Effect of Using Prickly Pear Seed Cake (Opuntia ficus indica L.) on Growth Performance, Digestibility, Physiological and Histometric Parameters in Rabbits
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
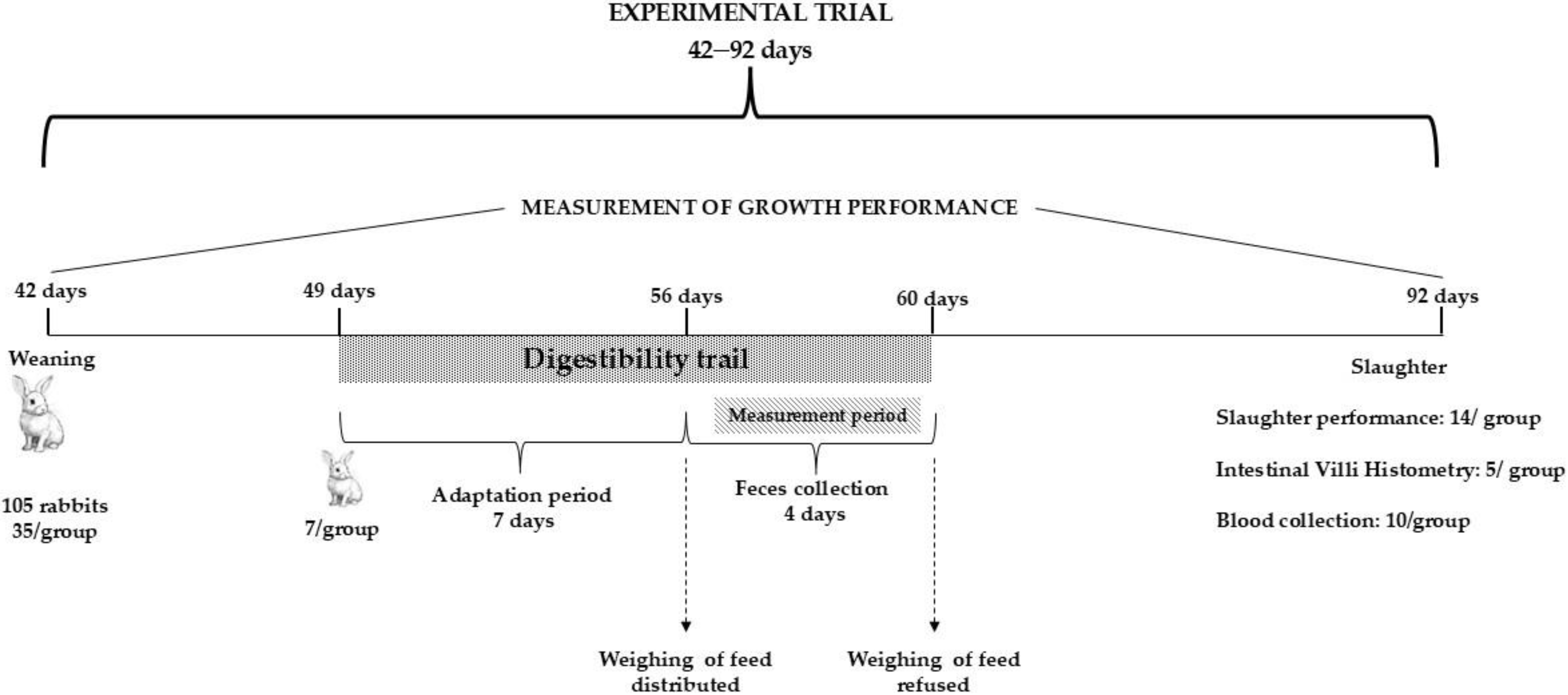
2.1. Animals and Management
2.2. Growth Performance Evaluation
2.3. Digestibility Trail
2.4. Chemical Analyses
2.5. Slaughter Performances
2.6. Blood Collection and Analyses
2.7. Intestinal Villi Histometry
2.8. Preparation of Prickly Pear Seed Cake
2.9. Statistical Analysis
3. Results
3.1. Digestibility of the Diets
3.2. Growth Performance
3.2.1. Body Weight and Weight Gain
3.2.2. Feed Intake and FCR
3.3. Carcass Characteristics
3.4. Digestive Tract
3.4.1. Weight of Digestive Tract
3.4.2. Histometry of Intestinal Villi
3.5. Blood Parameters
4. Discussion
4.1. Composition of Prickly Pear Seed Cake
4.2. Effect on Live Weight and Weight Gain
4.3. Effect on Feed Intake and Feed Conversion Ratio
4.4. Effect on Digestibility
4.5. Effect on Slaughter Yield and Carcass Characteristics
4.6. Effect on Digestive Tract Weight
4.7. Effect on Blood Biochemical Parameters
4.8. Effect on the Histometry of Intestinal Villi
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davies, R.R.; Davies, J.A. Rabbit gastrointestinal physiology. Vet. Clin. N. Am. Exot. Anim. Pract. 2003, 6, 139–153. [Google Scholar] [CrossRef]
- Gidenne, T. Dietary fibres in the nutrition of the growing rabbit and recommendations to preserve digestive health: A review. Animal 2015, 9, 227–242. [Google Scholar] [CrossRef] [PubMed]
- De Blas, J.C. Nutritional impact on health and performance in intensively reared rabbits. Animal 2013, 7, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.R.; Karuppusamy, S.; Sundaram, V. Unraveling the promise of agroindustrial byproducts as alternative feed source for sustainable rabbit meat production. Emerg. Anim. Species 2024, 10, 100044. [Google Scholar] [CrossRef]
- Bennegadi, N.; Gidenne, T.; Licois, D. Impact of fiber deficiency and sanitary status on non-specific enteropathy of the growing rabbit. Anim. Res. 2001, 50, 401–413. [Google Scholar] [CrossRef]
- Carabaño, R.; Badiola, I.; Chamorro, S.; García, J.; García-Ruiz, A.I.; García-Rebollar, P.; Gómez-Conde, M.; Gutiérrez, I.; Nicodemus, N.; Villamide, M.J.; et al. New trends in rabbit feeding: Influence of nutrition on intestinal health. Span. J. Agric. Res. 2008, 6, 15–25. [Google Scholar] [CrossRef]
- Lounaouci–Ouyed, G.; Lakabi, D.; Berchiche, M.; Lebas, F. Effets d’un apport de paille en complément d’un aliment granulé pauvre en fibres sur la digestion, la croissance et le rendement à l’abattage de lapins de population locale algérienne. In Proceedings of the 13th Journées de la Recherche Cunicole, Le Mans, France, 17–18 November 2009. [Google Scholar]
- Adaouri, M.; Dis, S.; Bouguera, A.; Tazka, H.; Zerrouki-Daoudi, N. Effect of incorporating local alfalfa hay or hay combined with wheat straw in the diet of growing rabbits (Algeria). Rev. Elev. Med. Vet. Pays Trop. 2023, 76, 36976. [Google Scholar] [CrossRef]
- Guermah, H.; Maertens, L.; Berchiche, M. Nutritive value of brewers’ grain and maize silage for fattening rabbits. World Rabbit Sci. 2016, 24, 183–189. [Google Scholar] [CrossRef]
