Electroencephalographic and Cardiovascular Assessments of Isoflurane-Anesthetized Dogs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
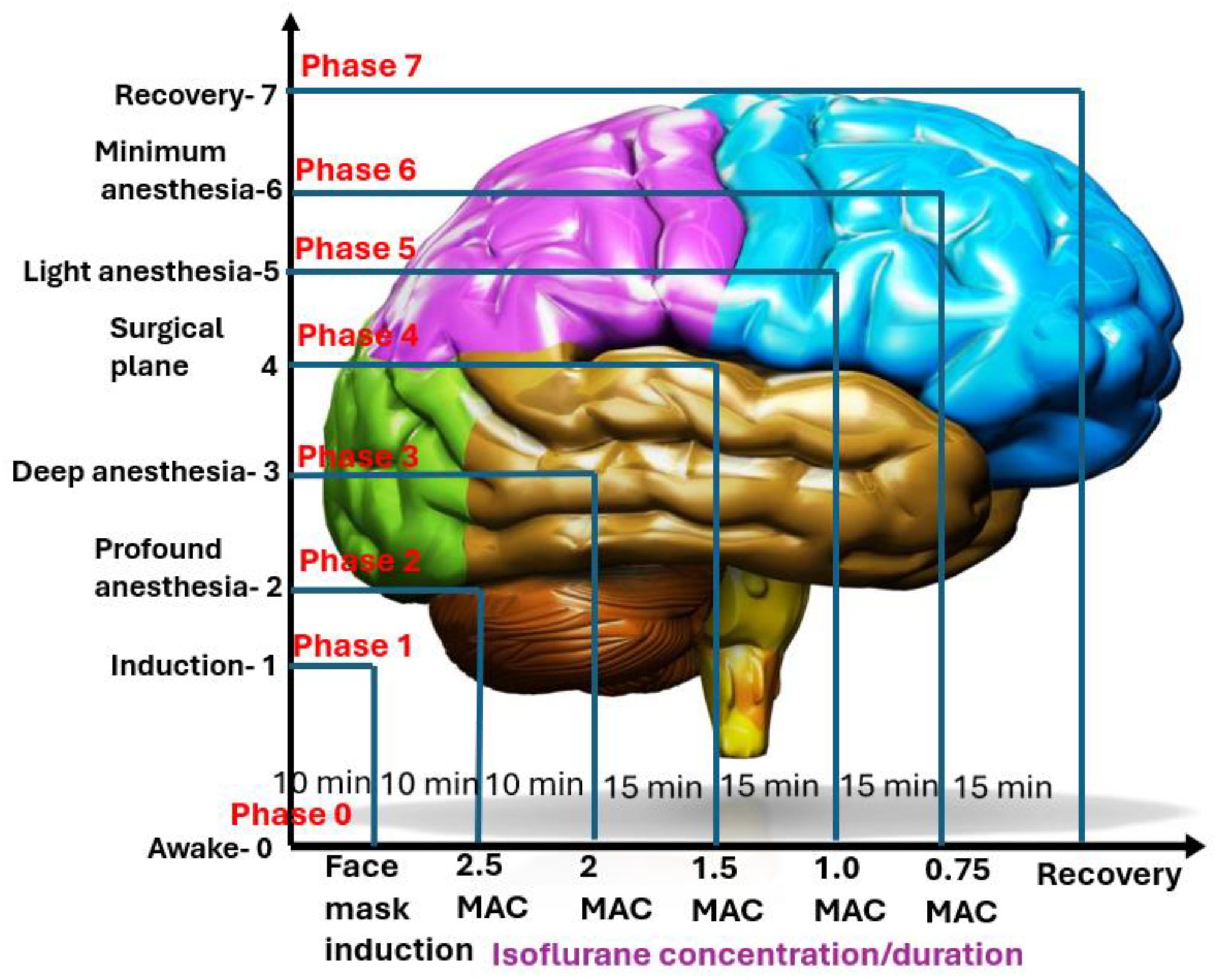
2.2. Experimental Design and Treatment Timeline
- o
- Phase 0: Awake BaselineThe treatment began by obtaining a short baseline measurement of the dog’s EEG and cardiorespiratory vital signs while the dog was fully awake. The procedures took approximately 10 min.
- o
- Phase 1: InductionIsoflurane was administered via a face mask to induce anesthesia in the instrumented dog. During this phase, the dog transitioned from a conscious state to an unconscious state while EEG was monitored whenever possible. Once endotracheal intubation was completed, the dog was placed on a mechanical ventilator, marking the end of this phase. The procedure took 10 min.
- o
- Phase 2: Profound anesthesiaA deep level of anesthesia was initiated by deepening the anesthetic dose until the end-tidal isoflurane concentration reached 2.5× MAC (3.3% end-tidal). To mitigate the profound anesthetic effect, this phase was limited to 10 min of maintenance instead of the usual 15 min for the other phases.
- o
- Phase 3: Deep anesthesiaThe level of anesthesia was reduced to a deep plane of 2× MAC (2.6% end-tidal) and maintained at this concentration for 15 min.
- o
- Phase 4: Surgical planeThe level of anesthesia was further reduced to a surgical plane, maintained using a concentration of 1.5 MAC (1.9% end-tidal) isoflurane. This phase also lasted for 15 min.
- o
- Phase 5: Light anesthesiaThe level of anesthesia was further reduced to a light anesthesia of 1× MAC (1.3% end-tidal), maintained during this phase for 15 min.
