Infectious Agents Associated with Abortion Outbreaks in Italian Pig Farms from 2011 to 2021
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
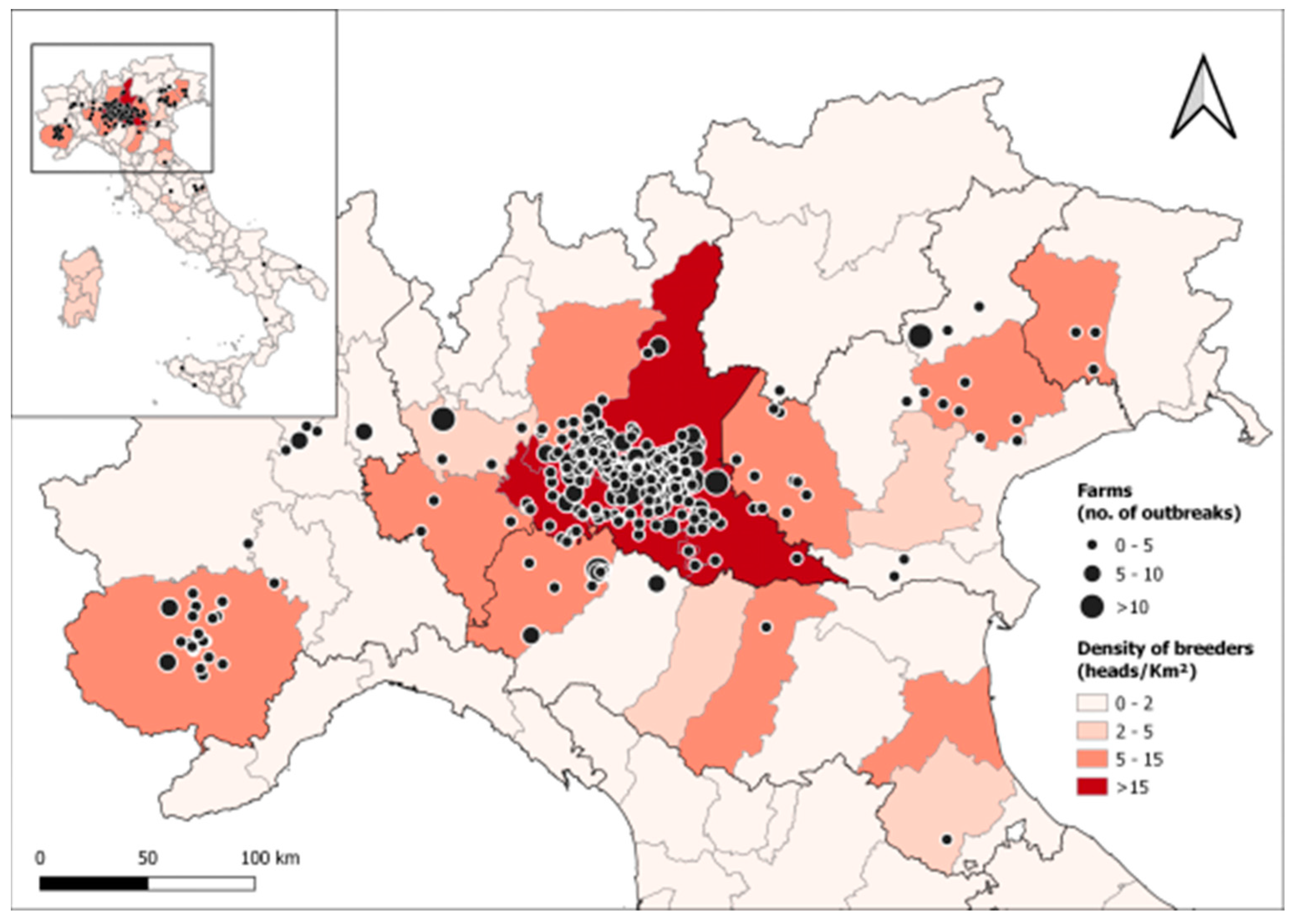
2.1. Sample Overview
2.2. Sample Processing and Pathogen Detection
2.3. Statistical Analysis
3. Results
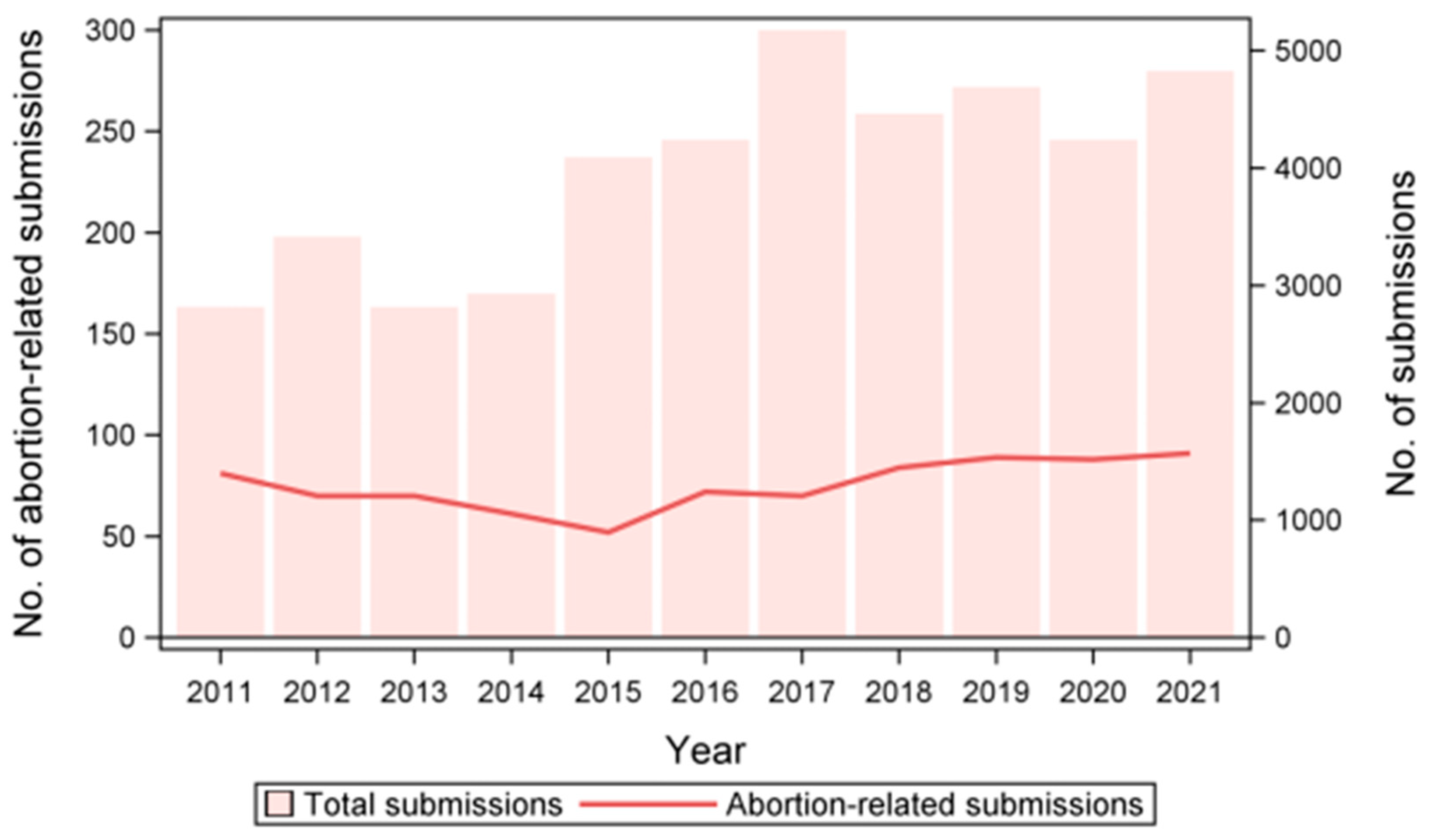
3.1. Temporal Trends in Abortion Outbreaks and Prevalence of Abortion-Inducing Pathogens
3.2. Factors Affecting Infection by Abortion-Inducing Pathogens
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christianson, W.T. Stillbirths, mummies, abortions, and early embryonic death. Vet. Clin. N. Am. Food Anim. Pract. 1992, 8, 623–639. [Google Scholar] [CrossRef] [PubMed]
- Maes, D.; Peltoniemi, O.; Malik, M. Abortion and fetal death in sows. Reprod. Domest. Anim. 2023, 58 (Suppl. S2), 125–136. [Google Scholar] [CrossRef]
- Holyoake, P.K.; Thompson, A. Isolation of [Actinobacillus] rossii from an aborted piglet. Aust. Vet. J. 2017, 95, 483–485. [Google Scholar] [CrossRef]
- Koketsu, Y.; Iida, R. Farm data analysis for lifetime performance components of sows and their predictors in breeding herds. Porc. Health Manag. 2020, 6, 24. [Google Scholar] [CrossRef]
- Althouse, G.C.; Kauffold, J.; Rossow, S. Diseases of the Reproductive System. In Diseases of Swine, 11th ed.; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schartz, K.J., Stevenson, G.W., Zhang, J., Eds.; State University Press: Ames, IA, USA, 2019; pp. 373–392. [Google Scholar]
- Koketsu, Y.; Iida, R. Sow housing associated with reproductive performance in breeding herds. Mol. Reprod. Dev. 2017, 84, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Vanderhaeghe, C.; Dewulf, J.; de Kruif, A.; Maes, D. Non-infectious factors associated with stillbirth in pigs: A review. Anim. Reprod. Sci. 2013, 139, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, J.J.; Dee, S.A.; Holtkamp, D.J.; Murtaugh, M.P.; Stadejek, T.; Stevenson, G.W.; Torremorell, M.; Yang, H.; Zhang, J. Porcine Reproductive and Respiratory Syndrome Viruses (Porcine Arteriviruses). In Diseases of Swine, 11th ed.; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schartz, K.J., Stevenson, G.W., Zhang, J., Eds.; State University Press: Ames, IA, USA, 2019; pp. 685–708. [Google Scholar]