- Kadi, S.A.; Belaidi-Gater, N.; Chebat, F. Inclusion of crude olive cake in growing rabbits diet: Effect on growth and slaughter yield. In Proceedings of the 8th World Rabbit Congress, Puebla, Mexico, 7–10 September 2004. [Google Scholar]
- Dorbane, Z.; Kadi, S.A.; Boudouma, D.; Gater-Belaid, N.; Bannelier, C.; Berchiche, M. Nutritive value of two types of olive cake (Olea Europaea L.) for growing rabbit. World Rabbit Sci. 2019, 27, 69–75. [Google Scholar] [CrossRef]
- Kadi, S.A.; Guermah, H.; Bannelier, C.; Berchiche, M.; Gidenne, T. Nutritive value of sun-dried sulla (Hedysarum flexuosum) and its effect on performance and carcass characteristics of growing rabbits. World Rabbit Sci. 2011, 19, 151–159. [Google Scholar] [CrossRef]
- Lounaouci–Ouyed, G.; Berchiche, M.; Gidenne, T. Effects of substitution of soybean meal-alfalfa-maize by a combination of field bean or pea with hard wheat bran on digestion and growth performance in rabbits in Algeria. World Rabbit Sci. 2014, 22, 137–146. [Google Scholar] [CrossRef][Green Version]
- Kadi, S.A.; Mouhous, A.; Djellal1, F.; Senhadji, Y.; Tiguemit, N.; Gidenne, T. Feuilles sèches de Figuier et foin de Sulla (Hedysarum flexuosum) en alimentation du lapin en engraissement. Livest. Res. Rural Dev. 2017, 29, 5. [Google Scholar]
- Harouz-Cherifi, Z.; Kadi, S.A.; Mouhous, A.; Bannellier, C.; Berchiche, M.; Gidenne, T. Effect of simplified feeding based only on wheat bran and brewer’s grain on rabbit performance and economic efficiency. World Rabbit Sci. 2018, 26, 27–34. [Google Scholar] [CrossRef]
- Al-Naqeb, G.; Fiori, L.; Ciolli, M.; Aprea, E. Prickly pear seed oil extraction, chemical characterization and potential health benefits. Molecules 2021, 26, 5018. [Google Scholar] [CrossRef]
- Morshedy, S.A.; Aymen, E.; Abdal Mohsen, A.E.; Mohamed, M.; Basyony, M.M.; Almeer, R.; Abdel-Daim, M.M.; El-Gindy, Y.M. Effect of prickly pear cactus peel supplementation on milk production, nutrient digestibility and rumen fermentation of sheep and the maternal effects on growth and physiological performance of suckling offspring. Animals 2020, 10, 1476. [Google Scholar] [CrossRef]
- El-Gindy, Y.M.; Hassan, A.A.; Basyony, M.M.; Morshedy, S.A. Milk yield and composition, feed efficiency, haemato-biochemical parameters and antioxidant status of lactating ewes fed diet supplemented with prickly pear cactus peels. Arch. Anim. Nutr. 2021, 75, 195–208. [Google Scholar] [CrossRef]
- Cherif, I.; Arbouche, R.; Arbouche, Y.; Mennani, A.; Arbouche, F. Dehydrated husks and cake of prickly pear (Opuntia ficus-indica) processing for broiler feed: Effects on growth performance, carcass characteristics, and meat quality. Vet. World 2022, 15, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, A.; Chaturved, O.H.; Thirumurugan, P.; Naqvi, S.M.K. Cactus: Ensuring round the year feed supply. Natl. Innov. Clim. Resilient Agric. 2017, 32. [Google Scholar]
- FAO. Élaboration D’36une Stratégie de Développement de la Filière du Figuier de Barbarie (Opuntia ficus-indica L.) en Algérie. 2022. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/4ad093a4-4251-4980-8231-dc57b11fc5cd/content (accessed on 1 September 2024).