- o
- Phase 6: Minimal anesthesiaThe level of anesthesia was reduced to a minimal level of 0.75× MAC (0.9% end-tidal) isoflurane and maintained for 15 min or until the dog showed signs of readiness for extubation, such as coughing or gagging against the endotracheal tube.
- o
- Phase 7: RecoveryDuring this final phase of anesthesia, isoflurane administration was terminated. If the dog was ready for extubation, it was extubated and allowed to recover. All extubated dogs were monitored until they regained awareness. Recovery behavior was noted for any untoward signs of rough behaviors, such as thrashing, paddling, and vocalization, with or without salivation.
2.3. Face Mask Induction, EEG, Cardiorespiratory, Analgesic, and Behavioral Monitoring
2.3.1. Isoflurane Face Mask Induction
2.3.2. Electroencephalography Instrumentation
2.3.3. Cardiorespiratory Monitoring
2.3.4. Nociceptive Assessment
2.3.5. Behavioral Assessment of the Anesthetic Level
2.4. EEG Data Acquisition, Analysis, and Correlation with Cardiovascular Parameters
3. Results
3.1. EEG Indices Changes with Different Isoflurane Anesthetic Concentrations (Phase 0–7)
3.2. Cardiovascular Parameters, Tolerance to Electrical Stimulation, and Subjective Anesthetic Depth Scores of the Dogs during the Various Depths of Isoflurane Anesthesia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Anesthetic Plane | Depth Score | Pupil Size | Globe Position | Eye Reflex (P: Palpebral; C: Corneal) | Heart Rate, Blood Pressure, Breathing | Third Eyelid Position | Jaw Tone, Body/Limb Movement, or Swallowing |
|---|---|---|---|---|---|---|---|
| Very light | 5 | Large or small, moving | Central | P: active C: active | Increased, spontaneous breathing attempts | Retracted | Strong |
| Medium–light | 4 | Large or small | Central or ventromedial | P: mildly depressed C: mildly depressed | Increased, spontaneous breathing attempts | Retracted or partially prolapsed | Medium |
| Medium | 3 | Small or medium | Ventromedial | P: markedly depressed C: mildly depressed | Normal | Completely prolapsed | None or barely |
| Medium–deep | 2 | Medium | Central | P: absent C: markedly depressed | Normal or depressed | Completely or partially prolapsed | None |
| Very deep | 1 | Large | Central | P: absent C: absent | Depressed | Partially depressed | None |
References