- Kim, J.H.; Kim, S.C.; Kim, H.J.; Jeong, C.G.; Park, G.S.; Choi, J.S.; Kim, W.I. Insight into the Economic Effects of a Severe Korean PRRSV1 Outbreak in a Farrow-to-Nursery Farm. Animals 2022, 12, 3024. [Google Scholar] [CrossRef] [PubMed]
- Pena, R.N.; Fernández, C.; Blasco-Felip, M.; Fraile, L.J.; Estany, J. Genetic Markers Associated with Field PRRSV-Induced Abortion Rates. Viruses 2019, 11, 706. [Google Scholar] [CrossRef]
- Segalés, J. Porcine circovirus type 2 (PCV2) infections: Clinical signs, pathology and laboratory diagnosis. Virus Res. 2012, 164, 10–19. [Google Scholar] [CrossRef]
- Streck, A.F.; Truyen, U. Porcine Parvovirus. Curr. Issues Mol. Biol. 2020, 37, 33–46. [Google Scholar] [CrossRef]
- Saporiti, V.; Valls, L.; Maldonado, J.; Perez, M.; Correa-Fiz, F.; Segalés, J.; Sibila, M. Porcine Circovirus 3 Detection in Aborted Fetuses and Stillborn Piglets from Swine Reproductive Failure Cases. Viruses 2021, 13, 264. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.G.; Giannitti, F.; Rossow, S.; Marthaler, D.; Knutson, T.P.; Li, L.; Deng, X.; Resende, T.; Vannucci, F.; Delwart, E. Detection of a novel circovirus PCV3 in pigs with cardiac and multi-systemic inflammation. Virol. J. 2016, 13, 184. [Google Scholar] [CrossRef] [PubMed]
- Palinski, R.; Piñeyro, P.; Shang, P.; Yuan, F.; Guo, R.; Fang, Y.; Byers, E.; Hause, B.M. A Novel Porcine Circovirus Distantly Related to Known Circoviruses Is Associated with Porcine Dermatitis and Nephropathy Syndrome and Reproductive Failure. J. Virol. 2017, 91, e01879-16. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.Y.; Lin, C.N.; Ooi, P.T. What do we know about porcine circovirus 3 (PCV3) diagnosis so far?: A review. Transbound. Emerg. Dis. 2021, 68, 2915–2935. [Google Scholar] [CrossRef]
- Vargas-Bermudez, D.S.; Mogollón, J.D.; Jaime, J. The Prevalence and Genetic Diversity of PCV3 and PCV2 in Colombia and PCV4 Survey during 2015–2016 and 2018–2019. Pathogens 2022, 11, 633. [Google Scholar] [CrossRef]
- Schautteet, K.; Vanrompay, D. Chlamydiaceae infections in pig. Vet. Res. 2011, 42, 29. [Google Scholar] [CrossRef]
- Ellis, W.A. Animal leptospirosis. Curr. Top. Microbiol. Immunol. 2015, 387, 99–137. [Google Scholar] [CrossRef] [PubMed]
