Description and Outcomes of an Ultrasound-Guided Technique for Catheter Placement in the Canine Quadratus Lumborum Plane: A Cadaveric Tomographic Study and Clinical Case Series
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. First Phase
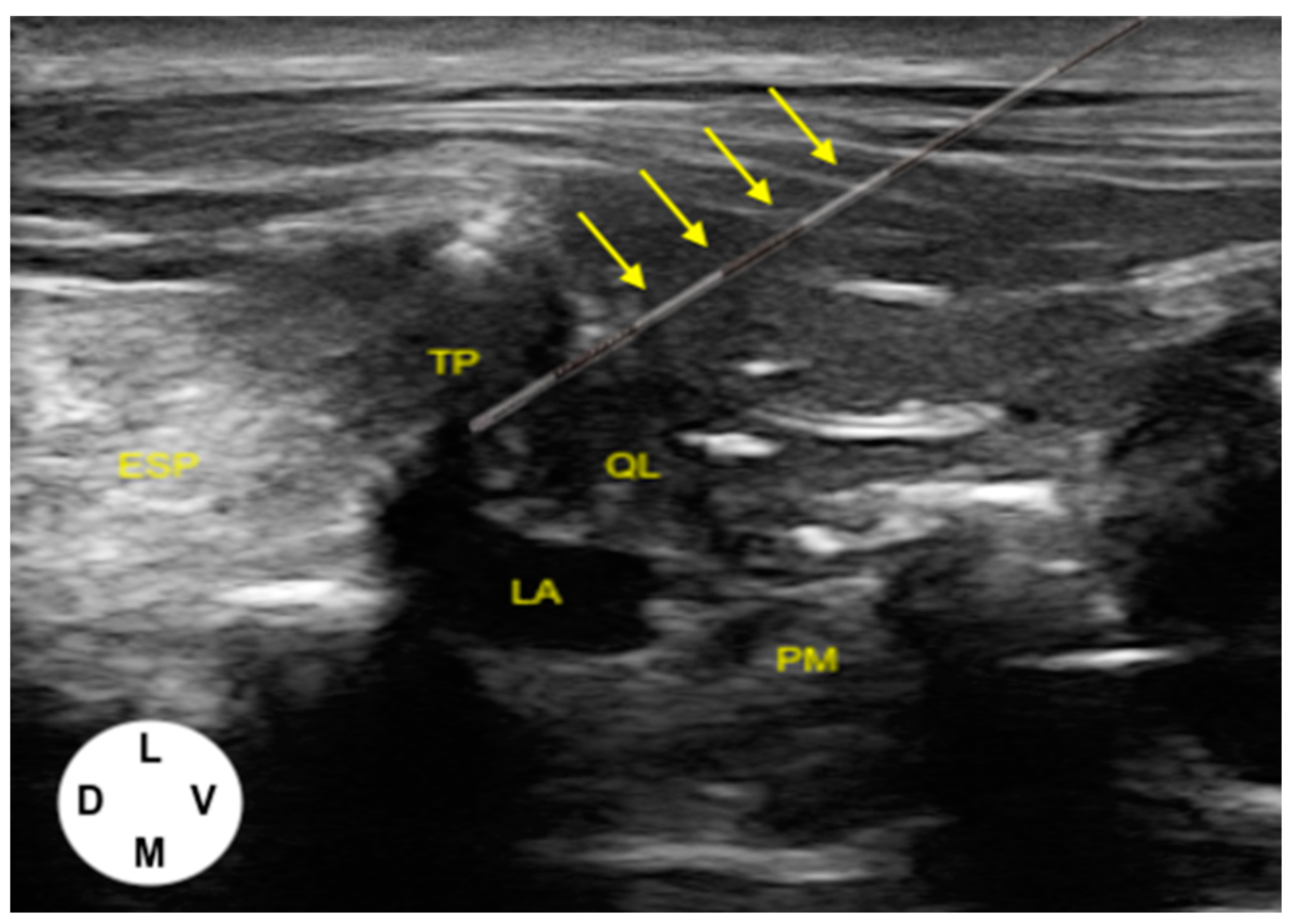
2.1.1. US-Guided Catheter Placement Technique
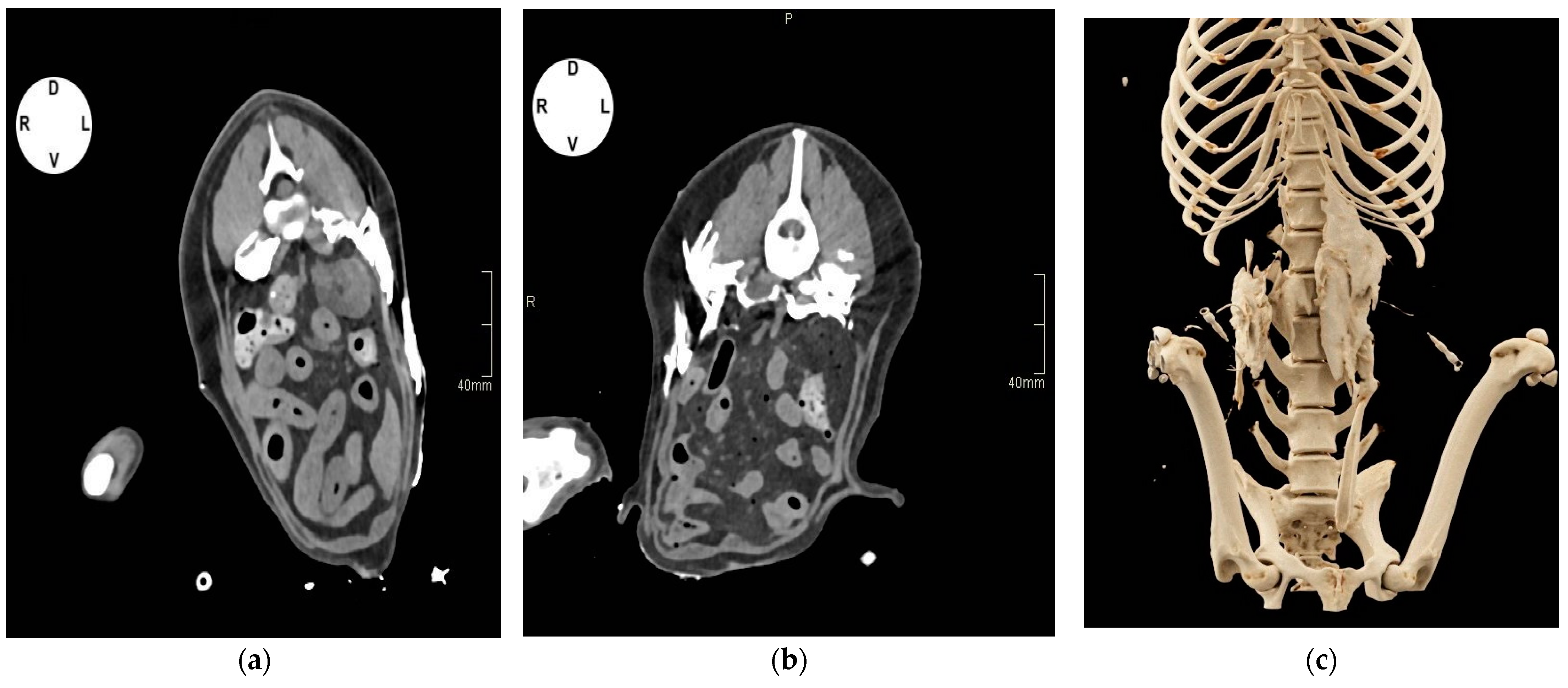
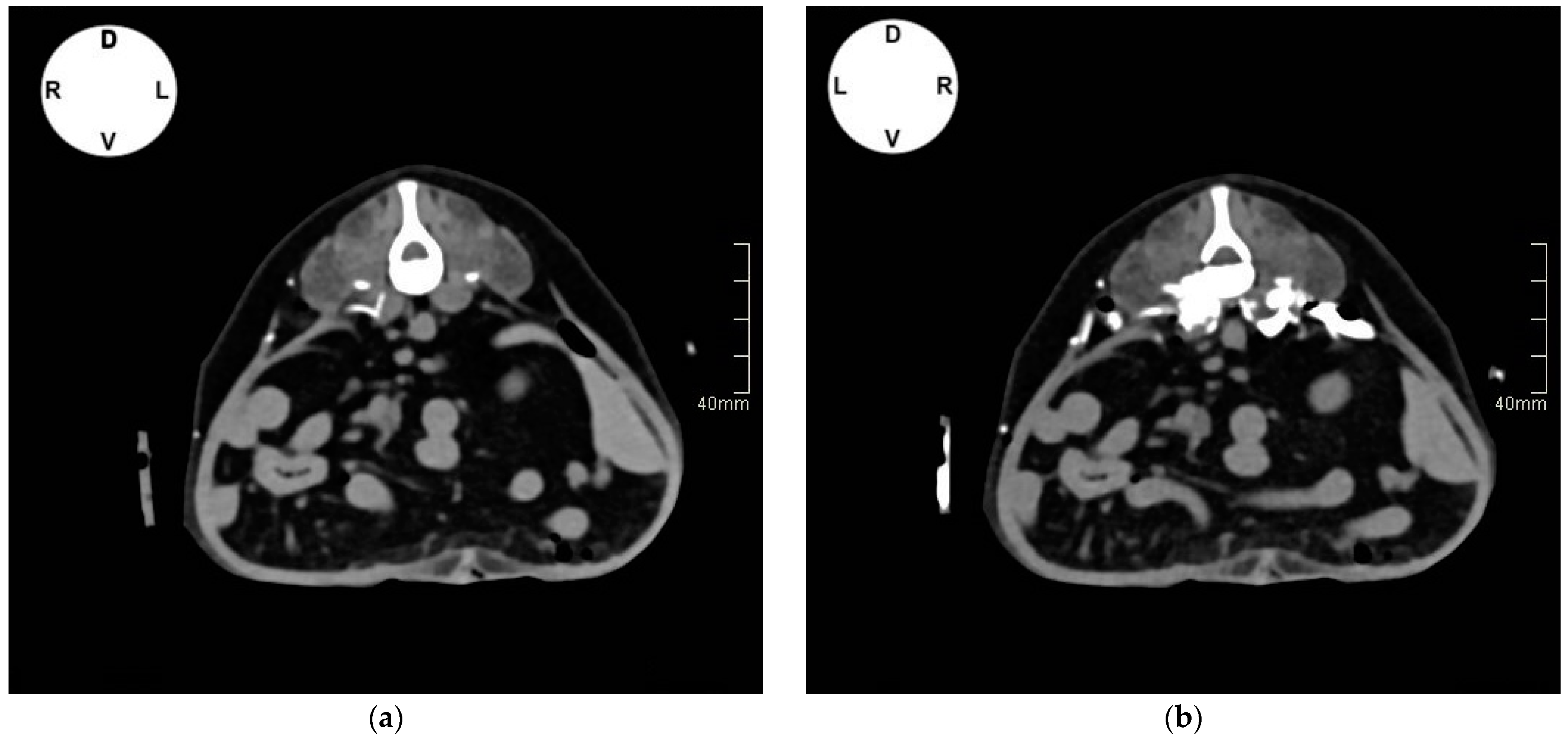
2.1.2. Tomographic Study
2.2. Second Phase
2.2.1. Cases Presentation
2.2.2. Catheter Placement and Postoperative Management