- Grubb, T.; Sager, J.; Gaynor, J.S.; Montgomery, E.; Parker, J.A.; Shafford, H.; Tearney, C. 2020 AAHA Anesthesia and Monitoring Guidelines for Dogs and Cats. J. Am. Anim. Hosp. Assoc. 2020, 56, 59–82. [Google Scholar] [CrossRef] [PubMed]
- AAFP Feline Anesthesia Guidelines. Available online: https://journals.sagepub.com/doi/epub/10.1177/1098612X18781391 (accessed on 13 August 2024).
- Pandya, A.N.; Majid, S.Z.; Desai, M.S. The Origins, Evolution, and Spread of Anesthesia Monitoring Standards: From Boston to Across the World. Anesth. Analg. 2021, 132, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Merry, A.F.; Cooper, J.B.; Soyannwo, O.; Wilson, I.H.; Eichhorn, J.H. International Standards for a Safe Practice of Anesthesia 2010. Can. J. Anaesth. 2010, 57, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Purdon, P.L.; Sampson, A.; Pavone, K.J.; Brown, E.N. Clinical Electroencephalography for Anesthesiologists. Anesthesiology 2015, 123, 937–960. [Google Scholar] [CrossRef]
- Brown, E.N.; Purdon, P.L.; Van Dort, C.J. General Anesthesia and Altered States of Arousal: A Systems Neuroscience Analysis. Annu. Rev. Neurosci. 2011, 34, 601–628. [Google Scholar] [CrossRef]
- Lobo, F.A.; Saraiva, A.P.; Nardiello, I.; Brandão, J.; Osborn, I.P. Electroencephalogram Monitoring in Anesthesia Practice. Curr. Anesth. Rep. 2021, 11, 169–180. [Google Scholar] [CrossRef]
- Sun, Y.; Wei, C.; Cui, V.; Xiu, M.; Wu, A. Electroencephalography: Clinical Applications During the Perioperative Period. Front. Med. 2020, 7, 251. [Google Scholar] [CrossRef]
- Bruhn, J.; Myles, P.S.; Sneyd, R.; Struys, M.M.R.F. Depth of anaesthesia monitoring: What’s available, what’s validated and what’s next? Br. J. Anaesth. 2006, 97, 85–94. [Google Scholar] [CrossRef]
- Brown, E.N.; Pavone, K.J.; Naranjo, M. Multimodal General Anesthesia. Anesth. Analg. 2018, 127, 1246–1258. [Google Scholar] [CrossRef]
- Mirra, A.; Casoni, D.; Barge, P.; Hight, D.; Levionnois, O.; Spadavecchia, C. Usability of the SedLine® electroencephalographic monitor of depth of anaesthesia in pigs: A pilot study. J. Clin. Monit. Comput. 2022, 36, 1635–1646. [Google Scholar] [CrossRef]
- Drewnowska, O.; Lisowska, B.; Turek, B. What Do We Know about the Use of EEG Monitoring during Equine Anesthesia: A Review. Appl. Sci. 2019, 9, 3678. [Google Scholar] [CrossRef]
