Epidemiologic and Clinicopathological Characterization of Feline Mammary Lesions
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Clinical and Epidemiological Characteristics
3.2. Frequency and Characterization of Mammary Lesions and Lymph Node Metastases
3.3. Analysis of Common Malignant Neoplasms for Macroscopic and Microscopic Features
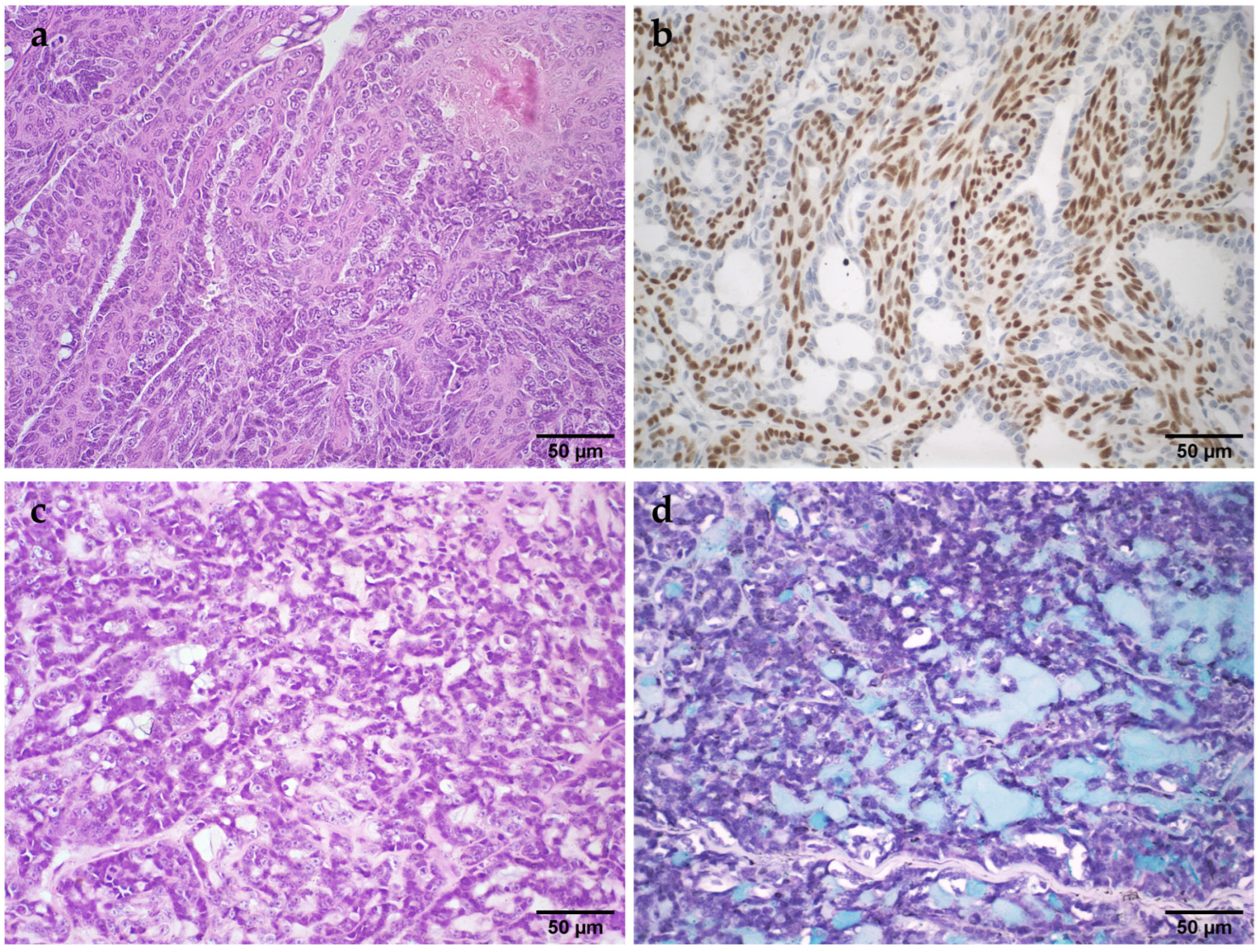
3.4. Identification and Histologic Characterization of Novel Lesions in the Feline Mammary Gland
- Sporotrichosis
- Benign phyllodes tumor
- Basaloid carcinoma
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dorn, C.R.; Taylor, D.O.; Schneider, R.; Hibbard, H.H.; Klauber, M.R. Survey of animal neoplasms in Alameda and Contra Costa Counties, California. II. Cancer morbidity in dogs and cats from Alameda County. J. Natl. Cancer Inst. 1968, 40, 307–318. [Google Scholar] [PubMed]
- Hayes, H.M., Jr.; Milne, K.L.; Mandell, C.P. Epidemiological features of feline mammary carcinoma. Vet. Rec. 1981, 108, 476–479. [Google Scholar] [CrossRef] [PubMed]
- Amorim, F.V.; Souza, H.J.M.; Ferreira, A.M.R.; Fonseca, A.B.M. Clinical, Cytological and Histopathological Evaluation of Mammary Masses in Cats from Rio de Janeiro, Brazil. J. Feline Med. Surg. 2006, 8, 379–388. [Google Scholar] [CrossRef]
- Simeonov, R.; Grozeva, I. Epidemiological Retrospective Studies of Feline Mammary Gland Tumours in Bulgaria. Bulg. J. Vet. Med. 2023. online first. [Google Scholar] [CrossRef]
- Togni, M.; Masuda, E.K.; Kommers, G.D.; Fighera, R.A.; Irigoyen, L.F. Estudo retrospectivo de 207 casos de tumores mamários em gatas. Pesq. Vet. Bras. 2013, 33, 353–358. [Google Scholar] [CrossRef]
- Morris, J. Mammary tumours in the cat: Size matters, so early intervention saves lives. J. Feline Med. Surg. 2013, 15, 391–400. [Google Scholar] [CrossRef]
- Misdorp, W.; Else, R.W.; Hellme’n, E.; Lipscomb, T.P. World Health Organization. International Histological Classification of Tumors of Domestic Animal, 2nd ed.; Armed Forces Institute of Pathology: Washington, DC, USA, 1999. [Google Scholar]
- Vail, D.M.; Thamm, D.H.; Liptak, J.M. Withrow & MacEwen’s Small Animal Clinical Oncology, 6th ed.; Elsevier: Philadelphia, PA, USA, 2020; pp. 604–625. [Google Scholar]