- Gomes de Araújo, H.; Limeira, C.H.; Ferreira de Aquino, V.V.; Vilela, V.L.R.; Alves, C.J.; Higino, S.S.d.S.; Santos, C.d.S.A.B.; de Azevedo, S.S. Global Seropositivity of Swine Leptospirosis: Systematic Review and Meta-Analysis. Trop. Med. Infect. Dis. 2023, 8, 158. [Google Scholar] [CrossRef]
- Nathues, H.; Tegeler, R.; Grummer, B.; Große Beilage, E. Retrospective analysis of laboratory examinations focusing on infectious agents in case of reproductive failure in swine herds. Tierarztl. Prax. Ausg. G Grosstiere Nutztiere 2011, 39, 155–161. [Google Scholar]
- Salogni, C.; Lazzaro, M.; Giacomini, E.; Giovannini, S.; Zanoni, M.; Giuliani, M.; Ruggeri, J.; Pozzi, P.; Pasquali, P.; Boniotti, M.B.; et al. Infectious agents identified in aborted swine fetuses in a high-density breeding area: A three-year study. J. Vet. Diagn. Investig. 2016, 28, 550–554. [Google Scholar] [CrossRef]
- King, J.M.; Roth-Johnson, L.; Newson, M.E. The Necropsy Book: A Guide for Veterinary Students, Residents, Clinicians, Pathologists, and Biological Researchers, 5th ed.; Charles Louis Davis DVM Foundation: Gurnee, IL, USA, 2007. [Google Scholar]
- Opriessnig, T.; Yu, S.; Gallup, J.M.; Evans, R.B.; Fenaux, M.; Pallares, F.; Thacker, E.L.; Brockus, C.W.; Ackermann, M.R.; Thomas, P.; et al. Effect of vaccination with selective bacterins on conventional pigs infected with type 2 porcine circovirus. Vet. Pathol. 2003, 40, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choi, C.; Han, D.U.; Chae, C. Simultaneous detection of porcine circovirus type 2 and porcine parvovirus in pigs with PMWS by multiplex PCR. Vet. Rec. 2001, 149, 304–305. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Jeong, C.G.; Nazki, S.; Lee, S.I.; Baek, Y.C.; Jung, Y.J.; Kim, W.I. Evaluation of a multiplex PCR method for the detection of porcine parvovirus types 1 through 7 using various field samples. PLoS ONE 2021, 16, e0245699. [Google Scholar] [CrossRef]
- De Puysseleyr, K.; De Puysseleyr, L.; Dhondt, H.; Geens, T.; Braeckman, L.; Morré, S.A.; Cox, E.; Vanrompay, D. Evaluation of the presence and zoonotic transmission of Chlamydia suis in a pig slaughterhouse. BMC Infect. Dis. 2014, 14, 560. [Google Scholar] [CrossRef][Green Version]