- Drewnowska, O.; Turek, B.; Lisowska, B.; Short, C.E. Preliminary Study of the Use of Root with Sedline® EEG Monitoring for Assessment of Anesthesia Depth in 6 Horses. Appl. Sci. 2020, 10, 1050. [Google Scholar] [CrossRef]
- Murillo, C.; Weng, H.-Y.; Weil, A.B.; Kreuzer, M.; Ko, J.C. Perioperative Brain Function Monitoring with Electroencephalography in Horses Anesthetized with Multimodal Balanced Anesthetic Protocol Subjected to Surgeries. Animals 2022, 12, 2851. [Google Scholar] [CrossRef] [PubMed]
- Murillo, C.; Weil, A.B.; Moore, G.E.; Kreuzer, M.; Ko, J.C. Electroencephalographic and Cardiovascular Changes Associated with Propofol Constant Rate of Infusion Anesthesia in Young Healthy Dogs. Animals 2023, 13, 664. [Google Scholar] [CrossRef]
- Ko, J.C.; Murillo, C.; Weil, A.B.; Kreuzer, M.; Moore, G.E. Ketamine–Propofol Coadministration for Induction and Infusion Maintenance in Anesthetized Dogs: Effects on Electroencephalography and Antinociception. Animals 2023, 13, 3391. [Google Scholar] [CrossRef]
- Sakai, D.M.; Trenholme, H.N.; Torpy, F.J.; Craig, H.A.; Reed, R.A. Evaluation of the electroencephalogram in awake, sedated, and anesthetized dogs. Res. Vet. Sci. 2023, 159, 66–71. [Google Scholar] [CrossRef]
- March, P.A.; Muir, W.W. Bispectral analysis of the electroencephalogram: A review of its development and use in anesthesia. Vet. Anaesth. Analg. 2005, 32, 241–255. [Google Scholar] [CrossRef]
- Kulka, A.M.; Otto, K.A.; Bergfeld, C.; Beyerbach, M.; Kästner, S.B.R. Effects of isoflurane anesthesia with and without dexmedetomidine or remifentanil on quantitative electroencephalographic variables before and after nociceptive stimulation in dogs. Am. J. Vet. Res. 2012, 73, 602–609. [Google Scholar] [CrossRef]
- Barletta, M.; Quandt, J.; Hofmeister, E. Determination of minimum alveolar concentration of isoflurane in dogs and cats using the up-and-down method. A preliminary study. Res. Vet. Sci. 2016, 106, 81–83. [Google Scholar] [CrossRef]
- Ko, J.C.; Lange, D.N.; Mandsager, R.E.; Payton, M.E.; Bowen, C.; Kamata, A.; Kuo, W.C. Effects of butorphanol and carprofen on the minimal alveolar concentration of isoflurane in dogs. J. Am. Vet. Med. Assoc. 2000, 217, 1025–1028. [Google Scholar] [CrossRef]