- Nebbache, S.; Chibani, A.; Chadli, R.; Bouznad, A. Chemical composition of Opuntia ficus-indica (L.) fruit. Afr. J. Biotechnol. 2009, 8, 1623–1624. [Google Scholar]
- Abidi, S.; BenSalem, H.; Martín-García, A.I.; Molina-Alcaide, E. Ruminal fermentation of spiny (Opuntia amyclae) and spineless (Opuntia ficus indica f. inermis) cactus cladodes and diets including cactus. Anim. Feed Sci. Technol. 2009, 149, 333–340. [Google Scholar] [CrossRef]
- Boudechiche, L. Valorization of the prickly pear in animal. Renc. Rech. Rumin. 2012, 19. [Google Scholar]
- Andreu, L.; Nuncio-Jáuregui, N.; Carbonell-Barrachina, A.A.; Legua, P.; Hernández, F. Antioxidant properties and chemical characterization of Spanish Opuntia ficus-indica Mill. cladodes and fruits. J. Sci. Food Agric. 2017, 98, 1566–1573. [Google Scholar] [CrossRef]
- Mena, P.; Tassotti, M.; Andreu, L.; Nuncio-Jáuregui, N.; Legua, P.; Del Rio, D.; Hernández, F. Phytochemical characterization of different prickly pear (Opuntia ficus-indica (L.) Mill.) cultivars and botanical parts: UHPLC-ESI-MSn metabolomics profiles and their chemometric analysis. Food Res. Int. 2018, 108, 301–308. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, H.I.; Elmelegy, A.A.; Eldesoky, S.E.; Safwat, G. Phytochemical screening, antimicrobial, antiaxidant, anticancer activities and nutritional values of cactus (Opuntia ficus indicia) pulp and peel. Fresenius Environ. Bull. 2019, 28, 1534–1551. [Google Scholar]
- Zenteno-Ramírez, G.; Juárez-Flores, B.I.; Aguirre-Rivera, J.R.; MonreaL-Montes, M.; Mérida García, J.; Pérez Serratosa, M.; Varo Santos, M.Á.; Ortiz Pérez, M.D.; Rendón-Huerta, J.A. Juices of prickly pear fruits (Opuntia spp.) as functional foods. Ital. J. Food Sci. 2018, 30, 614–627. [Google Scholar] [CrossRef]
- Inácio, J.G.; Conceição, M.G.; Santos, D.C.; Oliveira, J.C.V.; Chagas, J.C.C.; Moraes, G.S.O.; Santos Silva, E.T.; Andrade Ferreira, M. Nutritional and performance viability of cactus Opuntia-based diets with different concentrate levels for Girolando lactating dairy cows. Asian-Australas J. Anim. Sci. 2020, 33, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Djaballah-Chachoua, I.; Alane, F.; Soltani, N.; Gasmi, S. The nutritional value of Opuntia ficus indica meal and the effect of its inclusion in the diet of fattening lambs. Ann. Rom. Soc. Cell. Biol. 2022, 26, 3285–3302. [Google Scholar]
- Ragab, M.S. Replacing yellow corn with prickly pear peels in growing Japanese quail diets with or without enzyme supplementation. Fayoum J. Agric. Res. Dev. 2007, 21, 97–112. [Google Scholar]
- Moula, N.; Humbel, M.; Leterrier, M.; Lempereur, L.; Ait-Kaki, A.; Touazi, L.; Saidj, D.; Hornick, J.L. Effects of Opuntia ficus-indica on growth performance and serum parameters of broiler chicken in Algeria. Tropicultura 2019, 37, 2295–8010. [Google Scholar] [CrossRef]
- Mahrose, K.M. Prickly Pear (Opuntia spp.) in Animal and Poultry Feed. In Opuntia spp.: Chemistry, Bioactivity and Industrial Applications; Ramadan, M.F., Ayoub, T.E.M., Rohn, S., Eds.; Springer: Cham, Switzerland, 2021; pp. 827–839. [Google Scholar] [CrossRef]
- Ikanya, L.W.; Maina, J.G.; Owino, W.O.; Dubeux, J.C.B. Feed intake, milk yield and milk composition of Somali camels supplemented with Opuntia stricta and urea. Acta Hortic. 2022, 1343, 207–212. [Google Scholar] [CrossRef]
- Zabut, B.M.; Alqedra, I.A.; Abushammalah, K.N. Evaluation of cactus cladodes as a partial feed for growing rabbits in the Gaza Strip. Livest. Res. Rural Dev. 2007, 19, 10. [Google Scholar]
- Bakr, A.A. Impact of partial replacement of alfalfa hay with Prickly pear peels (ppps) on productive performance of rabbits. Egypt J. Nutr. Feeds. 2019, 22, 535–550. [Google Scholar] [CrossRef]
- Pascoal, L.A.F.; Silva, K.A.G.; Watanabe, P.H.; Brito, J.M.F.; Silva, J.F.; Dantas, J.P.R.; Silva, D.R.P.; Brito, M.S.; Bezerra, A.P.A.; Almeida, J.M.D.S. Forage cactus (Opuntia ficus-indica Mill) meal in rabbit diets in the growth phase. Rev. Bras. Saúde Prod. Anim. 2020, 21, 1–13. [Google Scholar] [CrossRef]
- Zeedan, K.H.I.; El-Neney, B.A.M.; Lateif, A.I.; Abd, E.L.; Acadian, N.B.; Ebeid, T.A. Effect of using residues prickly pear as a source of dietary feedstuffs on productive performance, biological traits and immune response of rabbit. 1-prickly pear cladodes. Egypt. Poult. Sci. 2015, 35, 933–953. [Google Scholar]
- Amer, F.; Mobaraz, S.M.; Basyony, M.M.; Khalid, M.; Mahrose, K.H.; El-Medany, S.A. Effect of using prickly pear and its by-products as Alternative feed resources on performance of growing rabbit. Egypt. J. Rabbit Sci. 2019, 29, 99–124. [Google Scholar] [CrossRef][Green Version]