2.3. Statistical Analysis
3. Results
3.1. First Phase
3.1.1. US-Guided Catheter Placement
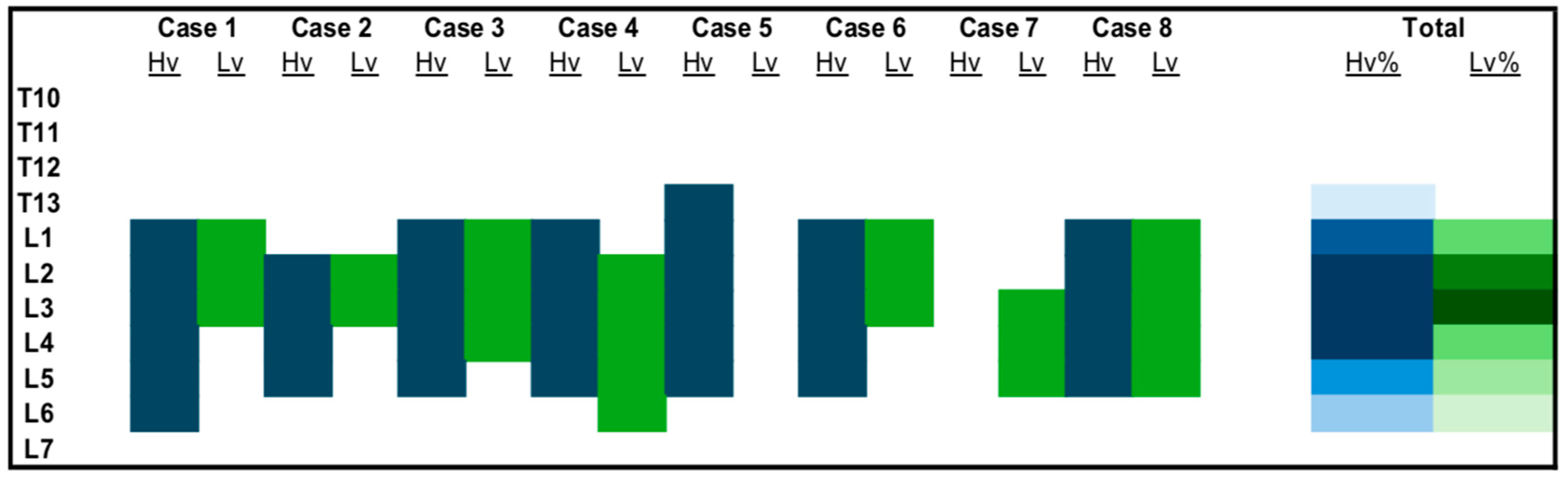
3.1.2. Tomographic Study
3.2. Second Phase
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garbin, M. Ultrasound-Guided Quadratus Lumborum Block. In Small Animal Regional Anesthesia and Analgesia, 2nd ed.; Reid, M., Campoy, L., Fischer, B., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2024; pp. 177–188. [Google Scholar]