- Stoddard, R.A.; Gee, J.E.; Wilkins, P.P.; McCaustland, K.; Hoffmaster, A.R. Detection of pathogenic Leptospira spp. through TaqMan polymerase chain reaction targeting the LipL32 gene. Diagn. Microbiol. Infect. Dis. 2009, 64, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Segalés, J.; Sibila, M. Revisiting Porcine Circovirus Disease Diagnostic Criteria in the Current Porcine Circovirus 2 Epidemiological Context. Vet. Sci. 2022, 9, 110. [Google Scholar] [CrossRef]
- da Silva, R.R.; da Silva, D.F.; da Silva, V.H.; de Castro, A.M.M.G. Porcine circovirus 3: A new challenge to explore. Front. Vet. Sci. 2024, 10, 1266499. [Google Scholar] [CrossRef]
- Broll, S.; Waldvogel, A.S.; Rosskopf, M.; Corboz, L.; Pospischil, A. The infectious causes of abortion and stillbirth in swine in Switzerland. J. Vet. Med. B 1993, 40, 641–653. [Google Scholar] [CrossRef]
- Hansen, M.S.; Hjulsager, C.K.; Bille-Hansen, V.; Haugegaard, S.; Dupont, K.; Høgedal, P.; Kunstmann, L.; Larsen, L.E. Selection of method is crucial for the diagnosis of porcine circovirus type 2 associated reproductive failures. Vet. Microbiol. 2010, 144, 203–209. [Google Scholar] [CrossRef]
- Jara, M.; Rasmussen, D.A.; Corzo, C.A.; Machado, G. Porcine reproductive and respiratory syndrome virus dissemination across pig production systems in the United States. Transbound. Emerg. Dis. 2021, 68, 667–683. [Google Scholar] [CrossRef]
- Kristensen, C.S.; Christiansen, M.G.; Pedersen, K.; Larsen, L.E. Production losses five months after outbreak with a recombinant of two PRRSV vaccine strains in 13 Danish sow herds. Porc. Health Manag. 2020, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Ruedas-Torres, I.; Sánchez-Carvajal, J.M.; Carrasco, L.; Pallarés, F.J.; Larenas-Muñoz, F.; Rodríguez-Gómez, I.M.; Gómez-Laguna, J. PRRSV-1 induced lung lesion is associated with an imbalance between costimulatory and coinhibitory immune checkpoints. Front. Microbiol. 2023, 13, 1007523. [Google Scholar] [CrossRef] [PubMed]
- Martín-Valls, G.E.; Cortey, M.; Allepuz, A.; Illas, F.; Tello, M.; Mateu, E. Introduction of a PRRSV-1 strain of increased virulence in a pig production structure in SpaIn Virus evolution and impact on production. Porc. Health Manag. 2023, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Romeo, C.; Parisio, G.; Scali, F.; Tonni, M.; Santucci, G.; Maisano, A.M.; Barbieri, I.; Boniotti, M.B.; Stadejek, T.; Alborali, G.L. Complex interplay between PRRSV-1 genetic diversity, coinfections and antimicrobial use influences performance parameters in post-weaning pigs. Vet. Microbiol. 2023, 284, 109830. [Google Scholar] [CrossRef] [PubMed]