- El-Neney, B.A.M.; Zeedan, K.I.; El-Kotamy, E.M.; Gad, G.G.; Abdou, A. Effect of using prickly pear as a source of dietary feedstuffs on productive performance, physiological traits and immune response of rabbit. 2- prickly pear peels. Egypt. J. Nutr. Feeds. 2019, 22, 91–106. [Google Scholar] [CrossRef]
- Ezzeroug, R.; Belabbas, R.; Argente, M.J.; Berbar, A.; Diss, S.; Boudjella, Z.; Talaziza, D.; Boudahdir, N.; García, M.L. Genetic correlations for reproductive and growth traits in rabbits. Can. J. Anim. Sci. 2020, 100, 317–322. [Google Scholar] [CrossRef]
- Pérez, J.M.; Lebas, F.; Gidenne, T.; Maertens, L.; Xiccato, G.; Parigi-Bini, R.; Dalle Zotte, A.; Cossu, M.E.; Carazzolo, A.; Villamide, M.J.; et al. European reference method for in vivo determination of diet digestibility in rabbits. Word Rabbit Sci. 1995, 3, 41–43. [Google Scholar] [CrossRef]
- AFNOR. Recueil de normes françaises. Méthodes d’analyses françaises et communautaires. In Aliments des Animaux, 2nd ed.; Association Française de Normalisation: Paris, France, 1985; pp. 47–170. [Google Scholar]
- Lebas, F. Estimation de la digestibilité des protéines et de la teneur en énergie digestible des matières premières pour le lapin, avec un système d’équations. In Proceedings of the 15th Journées de la Recherche Cunicole, Le Mans, France, 19–20 November 2013. [Google Scholar]
- Blasco, A.; Ouhayoun, J.; Maseoro, G. Harmonisation of criteria and terminology in rabbit meat research. World Rabbit Sci. 1993, 1, 3–10. [Google Scholar] [CrossRef]
- Gallois, M. Statut Nutritionnel du Lapereau: Maturation des Structures et des Fonctions Digestives et Sensibilité à une Infection par une Souche Entéropathogène d’Escherichia coli. Ph.D. Thesis, Sciences Ecologiques, Vétérinaires, Agronomiques et Bioingénieries, Institut National Polytechnique de Toulouse, Toulouse, France, 16 March 2006. [Google Scholar]
- Gabe, M. Techniques Histologiques; Edition Masson et Cie: Paris, France, 1968; pp. 11–88. [Google Scholar]
- De Blas, C.; Mateos, G.G. Feed formulation. In Nutrition of the Rabbit, 2nd ed.; De Blas, C., Wiseman, J., Eds.; CABI: Wallingford, UK, 2010; pp. 222–232. [Google Scholar]
- Farías-Kovac, C.; Nicodemus, N.; Delgado, R.; Ocasio-Vega, C.; Noboa, T.; Abdelrasoul, R.A.S.; Carabaño, R.; García, J. Effect of dietary insoluble and soluble fibre on growth performance, digestibility, and nitrogen, energy, and mineral retention efficiency in growing rabbits. Animals 2020, 10, 1346. [Google Scholar] [CrossRef]
- Masmoudi, M.; Baccouche, A.; Borchani, M.; Besbes, S.; Blecker, C.; Attiaa, H. Physico-chemical and antioxidant properties of oils and by-products obtained by cold press-extraction of Tunisian Opuntia spp. seeds. Appl. Food Res. 2021, 1, 100024. [Google Scholar] [CrossRef]
- Sáenz, C. Processing technologies: An alternative for cactus pear (Opuntia spp.) fruits and cladodes. J. Arid Environ. 2000, 46, 209–225. [Google Scholar] [CrossRef]
- Muñoz de Chávez, M.; Chávez, A.; Valles, V.; Roldán, J.A. The Nopal: A plant of manifold qualities. World Rev. Nutr. Diet. 1995, 77, 109–134. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, J. Cactus pear (Opuntia spp.) varieties: Main characteristics of Republica Argentina. Cactusnet Newsl. 2008, 8, 32. [Google Scholar]
- AbdelFattah, M.S.; Sherif, E.A.; Elsaid, A.S. Nutritive value and chemical composition of prickly pear seeds (Opuntia ficus indica L.) growing in Egypt. Int. J. Agric. Pol. Res. 2020, 81, 1–10. [Google Scholar] [CrossRef]
- Ghanemi, F.Z.; Nani, A.; Patoli, D.; Khaldi, D.; Mami, Z.; Belarbi, M. Evaluation of the nutritional value and antioxidant activity of Opuntia ficus indica seeds in the western region of Algeria. J. Nat. Prod. Res. App. 2022, 2, 5466. [Google Scholar] [CrossRef]
- Lebas, F. Recommandations pour la composition d’aliments destinés à des lapins en production intensive. Cunicult. Mag. 2004, 31, 2. [Google Scholar]
- Özcan, M.M.; Al Juhaimi, F.Y. Nutritive value and chemical composition of prickly pear seeds (Opuntia ficus indica L.) growing in Turkey. Int. J. Food Sci. Nutr. 2011, 62, 533–536. [Google Scholar] [CrossRef]
- Al-Juhaimi, F.; Özcan, M.M. Determination of some mineral contents of prickly pear (Opuntia ficus-indica L.) seed flours. Environ. Monit. Assess. 2013, 185, 3659–3663. [Google Scholar] [CrossRef]