- Jacobs, T.M.; Hoppe, B.R.; Poehlmann, C.E.; Ferracone, J.D.; Sorenmo, K.U. Mammary Adenocarcinomas in Three Male Cats Exposed to Medroxyprogesterone Acetate (1990–2006). J. Feline Med. Surg. 2010, 12, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Cunha, S.; Corgozinho, K.; Justen, H.; Silva, K.; Leite, J.; Ferreira, A.M. Survival and Disease-Free Interval of Cats with Mammary Carcinoma Treated with Chain Mastectomy. Acta Sci. Vet. 2016, 44, 8. [Google Scholar] [CrossRef][Green Version]
- Skorupski, K.A.; Overley, B.; Shofer, F.S.; Goldschmidt, M.H.; Miller, C.A.; Sørenmo, K.U. Clinical Characteristics of Mammary Carcinoma in Male Cats. J. Vet. Intern. Med. 2005, 19, 52–55. [Google Scholar] [CrossRef]
- Gemignani, F.; Mayhew, P.D.; Giuffrida, M.A.; Palaigos, J.; Runge, J.J.; Holt, D.E.; Robertson, N.A.; Seguin, B.; Walker, M.; Singh, A.; et al. Association of Surgical Approach with Complication Rate, Progression-Free Survival Time, and Disease-Specific Survival Time in Cats with Mammary Adenocarcinoma: 107 Cases (1991–2014). J. Am. Vet. Med. Assoc. 2018, 252, 1393–1402. [Google Scholar] [CrossRef]
- Simpson, G.M.; England, G.C.W.; Harvey, M. Manual of Small Animal Reproduction and Neonatology; British Small Animal Veterinary Association: Gloucester, UK, 1998. [Google Scholar]
- Giménez, F.; Hecht, S.; Craig, L.E.; Legendre, A.M. Early Detection, Aggressive Therapy: Optimizing the Management of Feline Mammary Masses. J. Feline Med. Surg. 2010, 12, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Zappulli, V.; De Zan, G.; Cardazzo, B.; Bargelloni, L.; Castagnaro, M. Feline Mammary Tumours in Comparative Oncology. J. Dairy Res. 2005, 72, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Zappulli, V.; Pena, L.; Rasotto, R.; Goldschmidt, M.H.; Gama, A.; Scruggs, J.L.; Kiupel, M. Volume 2: Mammary Tumors. In Surgical Pathology of Tumors of Domestic Animals; Davis-Thompson DVM Foundation: Washington, DC, USA, 2019; pp. 1–268. [Google Scholar]
- Cassali, G.; Jark, P.; Gamba, C.; Damasceno, K.; Estrela-Lima, A.; Nardi, A.; Ferreira, E.; Horta, R.; Firmo, B.; Sueiro, F.; et al. Consensus Regarding the Diagnosis, Prognosis and Treatment of Canine and Feline Mammary Tumors—2019. Braz. J. Vet. Pathol. 2020, 13, 555–574. [Google Scholar] [CrossRef]
- Zappulli, V.; Rasotto, R.; Caliari, D.; Mainenti, M.; Peña, L.; Goldschmidt, M.H.; Kiupel, M. Prognostic Evaluation of Feline Mammary Carcinomas: A Review of the Literature. Vet. Pathol. 2015, 52, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Seixas, F.; Palmeira, C.; Pires, M.A.; Lopes, C. Mammary Invasive Micropapillary Carcinoma in Cats: Clinicopathologic Features and Nuclear DNA Content. Vet. Pathol. 2007, 44, 842–848. [Google Scholar] [CrossRef]