- Von Dincklage, F.; Jurth, C.; Schneider, G.; García, P.S.; Kreuzer, M. Technical considerations when using the EEG export of the SEDLine Root device. J. Clin. Monit. Comput. 2021, 35, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Hagihira, S. Brain Mechanisms during Course of Anesthesia: What We Know from EEG Changes during Induction and Recovery. Front. Syst. Neurosci. 2017, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Hagihira, S. Changes in the electroencephalogram during anaesthesia and their physiological basis. Br. J. Anaesth. 2015, 115, i27–i31. [Google Scholar] [CrossRef] [PubMed]
- Hemmings, H.C.; Akabas, M.H.; Goldstein, P.A.; Trudell, J.R.; Orser, B.A.; Harrison, N.L. Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol. Sci. 2005, 26, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Sanders, R.D.; Tononi, G.; Laureys, S.; Sleigh, J.W.; Warner, D.S. Unresponsiveness ≠ Unconsciousness. Anesthesiology 2012, 116, 946–959. [Google Scholar] [CrossRef]
- Luo, T.; Leung, L.S. Involvement of Tuberomamillary Histaminergic Neurons in Isoflurane Anesthesia. Anesthesiology 2011, 115, 36–43. [Google Scholar] [CrossRef]
- Luo, T.; Leung, L.S. Basal Forebrain Histaminergic Transmission Modulates Electroencephalographic Activity and Emergence from Isoflurane Anesthesia. Anesthesiology 2009, 111, 725–733. [Google Scholar] [CrossRef]
- Šimić, G.; Tkalčić, M.; Vukić, V.; Mulc, D.; Španić, E.; Šagud, M.; Olucha-Bordonau, F.E.; Vukšić, M.; Hof, P.R. Understanding Emotions: Origins and Roles of the Amygdala. Biomolecules 2021, 11, 823. [Google Scholar] [CrossRef]
- Borsook, D.; Upadhyay, J.; Chudler, E.H.; Becerra, L. A Key Role of the Basal Ganglia in Pain and Analgesia-Insights Gained through Human Functional Imaging. Mol. Pain 2010, 6, 27. [Google Scholar] [CrossRef]
- Schönfeld, L.-M.; Wojtecki, L. Beyond Emotions: Oscillations of the Amygdala and Their Implications for Electrical Neuromodulation. Front. Neurosci. 2019, 13, 366. [Google Scholar] [CrossRef]
- Wakai, A.; Kohno, T.; Yamakura, T.; Okamoto, M.; Ataka, T.; Baba, H. Action of Isoflurane on the Substantia Gelatinosa Neurons of the Adult Rat Spinal Cord. Anesthesiology 2005, 102, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Constantinides, C.; Murphy, K. Molecular and Integrative Physiological Effects of Isoflurane Anesthesia: The Paradigm of Cardiovascular Studies in Rodents using Magnetic Resonance Imaging. Front. Cardiovasc. Med. 2016, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- Campagnol, D.; Neto, F.J.T.; Monteiro, E.R.; Beier, S.L.; Aguiar, A.J.A. Use of bispectral index to monitor depth of anesthesia