- De Wit, M.; Du Toit, A.; Osthoff, G.; Hugo, A. Prickly pear antioxidants: Comparison of fruit pulp, fruit rind, fruit seeds and cladodes from eight different prickly pear cultivars (Opuntia ficus-indica and Opuntia Robusta). J. Food Meas. Charact. 2019, 13, 2347–2356. [Google Scholar] [CrossRef]
- Motri, S.; Chaaben, H.; Elayeb, I. Etude des Propriétés Physico-chimiques et mycologiques des tourteaux de pépins de figue de barbarie. In Proceedings of the 1st International Congress of Environmental Science and Technology, ICEST, Niš, Serbia, 28–30 June 2017. [Google Scholar]
- Badr Sherif, E.A.; Abdel Fattah, M.S.; Salah, E. Productive performance and meat quality of commercial Cobb chicken fed diets containing different levels of prickly pear fruits (Opuntia ficus indica) peel. Bull. Natl. Res. Cent. 2019, 43, 1–12. [Google Scholar] [CrossRef]
- Benteboula, M.; Hornick, J.L.; Besseboua, O.; Ayad, A. Effect of partial dietary substitution of prickly pear (Opuntia ficus indica L.) seeds meal on growth performance and carcass characteristics of broiler chicken. Vet. Zootech. 2023, 81, 1–9. [Google Scholar]
- De Blas, J.C.; Santoma, G.; Carabaño, R.; Fraga, M.J. Fiber and starch levels in fattening rabbit diets. J. Anim. Sci. 1986, 63, 1897–1904. [Google Scholar] [CrossRef]
- De Blas, J.C.; Wiseman, J.; Fraga, M.J.; Villamide, M.J. Prediction of the digestible energy and digestiblity of gross energy of feeds for rabbits. 2. Mixed diets. Anim. Feed Sci. Technol. 1992, 39, 39–59. [Google Scholar] [CrossRef]
- Gidenne, T. Dietary fibres: Their analysis in animal feeding, and their role in rabbit nutrition and health. WARTAZOA 2013, 23, 195–213. [Google Scholar] [CrossRef]
- Gidenne, T.; Lebas, F. Role of dietary fibre in rabbit nutrition and in digestive troubles prevention. In Proceedings of the 2nd Rabbit Congress of the Americas, Habana City, Cuba, 19–22 June 2002. [Google Scholar]
- Gidenne, T. Effets d’une réduction de la teneur en fibres alimentaires sur le transit digestif du lapin. Comparaison et validation de modèles d’ajustement des cinétiques d’excrétion fécale des marqueurs. Reprod. Nutr. Dev. 1994, 34, 295–306. [Google Scholar] [CrossRef]
- Gidenne, T. Conséquences digestives de l’ingestion de fibres et d’amidon chez le lapin en croissance: Vers une meilleure définition des besoins. INRA Prod. Anim. 1996, 9, 243–254. [Google Scholar] [CrossRef]
- Gidenne, T.; Bellier, R.; Eys, J.V. Effect of the dietary fibre origin on the digestion and on the caecal fermentation pattern of the growing rabbit. J. Anim. Sci. 1998, 66, 509–517. [Google Scholar] [CrossRef]
- De Blas, C.; Javier García, J.; Carabano, R. Role of fibre in rabbit diets. A review. Ann. Zootech. 1999, 48, 3–13. [Google Scholar] [CrossRef][Green Version]
- Garcia, J.; Carabaño, R.; De Blas, C. Effect of fiber source on cell wall digestibility and rate of passage in rabbits. J. Anim. Sci. 1999, 77, 898–905. [Google Scholar] [CrossRef]
- Mehrez, A.Z.; Mousa, M.R.M. Growth performance of rabbits fed olive pulp in North Sinai. Asian J. Anim. Sci. 2011, 5, 317–329. [Google Scholar] [CrossRef][Green Version]
- Slavin, J.L. Dietary fiber and body weight. Nutrition 2005, 21, 411–418. [Google Scholar] [CrossRef]
- Kristensen, M.; Jensen, M.G. Dietary fibres in the regulation of appetite and food intake. Importance of viscosity. Appetite 2011, 56, 65–70. [Google Scholar] [CrossRef]
- Rasoamanana, R.; Even, P.C.; Darcel, N.; Tomé, D.; Fromentin, G. Dietary fibers reduce food intake by satiation without conditioned taste aversion in mice. Physiol. Behav. 2013, 110–111, 13–19. [Google Scholar] [CrossRef]
- Rui, L. Energy metabolism in the liver. Compr. Physiol. 2014, 4, 177–197. [Google Scholar] [CrossRef]
- Atti, N.; Mahouachi, M.; Rouissi, H. The effect of spineless cactus (Opuntia ficus-indica f. inermis) supplementation on growth, carcass, meat quality and fatty acid composition of male goat kids. Meat Sci. 2006, 73, 229–235. [Google Scholar] [CrossRef]
- Mahouachi, M.; Atti, N.; Hajji, H. Use of spineless cactus (Opuntia ficus indica f. inermis) for dairy goats and growing kids: Impacts on milk production, kid’s growth, and meat quality. Sci. World J. 2012, 2012, 1–4. [Google Scholar] [CrossRef]
- Aguilar–Yáñez, M.I.; Hernández–Mendo, O.; Guerrero–Legarreta, I.; Ramírez–Bribiesca, J.E.; Aranda–Osorio, G.; Crosby–Galvan, M.M. Productive response of lambs fed with fresh or dehydrated spineless cactus (Opuntia ficus-indica L.). J. PACD 2011, 13, 23–35. [Google Scholar] [CrossRef]
- Anguita, M.; Gasa, J.; Nofrarias, M.; Martín-Orúe, S.M.; Pérez, J.F. Effect of coarse ground corn, sugar beet pulp and wheat bran on the voluntary intake and physicochemical characteristics of digesta of growing pigs. Livest. Sci. 2007, 107, 182–191. [Google Scholar] [CrossRef]
