, P.J.; Mahan, S.M. A preliminary study to evaluate the immune responses induced by immunization of dogs with inactivated Ehrlichia canis organisms. Onderstepoort J. Vet. Res. 2005, 72, a207. [Google Scholar] [CrossRef][Green Version]
- Or, M.; Samish, M.; Waner, T.; Harrus, S. Attenuation of Ehrlichia canis by multiple passages in two different cultures. Clin. Microbiol. Infect. 2009, 15, 74–75. [Google Scholar] [CrossRef]
- Rudoler, N.; Baneth, G.; Eyal, O.; van Straten, M.; Harrus, S. Evaluation of an attenuated strain of Ehrlichia canis as a vaccine for canine monocytic ehrlichiosis. Vaccine 2012, 31, 226–233. [Google Scholar] [CrossRef]
- Thomas, S.; Thirumalapura, N.R.; Crocquet-Valdes, P.A.; Luxon, B.A.; Walker, D.H. Structure-based vaccines provide protection in a mouse model of ehrlichiosis. PLoS ONE 2011, 6, e27981. [Google Scholar] [CrossRef]
- Bidmos, F.A.; Siris, S.; Gladstone, C.A.; Langford, P.R. Bacterial vaccine antigen discovery in the reverse vaccinology 2.0 Era: Progress and challenges. Front. Immunol. 2018, 9, 2315. [Google Scholar] [CrossRef]
- Thirumalapura, N.R.; Crocquet-Valdes, P.A.; Saito, T.B.; Thomas, S.; McBride, J.W.; Walker, D.H. Recombinant Ehrlichia P29 protein induces a protective immune response in a mouse model of ehrlichiosis. Vaccine 2013, 31, 5960–5967. [Google Scholar] [CrossRef][Green Version]
- Crocquet-Valdes, P.A.; Thirumalapura, N.R.; Ismail, N.; Yu, X.; Saito, T.B.; Stevenson, H.L.; Walker, D.H. Immunization with Ehrlichia P28 outer membrane proteins confers protection in a mouse model of ehrlichiosis. Clin. Vaccine Immunol. 2011, 18, 2018–2025. [Google Scholar] [CrossRef] [PubMed]
- Nambooppha, B.; Rittipornlertrak, A.; Tattiyapong, M.; Tangtrongsup, S.; Tiwananthagorn, S.; Chung, Y.T.; Sthitmatee, N. Two different genogroups of Ehrlichia canis from dogs in Thailand using immunodominant protein genes. Infect. Genet. Evol. 2018, 63, 116–125. [Google Scholar] [CrossRef]
- Nambooppha, B.; Rittipornlertrak, A.; Muenthaisong, A.; Koonyosying, P.; Tangtrongsup, S.; Tiwananthagorn, S.; Sthitmatee, N. Effect of GP19 peptide hyperimmune antiserum on activated macrophage during Ehrlichia canis infection in canine macrophage-like cells. Animals 2021, 11, 2310. [Google Scholar] [CrossRef]
- Nambooppha, B.; Rittipornlertrak, A.; Muenthaisong, A.; Koonyosying, P.; Chomjit, P.; Sangkakam, K.; Sthitmatee, N. Recombinant Ehrlichia canis GP19 protein as a promising vaccine prototype providing a protective immune response in a mouse model. Vet. Sci. 2022, 9, 386. [Google Scholar] [CrossRef]
- Li, W.; Godzik, A. Cd-hit: A fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 2006, 22, 1658–1659. [Google Scholar] [CrossRef]
- Shawan, M.M.A.K.; Sharma, A.R.; Halder, S.K.; Arian, T.A.; Shuvo, M.N.; Sarker, S.R.; Hasan, M.A. Advances in computational and bioinformatics tools and databases for designing and developing a multi-epitope-based peptide vaccine. Int. J. Pept. Res. Ther. 2023, 29, 60. [Google Scholar] [CrossRef]
- Reche, P.A. Computational Vaccine Design, 1st ed.; Humana: New York, NY, USA, 2023; p. 535. [Google Scholar] [CrossRef]
- Adams, L.; Obiri-Yeboah, D.; Afiadenyo, M.; Hamidu, S.; Aning, A.; Ehun, E.; Shiels, K.; Joshi, A.; Mamfe Sakyimah, M.; Asamoah Kusi, K.; et al. An in vitro and in silico investigation of the antitrypanosomal activities of the stem bark extracts of Anopyxis klaineana (Pierre) Engl. Heliyon 2024, 10, e28025. [Google Scholar] [CrossRef]
- Zhang, N.; Bevan, M.J. CD8+ T cells: Foot soldiers of the immune system. Immunity 2011, 35, 161–168. [Google Scholar] [CrossRef]
- Embgenbroich, M.; Burgdorf, S. Current concepts of antigen crosspresentation. Front. Immunol. 2018, 9, 1643. [Google Scholar] [CrossRef]