in isoflurane-anesthetized dogs. Am. J. Vet. Res. 2007, 68, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Greene, S.A.; Benson, G.J.; Tranquilli, W.J.; Grimm, K.A. Relationship of canine bispectral index to multiples of sevoflurane minimal alveolar concentration, using patch or subdermal electrodes. Comp. Med. 2002, 52, 424–428. [Google Scholar] [PubMed]
- March, P.A.; Muir, W.W. Use of the bispectral index as a monitor of anesthetic depth in cats anesthetized with isoflurane. Am. J. Vet. Res. 2003, 64, 1534–1541. [Google Scholar] [CrossRef]
- Joyce, L.; Wenninger, A.; Kreuzer, M.; García, P.S.; Schneider, G.; Fenzl, T. Electroencephalographic monitoring of anesthesia during surgical procedures in mice using a modified clinical monitoring system. J. Clin. Monit. Comput. 2024, 38, 373–384. [Google Scholar] [CrossRef]
- Kim, D.; Ahn, J.H.; Heo, G.; Jeong, J.S. Comparison of Bispectral Index and Patient State Index values according to recovery from moderate neuromuscular block under steady-state total intravenous anesthesia. Sci. Rep. 2021, 11, 5908. [Google Scholar] [CrossRef]
- Klide, A.M. Cardiopulmonary effects of enflurane and isoflurane in the dog. Am. J. Vet. Res. 1976, 37, 127–131. [Google Scholar]
- Floriano, B.P.; Wagatsuma, J.T.; Ferreira, J.Z.; Abimussi, C.J.X.; Menegheti, T.M.; Santos, P.S.P.; Oliva, V.N.L.S. Effects on indicators of tissue perfusion in dogs anesthetized with isoflurane at two multiples of the minimum alveolar concentration. Am. J. Vet. Res. 2016, 77, 24–31. [Google Scholar] [CrossRef]
- Mutoh, T.; Nishimura, R.; Kim, H.Y.; Matsunaga, S.; Sasaki, N. Cardiopulmonary effects of sevoflurane, compared with halothane, enflurane, and isoflurane, in dogs. Am. J. Vet. Res. 1997, 58, 885–890. [Google Scholar] [CrossRef]
- Drover, D.; Ortega, H.R. Patient state index. Best Pr. Res. Clin. Anaesthesiol. 2006, 20, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.; Devereaux, P.J.; Garg, A.X.; Kurz, A.; Turan, A.; Rodseth, R.N.; Cywinski, J.; Thabane, L.; Sessler, D.I. Relationship between Intraoperative Mean Arterial Pressure and Clinical Outcomes after Noncardiac Surgery. Anesthesiology 2013, 119, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.-Y.; Chen, D.; Xu, Z.-X.; Wang, H.-L.; Sun, H. Comparison of bispectral index and patient state index as measures of sedation depth during surgeries using remimazolam tosilate. BMC Anesthesiol. 2023, 23, 208. [Google Scholar] [CrossRef] [PubMed]