- Libao-Mercado, A.J.; Zhu, C.L.; Fuller, M.F.; Rademacher, M.; Sève, B.; Lange, C.F.M. Effect of feeding fermentable fiber on synthesis of total and mucosal protein in the intestine of the growing pig. Livest. Sci. 2007, 109, 125–128. [Google Scholar] [CrossRef]
- Serena, A.; Hedemann, M.S.; Bach Knudsen, K.E. Feeding high fibre diets changes luminal environment and morphology in the intestine of sows. Livest. Sci. 2007, 109, 115–117. [Google Scholar] [CrossRef]
- Abaza, I.M.; Omara, M.E. Response of growing rabbits to diets containing different levels of wheat screening by-product with or without enzyme supplementation. J. Product. Dev. 2012, 17, 105–125. [Google Scholar] [CrossRef]
- Arce, O.; Alagón, G.; Ródenas, L.; Martínez-Paredes, E.; Javier Moya, V.; Cervera, C.; Pascual, J.J. Effect of dietary level of beet pulp, with or without molasses, on health status, growth performance, and carcass and digestive tract traits of rabbits. Animals 2022, 12, 3441. [Google Scholar] [CrossRef]
- Chao, H.Y.; Li, F.C. Effect of level of fibre on performance and digestion traits in growing rabbits. Anim. Feed Sci. Technol. 2008, 144, 279–291. [Google Scholar] [CrossRef]
- Wu, Z.; Zhou, H.; Li, F.; Zhang, N.; Zhu, Y. Effect of dietary fiber levels on bacterial composition with age in the cecum of meat rabbits. Open Microbiol. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- NanBin, Z.; Chao, S.Y.; Yu, Z.X.; Yan, L.G.; FuChang, L.; YanLi, Z. Effects of dietary different crude fiber levels on growth performance, slaughter performance, meat quality and gastrointestinal and immune organ development of growing meat rabbits. Chinese J. Anim. Nutr. 2018, 30, 1789–1797. [Google Scholar]
- Philippe, F.X.; Remience, V.; Dourmad, J.Y.; Cabaraux, J.F.; Vandenheede, M.; Nicks, B. Les fibres dans l’alimentation des truies gestantes: Effets sur la nutrition, le comportement, les performances et les rejets dans l’environnement. INRAE Prod. Anim. 2008, 21, 277–290. [Google Scholar] [CrossRef]
- Margüenda, I.; Nicodemus, N.; Vadillo, S.; Sevilla, L.; García-Rebollar, P.; Villarroel, M.; Romero, C.; Carabaño, R. 2012. Effect of dietary type and level of fibre on rabbit carcass yield and its microbiological characteristics. Livest. Sci. 2012, 145, 7–12. [Google Scholar] [CrossRef]
- Louacini, B.K.; Dellal, A.; Halbouche, M.; Ghazi, K. Effect of incorporation of the spineless Opuntia ficus indica in diets on biochemical parameters and its impact on the average weight of ewes during the maintenance. Glob. Vet. 2012, 8, 352–359. [Google Scholar]
- Wolfram, R.M.; Kritz, H.; Efthimiou, Y.; Stomatopoulos, J.; Sinzinger, H. Effect of prickly pear (Opuntia robusta) on glucose and lipid-metabolism in non-diabetics with hyperlipidemia: A pilot study. Wien. Klin. Wochenschr. 2002, 114, 840–846. [Google Scholar]
- Bennekum, A.M.V.; Nguyen, D.V.; Schulthess, G.; Helmut Hauser, H.; Phillips, C. Mechanisms of cholesterol-lowering effects of dietary insoluble fibres: Relationships with intestinal and hepatic cholesterol parameters. Br. J. Nutr. 2005, 94, 331–337. [Google Scholar] [CrossRef]
- Chiou, P.W.S.; Lu, T.W.; Hsu, J.C.; Yu, B. Effect of different sources of fiber on the intestinal morphology of domestic geese. Asian-Australas. J. Anim. Sci. 1996, 9, 539–550. [Google Scholar] [CrossRef]
- Satchithanandam, S.; Vargofcak-Apker, M.; Calvert, R.J.; Leeds, A.R.; Cassidy, M.M. Alteration of gastrointestinal mucin by fiber feeding in rats. J. Nutr. 1990, 120, 1179–1184. [Google Scholar] [CrossRef]
- Piel, C.; Montagne, L.; Seève, B.; Lalleès, J.P. Increasing digesta viscosity using carboxymethylcellulose in weaned piglets stimulates ileal goblet cell numbers and maturation. J. Nutr. 2005, 135, 86–91. [Google Scholar] [CrossRef]
- Alvarez, J.L.; Marguenda, I.; García-Rebollar, P.; Carabaño, R.; De Blas, C.; Corujo, A.; García-Ruiz, A.I. Effects of type and level of fibre on digestive physiology and performance in reproducing and growing rabbits. World Rabbit Sci. 2007, 15, 9–17. [Google Scholar] [CrossRef]
- Zanetti, L.H.; Murakami, A.E.; Diaz-Varga, M.S.; Garcia, A.F.Q.; Ospina-Rojas, I.C.; Rodrigues do Nascimento, G.R.; Dos Santos, T.C.; Matumoto Pintro, P.T. By-product of passion fruit seed (Passiflora edulis) in the diet of broilers. Can. J. Anim. Sci. 2018, 98, 109–118. [Google Scholar] [CrossRef]
| Items (%); kcal/kg et MJ/kg | DM | Ash | CP | EE | CF | NDF | ADF | ADL | DE * |
|---|---|---|---|---|---|---|---|---|---|