- Seixas, F.; Palmeira, C.; Pires, M.A.; Bento, M.J.; Lopes, C. Grade Is an Independent Prognostic Factor for Feline Mammary Carcinomas: A Clinicopathological and Survival Analysis. Vet. J. 2011, 187, 65–71. [Google Scholar] [CrossRef]
- Pickard Price, P.; Stell, A.; O’Neill, D.; Church, D.; Brodbelt, D. Epidemiology and Risk Factors for Mammary Tumours in Female Cats. J. Small Anim. Pract. 2023, 64, 313–320. [Google Scholar] [CrossRef]
- Elston, C.W.; Ellis, I.O. Systemic Pathology, 3rd ed.; Churchill Livingstone: London, UK, 1998; pp. 365–384. [Google Scholar]
- Meuten, D.J.; Moore, F.M.; George, J.W. Mitotic Count and the Field of View Area: Time to Standardize. Vet. Pathol. 2016, 53, 7–9. [Google Scholar] [CrossRef]
- Hayden, D.W.; Johnston, S.D.; Kiang, D.T.; Johnson, K.H.; Barnes, D.M. Feline Mammary Hypertrophy/Fibroadenoma Complex: Clinical and Hormonal Aspects. Am. J. Vet. Res. 1981, 42, 1699–1703. [Google Scholar]
- Ito, T.; Kadosawa, T.; Mochizuki, M.; Matsunaga, S.; Nishimura, R.; Sasaki, N. Prognosis of Malignant Mammary Tumor in 53 Cats. J. Vet. Med. Sci. 1996, 58, 723–726. [Google Scholar] [CrossRef]
- De Campos, C.B.; Nunes, F.C.; Lavalle, G.E.; Cassali, G.D. Use of Surgery and Carboplatin in Feline Malignant Mammary Gland Neoplasms with Advanced Clinical Staging. In Vivo 2014, 28, 863–866. [Google Scholar] [PubMed]
- Torrigiani, F.; Moccia, V.; Brunetti, B.; Millanta, F.; Valdivia, G.; Peña, L.; Cavicchioli, L.; Zappulli, V. Mammary Fibroadenoma in Cats: A Matter of Classification. Vet. Sci. 2022, 9, 253. [Google Scholar] [CrossRef] [PubMed]
- Vasiu, I.; Dąbrowski, R.; Wochnik, M.; Płusa, A.; Tvarijonaviciute, A. A Systematic Review of Mammary Gland Inflammations in Queens (Felis Catus). Anim. Reprod. Sci. 2023, 256, 107318. [Google Scholar] [CrossRef] [PubMed]
- Burrai, G.P.; Mohammed, S.I.; Miller, M.A.; Marras, V.; Pirino, S.; Addis, M.F.; Uzzau, S.; Antuofermo, E. Spontaneous Feline Mammary Intraepithelial Lesions as a Model for Human Estrogen Receptor- and Progesterone Receptor-Negative Breast Lesions. BMC Cancer 2010, 10, 156. [Google Scholar] [CrossRef]
- Brogi, E.; Hoda, S.A.; Koerner, F.C.; Rosen, P.P. Rosen’s Diagnosis of Breast Pathology by Needle Core Biopsy, 4th ed.; Wolters Kluwer Health: Philadelphia, PA, USA, 2017. [Google Scholar]
- Nakagaki, K.Y.; Nunes, M.M.; Garcia, A.P.V.; Nunes, F.C.; Schmitt, F.; Cassali, G.D. Solid Carcinoma of the Canine Mammary Gland: A Histological Type or Tumour Cell Arrangement? J. Comp. Pathol. 2021, 190, 1–12. [Google Scholar] [CrossRef]