- Faccini, S.; Barbieri, I.; Gilioli, A.; Sala, G.; Gibelli, L.R.; Moreno, A.; Sacchi, C.; Rosignoli, C.; Franzini, G.; Nigrelli, A. Detection and genetic characterization of Porcine circovirus type 3 in Italy. Transbound. Emerg. Dis. 2017, 64, 1661–1664. [Google Scholar] [CrossRef]
- Franzo, G.; Legnardi, M.; Tucciarone, C.M.; Drigo, M.; Klaumann, F.; Sohrmann, M.; Segales, J. Porcine circovirus type 3: A threat to the pig industry? Vet. Rec. 2018, 182, 83. [Google Scholar] [CrossRef]
- Franzo, G.; Legnardi, M.; Hjulsager, C.K.; Klaumann, F.; Larsen, L.E.; Segales, J.; Drigo, M. Full-genome sequencing of porcine circovirus 3 field strains from Denmark, Italy and Spain demonstrates a high within-Europe genetic heterogeneity. Transbound. Emerg. Dis. 2018, 65, 602–606. [Google Scholar] [CrossRef]
- Giudici, S.D.; Franzoni, G.; Bonelli, P.; Angioi, P.P.; Zinellu, S.; Deriu, V.; Carta, T.; Sechi, A.M.; Salis, F.; Balzano, F.; et al. Genetic Characterization of Porcine Circovirus 3 Strains Circulating in Sardinian Pigs and Wild Boars. Pathogens 2020, 9, 344. [Google Scholar] [CrossRef]
- Amoroso, M.G.; Serra, F.; Esposito, C.; D’Alessio, N.; Ferrara, G.; Cioffi, B.; Anzalone, A.; Pagnini, U.; De Carlo, E.; Fusco, G.; et al. Prevalence of Infection with Porcine Circovirus Types 2 and 3 in the Wild Boar Population in the Campania Region (Southern Italy). Animals 2021, 11, 3215. [Google Scholar] [CrossRef]
- Gillespie, J.; Opriessnig, T.; Meng, X.J.; Pelzer, K.; Buechner-Maxwell, V. Porcine circovirus type 2 and porcine circovirus-associated disease. J. Vet. Intern. Med. 2009, 23, 1151–1163. [Google Scholar] [CrossRef]
- Madson, D.M.; Opriessnig, T. Effect of porcine circovirus type 2 (PCV2) infection on reproduction: Disease, vertical transmission, diagnostics and vaccination. Anim. Health Res. Rev. 2011, 12, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, J.; Segalés, J.; Martínez-Puig, D.; Calsamiglia, M.; Riera, P.; Domingo, M.; Artigas, C. Identification of viral pathogens in aborted fetuses and stillborn piglets from cases of swine reproductive failure in Spain. Vet. J. 2005, 169, 454–456. [Google Scholar] [CrossRef] [PubMed]