| PPSC | 91.86 | 1.50 | 7.77 | 3.68 | 69.62 | 92.13 | 64.25 | 39.62 | 1159/4.89 |
| Alfalfa dehydrated | 91.57 | 9.78 | 18.38 | 1.97 | 31.63 | 68.14 | 34.91 | 15.28 | 2145/8.97 |
| C | 10PP | 20PP | |
|---|---|---|---|
| Ingredients (%) | |||
| Maize | 34.70 | 42.70 | 41.38 |
| Wheat bran | 20.00 | 4.00 | 7.00 |
| Soybean meal | 14.00 | 19.20 | 22.90 |
| Alfalfa dehydrated | 29.00 | 21.00 | 3.00 |
| Prickly pear seed cake (PPSC) | 0.00 | 10.45 | 22.10 |
| Limestone | 0.70 | 0.70 | 1.67 |
| Dicalcium phosphate | 0.45 | 0.80 | 0.80 |
| Salt | 0.15 | 0.15 | 0.15 |
| Premix * | 1.00 | 1.00 | 1.00 |
| Total | 100 | 100 | 100 |
| Chemical composition % (DM) | |||
| DM | 88.72 | 89.40 | 89.52 |
| CP | 17.98 | 16.85 | 16.78 |
| EE | 2.99 | 3.52 | 3.71 |
| Ash | 8.55 | 8.35 | 8.43 |
| CF | 13.53 | 14.75 | 16.50 |
| NDF | 32.82 | 34.45 | 34.94 |
| ADF | 18.72 | 20.22 | 22.08 |
| ADL | 5.29 | 5.58 | 6.15 |
| DE 1 (kcal/kg) | 2861 | 2804 | 2725 |
| DE (MJ/kg) | 11.97 | 11.73 | 11.40 |
| Groups | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | 10PP | 20PP | p Value | |||||||
| Parameters | Median | Q1 | Q3 | Median | Q1 | Q3 | Median | Q1 | Q3 | |
| DM, % | 72.01 ab | 71.53 | 72.60 | 73.81 b | 72.41 | 75.49 | 66.72 a | 64.35 | 69.54 | 0.003 |
| EE, % | 81.83 b | 81.17 | 83.02 | 78.25 a | 76.75 | 79.77 | 80.35 ab | 79.53 | 80.71 | 0.041 |
| CP, % | 81.02 | 80.01 | 82.54 | 82.05 | 79.66 | 84.45 | 78.07 | 75.43 | 80.86 | 0.091 |
| CF, % | 26.77 ab | 22.16 | 29.17 | 33.69 b | 28.11 | 36.38 | 22.62 a | 21.90 | 26.15 | 0.009 |
| Ash, % | 73.44 a | 72.11 | 73.95 | 77.82 b | 77.32 | 80.08 | 75.38 ab | 69.18 | 78.90 | 0.026 |
| DP * (g/100 g) | 13.00 | 12.61 | 13.86 | 13.14 | 12.76 | 13.62 | 12.32 | 11.86 | 12.75 | 0.129 |
| Day | Group | |||||
|---|---|---|---|---|---|---|
| C | 10PP | 20PP | ||||
| Mean | SE | Mean | SE | Mean | SE | |
| 42 | 1013 | 21 | 1006 | 22 | 998 | 20 |
| 49 | 1227 | 23 | 1251 | 26 | 1240 | 24 |
| 56 | 1449 | 25 | 1485 | 34 | 1455 | 29 |
| 63 | 1663 | 30 | 1738 | 30 | 1694 | 28 |
| 70 | 1834 a | 40 | 1966 b | 33 | 1876 a | 30 |
| 77 | 2031 | 33 | 2128 | 32 | 2040 | 37 |
| 84 | 2203 | 33 | 2284 | 35 | 2223 | 33 |
| 91 | 2344 | 35 | 2444 | 32 | 2377 | 35 |
| Time | Group | |||||
|---|---|---|---|---|---|---|
| C | 10PP | 20PP | ||||
| Mean | SE | Mean | SE | Mean | SE | |
| 42–48 days | 29.78 | 1.53 | 35.18 | 1.18 | 34.54 | 2.74 |
| 49–55 days | 31.74 | 1.51 | 32.73 | 2.66 | 30.71 | 2.39 |
| 56–62 days | 30.63 | 1.47 | 32.91 | 2.00 | 33.77 | 1.91 |
| 63–69 days | 24.33 | 3.65 | 31.00 | 1.97 | 27.50 | 2.32 |
| 70–76 days | 24.59 | 1.28 | 23.12 | 1.24 | 23.14 | 2.32 |
| 77–83 days | 24.56 | 1.57 | 22.22 | 1.48 | 26.22 | 1.89 |
| 84–91 days | 20.23 | 1.80 | 22.83 | 1.71 | 22.01 | 2.28 |
| Time | Group | |||||
|---|---|---|---|---|---|---|
| C | 10PP | 20PP | ||||
| Mean | SE | Mean | SE | Mean | SE | |
| 42–48 days | 81 | 2 | 90 | 2 | 92 | 4 |
| 49–55 days | 93 | 2 | 97 | 1 | 92 | 2 |
| 56–62 days | 103 | 3 | 107 | 5 | 104 | 4 |
| 63–69 days | 105 | 4 | 110 | 5 | 98 | 7 |
| 70–76 days | 103 | 2 | 106 | 2 | 105 | 7 |
| 77–83 days | 115 | 2 | 114 | 3 | 117 | 5 |
| 84–91 days | 125 a | 8 | 110 b | 8 | 121 ab | 9 |
| Time | Group | |||||
|---|---|---|---|---|---|---|
| C | 10PP | 20PP | ||||
| Mean | SE | Mean | SE | Mean | SE | |
| 42–48 days | 2.7 | 0.1 | 2.6 | 0.0 | 2.7 | 0.2 |
| 49–55 days | 2.9 | 0.1 | 3.1 | 0.2 | 3.2 | 0.3 |
| 56–62 days | 3.4 | 0.2 | 3.3 | 0.2 | 3.1 | 0.1 |
| 63–69 days | 3.8 | 0.1 | 3.6 | 0.2 | 3.6 | 0.3 |
| 70–76 days | 4.4 | 0.3 | 4.8 | 0.4 | 4.9 | 0.4 |
| 77–83 days | 4.7 | 0.1 | 5.3 | 0.3 | 4.6 | 0.4 |
| 84–91 days | 6.6 a | 0.7 | 5.0 b | 0.4 | 4.9 b | 0.5 |
| Group | |||||||
|---|---|---|---|---|---|---|---|
| C | 10PP | 20PP | p Value | ||||
| Parameters | Mean | SE | Mean | SE | Mean | SE | |
| BW (g) | 2412.71 | 47.94 | 2460.92 | 19.50 | 2455.50 | 31.29 | 0.553 |
| SW (g) | 241.40 | 6.73 | 255.41 | 8.40 | 253.10 | 6.22 | 0.328 |
| SW/BW% | 10.13 | 0.32 | 10.54 | 0.22 | 10.30 | 0.18 | 0.465 |
| WHC (g) | 1661.32 | 43.16 | 1780.13 | 25.25 | 1718.68 | 52.44 | 0.138 |
| HC/BW | 71.54 | 0.59 | 72.38 | 0.59 | 71.65 | 0.67 | 0.553 |
| WL (g) | 63.40 | 2.39 | 61.85 | 2.09 | 61.64 | 2.25 | 0.822 |
| WL/BW% | 3.80 | 0.16 | 3.73 | 0.12 | 3.67 | 0.15 | 0.813 |
| WCC (g) | 1626.46 | 27.17 | 1665.53 | 41.14 | 1688.46 | 30.41 | 0.424 |
| WCC/BW% | 69.48 | 0.87 | 69.26 | 0.52 | 68.21 | 0.42 | 0.322 |
| WPF (g) | 32.34 | 1.64 | 31.24 | 1.58 | 27.59 | 1.28 | 0.064 |
| WPF/BW | 1.34 b | 0.06 | 1.28 ab | 0.05 | 1.12 a | 0.04 | 0.013 |