- Ali, M.M.; Hassan, B.B.; Al-Mokaddem, A.K.; Shaheed, I.B. Histological Characterization of Some Feline Mammary Gland Tumors with Whole Slide Images Scan as a Trial of Remote Diagnosis. Adv. Anim. Vet. Sci. 2020, 9, 117–123. [Google Scholar] [CrossRef]
- De Campos, C.B.; Gamba, C.O.; Damasceno, K.A.; Lavalle, G.E.; Cassali, G.D. Malignant adenomyo-epithelioma in a feline mammary gland. Online J. Vet. Res. 2015, 19, 155–161. [Google Scholar]
- Nunes, M.M.; Garcia, A.P.V.; Nakagaki, K.Y.R.; Cassali, G.D. Histopathological and Immunohistochemical Characteristics of Malignant Adenomyoepithelioma in a Cat: Case Report. Arq. Bras. Med. Vet. Zootec. 2021, 73, 1351–1356. [Google Scholar] [CrossRef]
- Ahmadi, N.; Negahban, S.; Aledavood, A.; Daneshbod, K.; Daneshbod, Y. Malignant Adenomyoepithelioma of the Breast: A Review. Breast J. 2015, 21, 291–296. [Google Scholar] [CrossRef]
- Matsuda, K.; Kobayashi, S.; Yamashita, M.; Hirayama, K.; Kadosawa, T.; Taniyama, H. Tubulopapillary Carcinoma with Spindle Cell Metaplasia of the Mammary Gland in a Cat. J. Vet. Med. Sci. 2008, 70, 479–481. [Google Scholar] [CrossRef]

| Malignant Neoplasm (MN) | Benign Neoplasms (BN) | Non-Neoplastic Lesions (NNL) | p Value | |
|---|---|---|---|---|
| Sex | ||||
| Female | 369 (99.5%) | 9 (100%) | 38 (100%) | 0.88 |
| Male | 2 (0.5%) | 0 (0.0%) | 0 (0.0%) | |
| Total | 371 | 9 | 38 | |
| Age | ||||
| Kitten (1 year or less) | 3 (0.8%) | 2 (22.2%) | 8 (21.1%) | <0.001 |
| Young adult (1–6 years) | 34 (9.2%) | 1 (11.1%) | 11 (28.9%) | |
| Mature adult (7–10 years) | 128 (34.6%) | 1 (11.1%) | 6 (15.8%) | |
| Senior (>10 years) | 137 (37.0%) | 2 (22.2%) | 4 (10.5%) | |
| Total | 302 | 6 | 29 | |
| Breed | ||||
| Purebred | 69 (18.6%) | 0 (0.0%) | 6 (15.8%) | 0.38 |
| Crossbreed | 260 (70.1%) | 7 (77.8%) | 25 (65.8%) | |
| Total | 329 | 7 | 31 | |
| Tumor location * | ||||
| T1 | 25 (6.7%) | 0 (0.0%) | 3 (7.9%) | 0.07 |
| T2 | 30 (8.1%) | 0 (0.0%) | 1 (2.6%) | |
| A1 | 34 (9.2%) | 3 (33.3%) | 5 (13.2%) | |
| A2 | 50 (13.5%) | 1 (11.1%) | 1 (2.6%) | |
| Multicenter | 103 (27.8%) | 2 (22.2%) | 7 (18.4%) | |
| Total | 242 | 6 | 17 | |
| Side of the lesion | ||||
| Right | 82 (22.1%) | 4 (44.4%) | 10 (26.3%) | 0.18 |
| Left | 95 (25.6%) | 2 (22.2%) | 3 (7.9%) | |
| Bilateral | 55 (14.8%) | 0 (0.0%) | 4 (10.5%) | |
| Total | 232 | 6 | 17 | |
| Surgical technique | ||||