- Segalés, J.; Allan, G.M.; Domingo, M. Circoviruses. In Diseases of Swine, 11th ed.; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schartz, K.J., Stevenson, G.W., Zhang, J., Eds.; State University Press: Ames, IA, USA, 2019; pp. 473–487. [Google Scholar]
- Chae, C. Commercial porcine circovirus type 2 vaccines: Efficacy and clinical application. Vet. J. 2012, 194, 151–157. [Google Scholar] [CrossRef]
- Di Francesco, A.; Baldelli, R.; Cevenini, R.; Magnino, S.; Pignanelli, S.; Salvatore, D.; Galuppi, R.; Donati, M. Seroprevalence to Chlamydiae in pigs in Italy. Vet. Rec. 2006, 159, 849–850. [Google Scholar]
- Sheng, C.Y.; Gong, Q.L.; Ma, B.Y.; Liu, Y.; Ge, G.Y.; Li, D.L.; Luan, M.H.; Diao, N.C.; Li, J.M.; Shi, K.; et al. Prevalence of Chlamydia in Pigs in China from 1985 to 2020: A Systematic Review and Meta-Analysis. Vector Borne Zoonotic Dis. 2021, 21, 517–533. [Google Scholar] [CrossRef]
- Hoffmann, K.; Schott, F.; Donati, M.; Di Francesco, A.; Hässig, M.; Wanninger, S.; Sidler, X.; Borel, N. Prevalence of Chlamydial Infections in Fattening Pigs and Their Influencing Factors. PLoS ONE 2015, 10, e0143576. [Google Scholar] [CrossRef]
- Foni, E.; Gualandi, G. A serological survey of swine parvovirus infection in Italy. Microbiologica 1989, 12, 241–245. [Google Scholar] [PubMed]
- Streck, A.F.; Canal, C.W.; Truyen, U. Molecular epidemiology and evolution of porcine parvoviruses. Infect. Genet. Evol. 2015, 36, 300–306. [Google Scholar] [CrossRef]
- Mészáros, I.; Olasz, F.; Cságola, A.; Tijssen, P.; Zádori, Z. Biology of Porcine Parvovirus (Ungulate parvovirus 1). Viruses 2017, 9, 393. [Google Scholar] [CrossRef]
- Macaluso, G.; Torina, A.; Blanda, V.; Guercio, A.; Lastra, A.; Giacchino, I.; D’Agostino, R.; Sciacca, C.; D’Incau, M.; Bertasio, C.; et al. Leptospira in Slaughtered Fattening Pigs in Southern Italy: Serological Survey and Molecular Typing. Animals 2022, 12, 585. [Google Scholar] [CrossRef]
- Bertasio, C.; Papetti, A.; Scaltriti, E.; Tagliabue, S.; D’incau, M.; Boniotti, M.B. Serological Survey and Molecular Typing Reveal New Leptospira Serogroup Pomona Strains among Pigs of Northern Italy. Pathogens 2020, 9, 332. [Google Scholar] [CrossRef] [PubMed]