- Pedemonte, J.C.; Plummer, G.S.; Chamadia, S.; Locascio, J.J.; Hahm, E.; Ethridge, B.; Gitlin, J.; Ibala, R.; Mekonnen, J.; Colon, K.M.; et al. Electroencephalogram Burst-suppression during Cardiopulmonary Bypass in Elderly Patients Mediates Postoperative Delirium. Anesthesiology 2020, 133, 280–292. [Google Scholar] [CrossRef]
- Reese, M.; Christensen, S.; Anolick, H.; Roberts, K.C.; Wong, M.K.; Wright, M.C.; Acker, L.; Browndyke, J.N.; Woldorff, M.G.; Berger, M.; et al. EEG pre-burst suppression: Characterization and inverse association with preoperative cognitive function in older adults. Front. Aging Neurosci. 2023, 15, 1229081. [Google Scholar] [CrossRef]
- Leung, J.M.; Cole, D.J. Reducing Postoperative Delirium with Intraoperative Processed EEG. ASA Monit. 2021, 85, 1–12. [Google Scholar] [CrossRef]
- Acker, L.; Ha, C.; Zhou, J.; Manor, B.; Giattino, C.M.; Roberts, K.; Berger, M.; Wright, M.C.; Colon-Emeric, C.; Devinney, M.; et al. Electroencephalogram-Based Complexity Measures as Predictors of Post-operative Neurocognitive Dysfunction. Front. Syst. Neurosci. 2021, 15, 718769. [Google Scholar] [CrossRef]
- Vicenti, C.; Romagnoli, N.; Stabile, M.; Lambertini, C.; Piemontese, C.; Spaccini, F.; Foglia, A.; Lacitignola, L.; Crovace, A.; Staffieri, F. The Pleth Variability Index as a Guide to Fluid Therapy in Dogs Undergoing General Anesthesia: A Preliminary Study. Vet. Sci. 2024, 11, 396. [Google Scholar] [CrossRef]

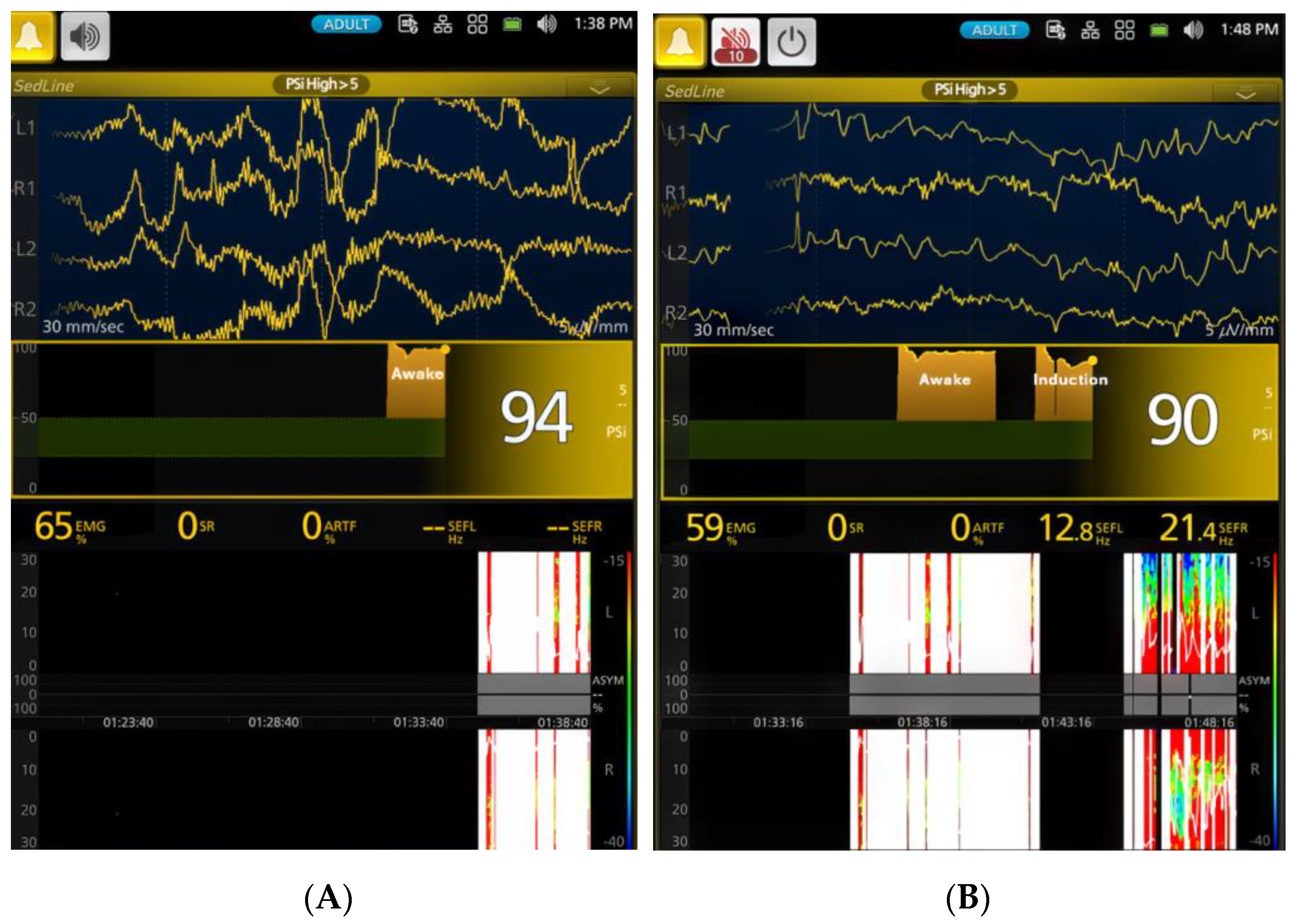
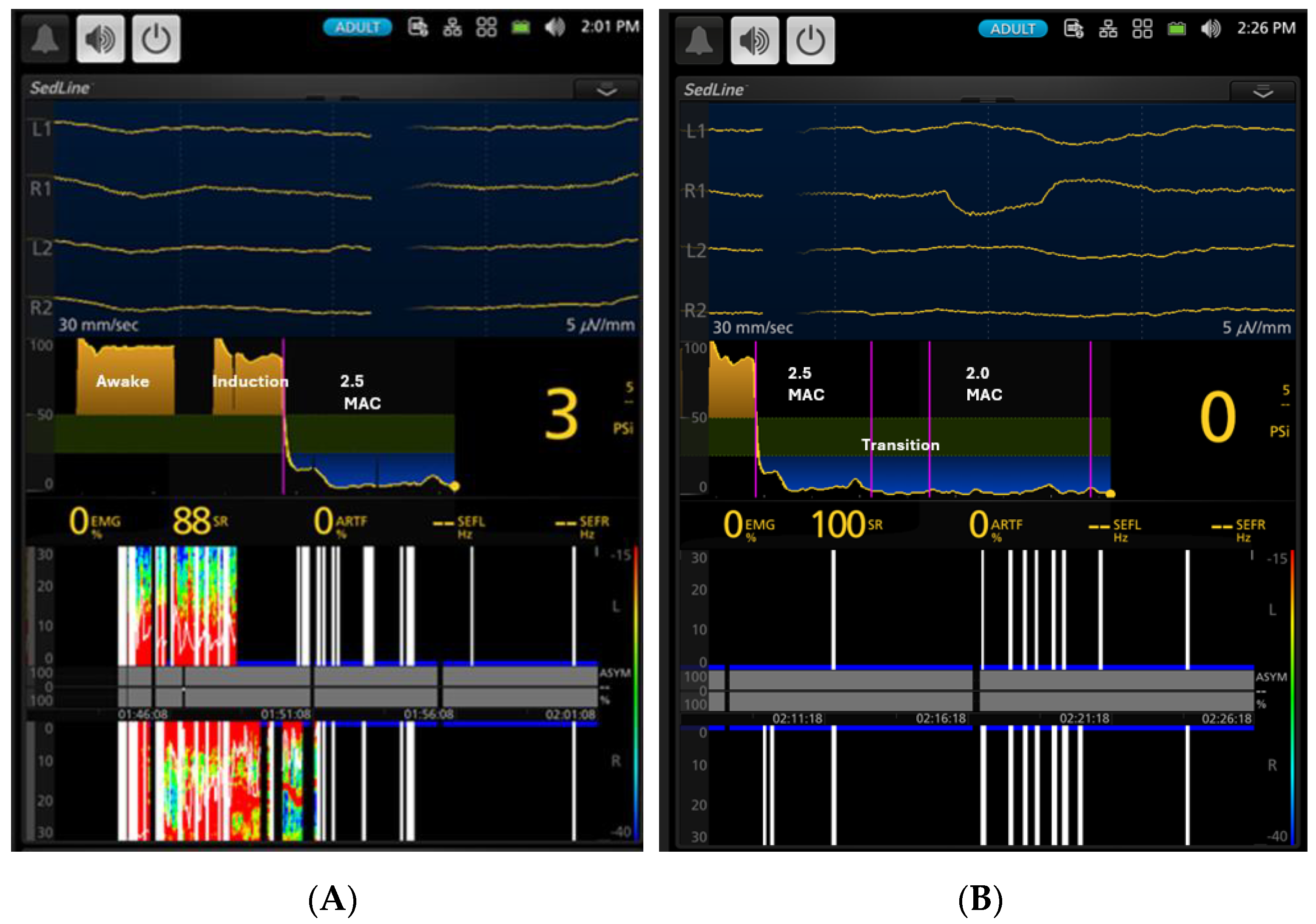
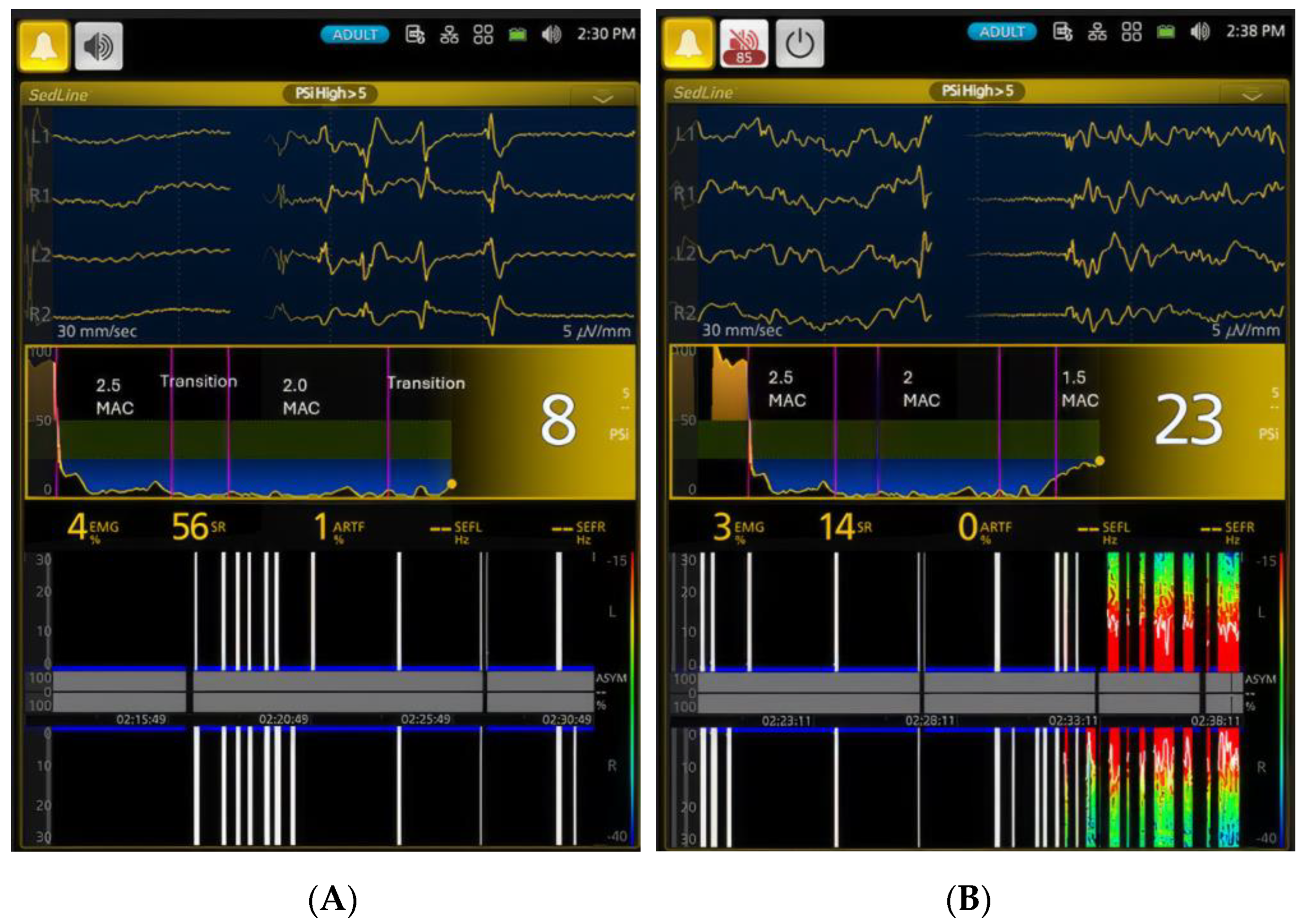

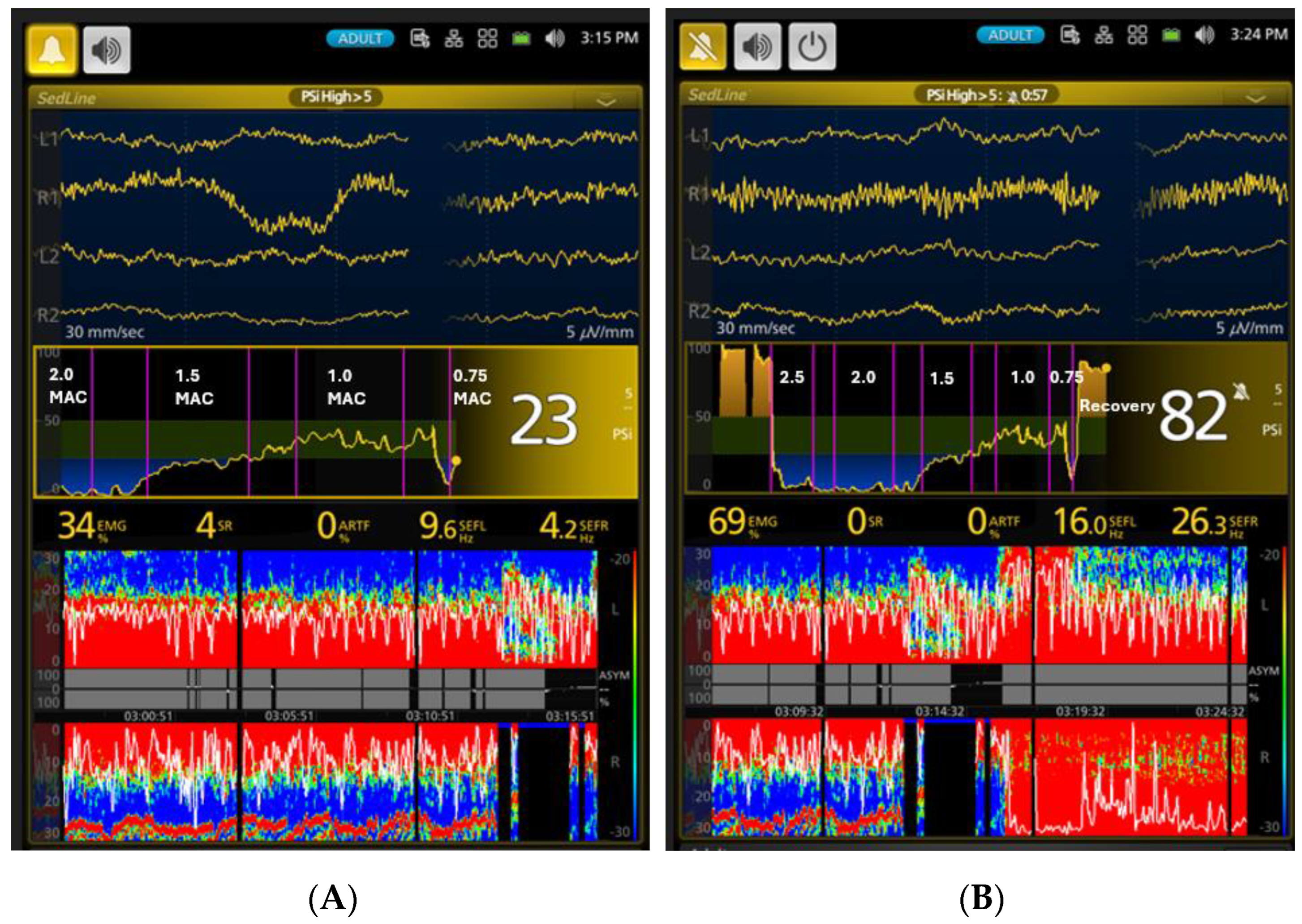
| Phases | PSI * | SR% * | SEF-L | SEF-R | EMG * | ART * |
|---|---|---|---|---|---|---|
| Baseline (0) | 72.8 ± 29.1 a | 5.3 ± 6.5 a | 9.3 ± 7.6 | 6.9 ± 6.1 | 52.7 ± 33.5 a | 12.5 ± 18.1 a |
| Induction (1) | 65.0 ± 22.5 a | 3.9 ± 5.1 a | 7.7 ± 4.8 | 7.3 ± 6.3 | 27.6 ± 27.0 b | 21.6 ± 26.3 a |
| 2.5× MAC (2) | 6.5 ± 10.8 b | 78.3 ± 24.0 b | 10.8 ± 4.3 | 8.3 ± 5.3 | 7.4 ± 11.6 c | 5.2 ± 12.4 b |
| 2× MAC (3) | 10.5 ± 13.6 b | 63.0 ± 33.3 b | 15.2 ± 7.8 | 15.9 ± 7.5 | 6.4 ± 12.9 c | 17.2 ± 35.4 a |
| 1.5× MAC (4) | 31.8 ± 12.4 c | 9.6 ± 20.6 a | 13.5 ± 4.0 | 11.4 ± 4.3 | 3.9 ± 4.4 c | 2.2 ± 4.8 b |
| 1× MAC (5) | 47.8 ± 12.6 a | 0.5 ± 2.5 a | 14.3 ± 4.8 | 12.2 ± 5.5 | 9.7 ± 15.9 c | 0.2 ± 1.b |