- Budachetri, K.; Teymournejad, O.; Lin, M.; Yan, Q.; Mestres-Villanueva, M.; Brock, G.N.; Rikihisa, Y. An entry-triggering protein of Ehrlichia is a new vaccine candidate against tick-borne human monocytic ehrlichiosis. MBio 2020, 11, 10–1128. [Google Scholar] [CrossRef]
- Budachetri, K.; Lin, M.; Chien, R.C.; Zhang, W.; Brock, G.N.; Rikihisa, Y. Efficacy and immune correlates of OMP-1B and virB2-4 vaccines for protection of dogs from tick transmission of Ehrlichia chaffeensis. MBio 2022, 13, e02140-22. [Google Scholar] [CrossRef] [PubMed]
- Kowalzik, F.; Schreiner, D.; Jensen, C.; Teschner, D.; Gehring, S.; Zepp, F. mRNA-based vaccines. Vaccines 2021, 9, 390. [Google Scholar] [CrossRef] [PubMed]
- Latif, A.A.; Steyn, H.C.; Josemans, A.I.; Marumo, R.D.; Pretorius, A.; Troskie, P.C.; Mans, B.J. Safety and efficacy of an attenuated heartwater (Ehrlichia ruminantium) vaccine administered by the intramuscular route in cattle, sheep and Angora goats. Vaccine 2020, 38, 7780–7788. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.L.; Oliva Chavez, A.S.; Martinez, D.; Vachiery, N.; Meyer, D.F. Possible biased virulence attenuation in the Senegal strain of Ehrlichia ruminantium by ntrX gene conversion from an inverted segmental duplication. PLoS ONE 2023, 18, e0266234. [Google Scholar] [CrossRef] [PubMed]
- Madesh, S.; McGill, J.; Jaworski, D.C.; Ferm, J.; Liu, H.; Fitzwater, S.; Ganta, R.R. Long-Term Protective Immunity against Ehrlichia chaffeensis Infection Induced by a Genetically Modified Live Vaccine. Vaccines 2024, 12, 903. [Google Scholar] [CrossRef]
- Tshilwane, S.I.; Thema, N.; Steyn, H.C.; Van Kleef, M.; Pretorius, A. A multi-epitope DNA vaccine co-administered with monophosphoryl lipid A adjuvant provides protection against tick transmitted Ehrlichia ruminantium in sheep. Vaccine 2019, 37, 4354–4363. [Google Scholar] [CrossRef]
- Pretorius, A.; Nefefe, T.; Thema, N.; Liebenberg, J.; Steyn, H.; van Kleef, M. Screening for immune biomarkers associated with infection or protection against Ehrlichia ruminantium by RNA-sequencing analysis. Microb. Pathog. 2024, 189, 106588. [Google Scholar] [CrossRef]
- Dias, F.; Couto, J.; Ferrolho, J.; Seron, G.S.; Bell-Sakyi, L.; Antunes, S.; Domingos, A. Folate pathway modulation in Rhipicephalus ticks in response to infection. Transbound. Emerg. Dis. 2020, 67 (Suppl. S2), 94–99. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves-Ribeiro, B.S.; Duarte, R.B.; Assis-Silva, Z.M.d.; Gomes, A.P.C.; Silva, Y.A.; Fernandes-Silva, L.; Rocha, A.C.d.S.; Moraes, I.d.S.; Saturnino, K.C.; Ramos, D.G.d.S.; et al. Ehrlichia canis Vaccine Development: Challenges and Advances. Vet. Sci. 2024, 11, 624. https://doi.org/10.3390/vetsci11120624
Alves-Ribeiro BS, Duarte RB, Assis-Silva ZMd, Gomes APC, Silva YA, Fernandes-Silva L, Rocha ACdS, Moraes IdS, Saturnino KC, Ramos DGdS, et al. Ehrlichia canis Vaccine Development: Challenges and Advances. Veterinary Sciences. 2024; 11(12):624. https://doi.org/10.3390/vetsci11120624
Chicago/Turabian StyleAlves-Ribeiro, Bruna Samara, Raiany Borges Duarte, Zara Mariana de Assis-Silva, Ana Paula Carvalho Gomes, Yasodaja Assis Silva, Lizandra Fernandes-Silva, Alice Caroline da Silva Rocha, Iago de Sá Moraes, Klaus Casaro Saturnino, Dirceu Guilherme de Souza Ramos, and et al. 2024. "Ehrlichia canis Vaccine Development: Challenges and Advances" Veterinary Sciences 11, no. 12: 624. https://doi.org/10.3390/vetsci11120624
APA StyleAlves-Ribeiro, B. S., Duarte, R. B., Assis-Silva, Z. M. d., Gomes, A. P. C., Silva, Y. A., Fernandes-Silva, L., Rocha, A. C. d. S., Moraes, I. d. S., Saturnino, K. C., Ramos, D. G. d. S., Taques, I. I. G. G., & Braga, Í. A. (2024). Ehrlichia canis Vaccine Development: Challenges and Advances. Veterinary Sciences, 11(12), 624. https://doi.org/10.3390/vetsci11120624