| Nodulectomy/Lumpectomy | 54 (14.6%) | 3 (33.3%) | 11 (28.9%) | 0.37 |
| Simple mastectomy | 84 (22.6%) | 1 (11.1%) | 8 (21.1%) | |
| Regional mastectomy | 47 (12.7%) | 3 (33.3%) | 3 (7.9%) | |
| Combined | 8 (2.2%) | 0 (0.0%) | 0 (0.0%) | |
| Total | 25 (6.5%) | 0 (0.0%) | 2 (5.3%) | |
| Tumor size | 218 | 7 | 24 | |
| T1 (<2 cm) | 123 (33.2%) | 2 (22.2%) | 14 (36.8%) | 0.08 |
| T2 (2–3 cm) | 101 (27.2%) | 2 (22.2%) | 2 (5.3%) | |
| T3 (>3 cm) | 133 (35.8%) | 5 (55.6%) | 20 (52.6%) | |
| Total | 357 | 9 | 36 |
| Groups | Histological Classification | n | % |
|---|---|---|---|
| Malignant neoplasms n = 591 | Tubulopapillary carcinoma | 147 | 17.1% |
| Cribriform carcinoma | 144 | 16.8% | |
| Malignant adenomyoepithelioma | 104 | 12.1% | |
| Carcinoma in situ | 45 | 5.2% | |
| Tubular carcinoma | 42 | 4.9% | |
| Papillary carcinoma (invasive and noninvasive) | 32 | 3.7% | |
| Solid papillary carcinoma | 11 | 1.3% | |
| Solid carcinoma | 11 | 1.3% | |
| Mucinous carcinoma | 10 | 1.2% | |
| Micropapillary carcinoma | 9 | 1.0% | |
| Basaloid carcinoma | 6 | 0.7% | |
| Papilloma with ductal carcinoma in situ | 6 | 0.7% | |
| Carcinoma with solid pattern | 6 | 0.7% | |
| Carcinoma in a mixed tumor | 6 | 0.7% | |
| Apocrine carcinoma | 4 | 0.5% | |
| Neuroendocrine carcinoma | 2 | 0.2% | |
| Secretory carcinoma | 2 | 0.2% | |
| Carcinosarcoma | 2 | 0.2% | |
| Carcinoma with sebaceous differentiation | 1 | 0.1% | |
| Lipid-rich carcinoma | 1 | 0.1% | |
| Non-neoplastic lesions n = 212 | Mastitis | 69 | 8.0% |
| Usual ductal hyperplasia (UDH) | 62 | 7.2% | |
| Fibroadenomatous hyperplasia (fibroepithelial hyperplasia) | 39 | 4.5% | |
| Columnar cell alteration | 16 | 1.9% | |
| Duct ectasia | 11 | 1.3% | |
| Adenosis | 4 | 0.5% | |
| Mastitis obliterans | 4 | 0.5% | |
| Atypical ductal hyperplasia (ADH) | 3 | 0.3% | |
| Lobular hyperplasia | 3 | 0.3% | |
| Sporotrichosis (Sporothrix sp.) | 1 | 0.1% | |
| Benign neoplasms n = 55 | Adenoma (tubular/ductal/basaloid) | 28 | 3.3% |
| Ductal papiloma | 16 | 1.9% | |
| Benign adenomyoepithelioma | 4 | 0.5% | |
| Benign phyllodes tumor | 3 | 0.3% | |
| Sclerosing papilloma | 2 | 0.2% | |
| Fibroadenoma | 1 | 0.1% | |
| Benign mixed tumor | 1 | 0.1% | |
| Total | 858 | 100% |
| Tubulopapillary Carcinoma | Cribriform Carcinoma | Malignant Adenomyoepithelioma | p Value | |
|---|---|---|---|---|
| Ulceration (Gross morphology) | ||||
| Present | 30 (21.0%) | 28 (20.3%) | 13 (12.7%) | 0.211 |
| Absent | 113 (79.0%) | 110 (79.7%) | 89 (87.3%) | |