| 0.75× MAC (6) | 69.0 ± 18.8 a | 3.2 ± 12.9 a | 15.4 ± 5.4 | 18.5 ± 7.3 | 33.2 ± 35.5 b | 0.1 ± 0.2 b |
| Recovery (7) | 54.1 ± 29.1 a | 11.9 ± 13.0 a | 13.8 ± 9.6 | 13.5 ± 8.6 | 45.6 ± 37.2 a | 9.7 ± 13.7 a |
| Phase | End-Tidal * Isoflurane Concentration (%) | HR (bpm) * | SBP * (mmHg) | MBP * (mmHg) | DBP * (mmHg) | * Tolerance to Electrical Stimulation (Hz, Maxim 900 Hz) | Depth Score (1—Deep, 5—Light) |
|---|---|---|---|---|---|---|---|
| Awake baseline (0) | NA | 97.1 ± 20.8 a | 156 ± 23.4 a | 108.5 ± 16.7 a | 90.9 ± 18.4 a | NA | NA |
| Isoflurane induction (1) | 2.8 ± 1.3 a | 119.5 ± 26.2 b | 91.7 ± 16.2 b | 60.0 ± 4.4 b | 48.3 ± 5.5 b | NA | 3.0 ± 0.0 |
| 2.5× MAC (2) | 3.3 ± 0.1 b | 121.4 ± 13.6 b | 67.7 ± 20.9 c | 45.6 ± 16.4 c | 41.3 ± 17.9 c | 888.0 ± 33.2 a | 2.1 ± 0.7 |
| 2× MAC (3) | 2.6 ± 0.1 a | 120.9 ± 12.4 b | 76.9 ± 14.1 c | 50.4 ± 7.4 c | 40.6 ± 6.9 c | 888.6 ± 32.3 a | 2.3 ± 0.7 |
| 1.5× MAC (4) | 1.9 ± 0.1 c | 126.8 ± 15.2 b | 116.1 ± 8.9 d | 78.8 ± 8.9 d | 60.0 ± 7.1 d | 880.6 ± 62.4 a | 2.8 ± 0.6 |
| 1× MAC (5) | 1.3 ± 0.1 d | 132.3 ± 19.3 c | 139.2 ± 12.4 e | 99.8 ± 13.2 e | 82.1 ± 11.0 e | 553.7 ± 234.1 b | 3.7 ± 0.6 |
| 0.75× MAC (6) | 0.9 ± 0.1 e | 139.2 ± 19.2 c | 156 ± 16.1 a | 114.7 ± 13.6 a | 98 ± 13.3 a | NA | 4.0 ± 0.0 |
| Recovery (7) | 0.5 ± 0.2 e | 153.6 ± 19.3 d | 162.7 ± 21.2 a | 122.9 ± 25.9 a | 105 ± 26.4 a | NA | 4.7 ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, J.C.; Murillo, C.; Weil, A.B.; Kreuzer, M.; Moore, G.E. Electroencephalographic and Cardiovascular Assessments of Isoflurane-Anesthetized Dogs. Vet. Sci. 2024, 11, 514. https://doi.org/10.3390/vetsci11100514
Ko JC, Murillo C, Weil AB, Kreuzer M, Moore GE. Electroencephalographic and Cardiovascular Assessments of Isoflurane-Anesthetized Dogs. Veterinary Sciences. 2024; 11(10):514. https://doi.org/10.3390/vetsci11100514
Chicago/Turabian StyleKo, Jeff C., Carla Murillo, Ann B. Weil, Matthias Kreuzer, and George E. Moore. 2024. "Electroencephalographic and Cardiovascular Assessments of Isoflurane-Anesthetized Dogs" Veterinary Sciences 11, no. 10: 514. https://doi.org/10.3390/vetsci11100514
APA StyleKo, J. C., Murillo, C., Weil, A. B., Kreuzer, M., & Moore, G. E. (2024). Electroencephalographic and Cardiovascular Assessments of Isoflurane-Anesthetized Dogs. Veterinary Sciences, 11(10), 514. https://doi.org/10.3390/vetsci11100514