- Camenisch, U.; Lu, Z.H.; Vaughan, L.; Pospischil, A.; Sydler, T.; Corboz, L.; Wittenbrink, M.M.; Zimmermann, D.R. Diagnostic investigation into the role of Chlamydiae in cases of increased rates of return to oestrus in pigs. Vet. Rec. 2004, 155, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.G.; Chen, G.D.; Huang, Y.; Ding, L.; Li, Z.C.; Chang, C.D.; Wang, C.Y.; Tong, D.W.; Liu, H.J. Development of multiplex PCR for simultaneous detection of six swine DNA and RNA viruses. J. Virol. Methods 2012, 183, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Ge, L.; Tan, S.; Zhang, H.; Yang, Y.; Zhang, L.; Deng, Z. Epidemiological Survey of Four Reproductive Disorder Associated Viruses of Sows in Hunan Province during 2019–2021. Vet. Sci. 2022, 9, 425. [Google Scholar] [CrossRef]
- Mak, C.K.; Yang, C.; Jeng, C.R.; Pang, V.F.; Yeh, K.S. Reproductive failure associated with coinfection of porcine circovirus type 2 and porcine reproductive and respiratory syndrome virus. Can. Vet. J. 2018, 59, 525–530. [Google Scholar]
- Eddicks, M.; Gründl, J.; Seifert, A.; Eddicks, L.; Reese, S.; Tabeling, R.; Swam, H.; Strutzberg-Minder, K.; Ritzmann, M.; Fux, R. Examination on the Occurrence of Coinfections in Diagnostic Transmittals in Cases of Stillbirth, Mummification, Embryonic Death, and Infertility (SMEDI) Syndrome in Germany. Microorganisms 2023, 11, 1675. [Google Scholar] [CrossRef]


| Pathogen | Period | Material | Method | References | Positivity Cut-Off |
|---|---|---|---|---|---|
| PCV2 | 2011–2021 | pooled organs (heart, lung, and liver) | quantitative real-time PCR | [24] | LoQ 105 copies/mL of homogenate |
| PCV3 | 2015–2021 | pooled organs (heart, lung, and liver) | real-time PCR | primers described in [15]; probe described in Table S1 | Cq 38 |
| PPV | 2011–2014 | pooled organs (heart, lung, and liver) | PCR | [25] | |
| PPV | 2015–2021 | pooled organs (heart, lung, and liver) | real-time PCR | [26] | Cq 38 |
| PRRSV | 2011–2021 | pooled organs (heart, lung, and liver) | real-time RT-PCR | Virotype PRRSV RT-PCR Kit, Indical®, Leipzig, Germany | Cq 37 |
| Chlamydia spp. | 2015–2021 | pooled organs (heart, lung, and liver) | real-time PCR | [27] | Cq 38 |
| Leptospira spp. | 2015–2021 | kidney tissue | real-time PCR | [28] | Cq 40 |
| Pathogens | No. of Outbreaks Tested | No. of Positive Outbreaks | Prevalence (%) | 95% CI (%) |
|---|---|---|---|---|
| PCV2 | 790 | 91 | 11.5 | 9.3–13.7 |
| PCV3 a | 438 | 86 | 19.6 | 15.9–23.4 |