| Cystic spaces (Gross morphology) | ||||
| Present | 36 (25.2%) | 36 (26.1%) | 27 (26.5%) | 0.971 |
| Absent | 107 (74.8%) | 102 (73.9%) | 75 (73.5%) | |
| Necrosis | ||||
| Present | 85 (59.4%) | 81 (58.7%) | 42 (40.4%) | 0.005 |
| Absent | 58 (40.6%) | 57 (41.3%) | 62 (59.6%) | |
| Ulceration | ||||
| Present | 26 (18.2%) | 24 (17.4%) | 9 (8.7%) | 0.085 |
| Absent | 117 (81.8%) | 114 (82.6%) | 95 (91.3%) | |
| Lymphovascular invasion | ||||
| Present | 32 (22.4%) | 42 (30.4%) | 6 (5.8%) | <0.001 |
| Absent | 111 (77.6%) | 96 (69.6%) | 98 (94.2%) | |
| Resection Margins | ||||
| Clean | 54 (37.8%) | 56 (40.6%) | 76 (73.1%) | <0.001 |
| Infiltrated | 40 (28.0%) | 29 (21.0%) | 12 (11.5%) | |
| Close | 49 (34.3%) | 53 (38.4%) | 16 (15.4%) | |
| Anisocytosis | ||||
| Mild | 2 (1.4%) | 0 (0.0%) | 6 (5.8%) | <0.001 |
| Moderate | 70 (49.0%) | 60 (43.5%) | 65 (63.1%) | |
| Marked | 71 (49.7%) | 78 (56.5%) | 32 (31.1%) | |
| Anisokaryosis | ||||
| Mild | 2 (1.4%) | 0 (0.0%) | 6 (5.8%) | <0.001 |
| Moderate | 70 (49.0%) | 60 (43.5%) | 64 (62.1%) | |
| Marked | 71 (49.7%) | 78 (56.5%) | 33 (32.0%) | |
| Mitotic counts | ||||
| Score 1 (0–7) | 33 (23.1%) | 12 (8.7%) | 57 (54.8%) | |
| Score 2 (8–16) | 51 (35.7%) | 36 (26.1%) | 29 (27.9%) | <0.001 |
| Score 3 (>16) | 59 (41.3%) | 90 (65.2%) | 18 (17.3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, F.R.; Moreira, I.S.; Dariva, A.A.; Nakagaki, K.Y.R.; Abreu, C.C.; Balabram, D.; Cassali, G.D. Epidemiologic and Clinicopathological Characterization of Feline Mammary Lesions. Vet. Sci. 2024, 11, 549. https://doi.org/10.3390/vetsci11110549
Souza FR, Moreira IS, Dariva AA, Nakagaki KYR, Abreu CC, Balabram D, Cassali GD. Epidemiologic and Clinicopathological Characterization of Feline Mammary Lesions. Veterinary Sciences. 2024; 11(11):549. https://doi.org/10.3390/vetsci11110549
Chicago/Turabian StyleSouza, Fernanda R., Isabella S. Moreira, Artur A. Dariva, Karen Y. R. Nakagaki, Camila C. Abreu, Débora Balabram, and Geovanni D. Cassali. 2024. "Epidemiologic and Clinicopathological Characterization of Feline Mammary Lesions" Veterinary Sciences 11, no. 11: 549. https://doi.org/10.3390/vetsci11110549
APA StyleSouza, F. R., Moreira, I. S., Dariva, A. A., Nakagaki, K. Y. R., Abreu, C. C., Balabram, D., & Cassali, G. D. (2024). Epidemiologic and Clinicopathological Characterization of Feline Mammary Lesions. Veterinary Sciences, 11(11), 549. https://doi.org/10.3390/vetsci11110549