| WIF | 8.05 b | 0.35 | 7.79 ab | 0.34 | 6.90 a | 0.33 | 0.043 |
| WIF/BW | 0.34 b | 0.02 | 0.32 ab | 0.01 | 0.28 a | 0.01 | 0.011 |
| WDT | 343.21 | 15.91 | 338.19 | 11.75 | 357.58 | 10.27 | 0.523 |
| WDT/BW% | 14.25 | 0.61 | 13.99 | 0.42 | 14.59 | 0.44 | 0.674 |
| Groups | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | 10PP | 20PP | ||||||||
| Parameters | Md | Q1 | Q3 | Md | Q1 | Q3 | Md | Q1 | Q3 | p Value |
| BW(g) | 2317 | 2257 | 2352 | 2455 | 2449 | 2455 | 2475 | 2465 | 2498 | 0.102 |
| WES(g) | 19.70 | 19.60 | 22.20 | 16.40 | 15.90 | 18.80 | 18.00 | 16.70 | 18.80 | 0.087 |
| WES/BW | 0.87 | 0.84 | 0.96 | 0.69 | 0.65 | 0.73 | 0.72 | 0.67 | 0.74 | 0.037 |
| WESI(g) | 50.15 | 48.20 | 54.50 | 50.00 | 47.00 | 53.20 | 51.40 | 50.40 | 52.10 | 0.763 |
| WESI/BW | 2.16 | 2.09 | 2.30 | 2.09 | 1.92 | 2.09 | 2.04 | 1.91 | 2.08 | 0.196 |
| WEC(g) | 32.70 | 31.20 | 32.80 | 31.30 | 30.80 | 35.10 | 34.60 | 34.30 | 42.90 | 0.230 |
| WEC/BW | 1.42 | 1.38 | 1.53 | 1.25 | 1.22 | 1.43 | 1.40 | 1.26 | 1.75 | 0.379 |
| WECol(g) | 28.30 | 26.30 | 32.60 | 27.60 | 27.00 | 28.10 | 33.20 | 30.80 | 35.50 | 0.249 |
| WECol/BW | 1.22 | 1.01 | 1.39 | 1.13 | 1.10 | 1.18 | 1.30 | 1.26 | 1.35 | 0.330 |
| Parameter | Group | |||||||
|---|---|---|---|---|---|---|---|---|
| C | 10PP | 20PP | p-Value | |||||
| Segments | Mean | SE | Mean | SE | Mean | SE | ||
| Duodenum | High (µm) | 3132.79 b | 73.55 | 4004.77 a | 65.09 | 3631.79 b | 60.22 | <0.001 |
| Base (µm) | 502.37 b | 12.64 | 580.77 a | 11.60 | 566.10 ab | 12.24 | 0.006 | |
| Area (mm2) | 4961.99 b | 177.96 | 7255.58 a | 158.99 | 6428.76 b | 152.67 | <0.001 | |
| Jejunum | High (µm) | 3460.74 b | 67.86 | 4210.97 a | 90.47 | 3777.86 b | 61.64 | <0.001 |
| Base (µm) | 412.45 b | 9.91 | 558.72 a | 11.79 | 541.76 a | 8.09 | <0.001 | |
| Area (mm2) | 4522.49 b | 157.58 | 7313.47 a | 176.74 | 6418.08 b | 137.18 | <0.001 | |
| Ileum | High (µm) | 2077.95 b | 43.72 | 3087.31 a | 50.01 | 2344.15 b | 47.87 | <0.001 |
| Base (µm) | 444.45 b | 8.46 | 510.76 a | 9.40 | 489.94 b | 9.34 | 0.013 | |
| Area (mm2) | 2901.31 b | 83.57 | 4910.05 a | 86.59 | 3609.52 b | 103.45 | <0.001 | |
| Groups | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | 10PP | 20PP | p Value | |||||||
| Parameters | Median | Q1 | Q3 | Median | Q1 | Q3 | Median | Q1 | Q3 | |
| Glucose (mmol/L) | 14.33 | 12.93 | 16.56 | 14.14 | 10.95 | 15.35 | 14.51 | 14.26 | 15.37 | 0.786 |
| Triglycerides (mmol/L) | 1.49 b | 1.44 | 1.51 | 1.45 b | 1.41 | 1.49 | 1.09 a | 1.04 | 1.12 | <0.001 |
| Cholesterol (mmol/L) | 2.31 b | 2.09 | 2.53 | 2.06 ab | 1.94 | 2.10 | 1.92 a | 1.78 | 2.06 | 0.002 |
| Total proteins (g/L) | 60.00 | 58.80 | 64.60 | 63.05 | 57.20 | 68.30 | 55.75 | 54.20 | 59.90 | 0.098 |
| Urea (mmol/L) | 12.20 | 12.04 | 12.37 | 12.24 | 11.95 | 12.85 | 12.62 | 12.40 | 12.78 | 0.308 |
| Creatinine (mg/L) | 20.00 | 18.33 | 21.67 | 21.67 | 20.00 | 21.67 | 21.67 | 20.00 | 23.33 | 0.187 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benali, N.; Belabbas, R.; Sais, M.; AinBaziz, H.; Djellout, B.; Ettouahria, F.N.; Oulebsir, N.; Brecchia, G.; Quattrone, A.; Curone, G.; et al. Effect of Using Prickly Pear Seed Cake (Opuntia ficus indica L.) on Growth Performance, Digestibility, Physiological and Histometric Parameters in Rabbits. Vet. Sci. 2024, 11, 513. https://doi.org/10.3390/vetsci11100513
Benali N, Belabbas R, Sais M, AinBaziz H, Djellout B, Ettouahria FN, Oulebsir N, Brecchia G, Quattrone A, Curone G, et al. Effect of Using Prickly Pear Seed Cake (Opuntia ficus indica L.) on Growth Performance, Digestibility, Physiological and Histometric Parameters in Rabbits. Veterinary Sciences. 2024; 11(10):513. https://doi.org/10.3390/vetsci11100513
Chicago/Turabian StyleBenali, Nadia, Rafik Belabbas, Mounira Sais, Hacina AinBaziz, Baya Djellout, Fatima Nouara Ettouahria, Nadira Oulebsir, Gabriele Brecchia, Alda Quattrone, Giulio Curone, and et al. 2024. "Effect of Using Prickly Pear Seed Cake (Opuntia ficus indica L.) on Growth Performance, Digestibility, Physiological and Histometric Parameters in Rabbits" Veterinary Sciences 11, no. 10: 513. https://doi.org/10.3390/vetsci11100513
APA StyleBenali, N., Belabbas, R., Sais, M., AinBaziz, H., Djellout, B., Ettouahria, F. N., Oulebsir, N., Brecchia, G., Quattrone, A., Curone, G., & Menchetti, L. (2024). Effect of Using Prickly Pear Seed Cake (Opuntia ficus indica L.) on Growth Performance, Digestibility, Physiological and Histometric Parameters in Rabbits. Veterinary Sciences, 11(10), 513. https://doi.org/10.3390/vetsci11100513