| PPV | 680 | 27 | 4.0 | 2.5–5.4 |
| PRRSV | 817 | 204 | 25.0 | 22.0–27.9 |
| Chlamydia spp. a | 537 | 30 | 5.6 | 3.6–7.5 |
| Leptospira spp. a | 490 | 13 | 2.6 | 1.2–4.1 |
| Pathogens | No. of Pathogens | No. of Positive Outbreaks | % |
|---|---|---|---|
| PRRSV + PCV2 | 2 | 28 | 32.2 |
| PRRSV + PCV3 | 2 | 20 | 23.0 |
| PRRSV + Chlamydia spp. | 2 | 6 | 6.9 |
| PCV2 + PCV3 | 2 | 4 | 4.6 |
| PCV3 + Chlamydia spp. | 2 | 4 | 4.6 |
| PRRSV + PPV | 2 | 4 | 4.6 |
| PRRSV + PCV2 + PPV | 3 | 4 | 4.6 |
| PCV3 + Leptospira spp. | 2 | 3 | 3.4 |
| PRRSV + PCV2 + PCV3 | 3 | 3 | 3.4 |
| PCV2 + PPV | 2 | 2 | 2.3 |
| PCV2 + PCV3 + Chlamydia spp. | 3 | 2 | 2.3 |
| PCV2 + Leptospira spp. | 2 | 1 | 1.1 |
| PCV2 + PCV3 + PPV | 3 | 1 | 1.1 |
| PCV3 + Leptospira spp. + Chlamydia spp. | 3 | 1 | 1.1 |
| PCV3 + PPV | 2 | 1 | 1.1 |
| PRRSV + Leptospira spp. | 2 | 1 | 1.1 |
| PRRS + PPV + Leptospira spp. | 3 | 1 | 1.1 |
| PRRS + PCV3 + PPV | 3 | 1 | 1.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donneschi, A.; Recchia, M.; Romeo, C.; Pozzi, P.; Salogni, C.; Maisano, A.M.; Santucci, G.; Scali, F.; Faccini, S.; Boniotti, M.B.; et al. Infectious Agents Associated with Abortion Outbreaks in Italian Pig Farms from 2011 to 2021. Vet. Sci. 2024, 11, 496. https://doi.org/10.3390/vetsci11100496
Donneschi A, Recchia M, Romeo C, Pozzi P, Salogni C, Maisano AM, Santucci G, Scali F, Faccini S, Boniotti MB, et al. Infectious Agents Associated with Abortion Outbreaks in Italian Pig Farms from 2011 to 2021. Veterinary Sciences. 2024; 11(10):496. https://doi.org/10.3390/vetsci11100496
Chicago/Turabian StyleDonneschi, Anna, Matteo Recchia, Claudia Romeo, Paolo Pozzi, Cristian Salogni, Antonio Marco Maisano, Giovanni Santucci, Federico Scali, Silvia Faccini, Maria Beatrice Boniotti, and et al. 2024. "Infectious Agents Associated with Abortion Outbreaks in Italian Pig Farms from 2011 to 2021" Veterinary Sciences 11, no. 10: 496. https://doi.org/10.3390/vetsci11100496
APA StyleDonneschi, A., Recchia, M., Romeo, C., Pozzi, P., Salogni, C., Maisano, A. M., Santucci, G., Scali, F., Faccini, S., Boniotti, M. B., D’Incau, M., Maes, D., & Alborali, G. L. (2024). Infectious Agents Associated with Abortion Outbreaks in Italian Pig Farms from 2011 to 2021. Veterinary Sciences, 11(10), 496. https://doi.org/10.3390/vetsci11100496