- Akerman, M.; Pejčić, N.; Veličković, I. A Review of the Quadratus Lumborum Block and ERAS. Front. Med. 2018, 5, 44. [Google Scholar] [CrossRef] [PubMed]
- Uppal, V.; Retter, S.; Kehoe, E.; McKeen, D.M. Quadratus Lumborum Block for Postoperative Analgesia: A Systematic Review and Meta-Analysis. Can. J. Anaesth. 2020, 67, 1557–1575. [Google Scholar] [CrossRef] [PubMed]
- Viscasillas, J.; Sanchis-Mora, S.; Burillo, P.; Esteve, V.; Del Romero, A.; Lafuente, P.; Redondo, J.I. Evaluation of Quadratus Lumborum Block as Part of an Opioid-Free Anaesthesia for Canine Ovariohysterectomy. Animals 2021, 11, 3424. [Google Scholar] [CrossRef] [PubMed]
- Degani, M.; Di Franco, C.; Tayari, H.; Fages Carcéles, A.; Figà Talamanca, G.; Sandersen, C.; Briganti, A. Postoperative Analgesic Effect of Bilateral Quadratus Lumborum Block (QLB) for Canine Laparoscopic Ovariectomy: Comparison of Two Concentrations of Ropivacaine. Animals 2023, 13, 3604. [Google Scholar] [CrossRef]
- Dos-Santos, J.D.; Ginja, M.; Martins, J.; Cabral, P.; Alves-Pimenta, S.; Ribeiro, L.; Otero, P.E.; Colaço, B. Comparison between Bilateral Ultrasound-Guided Quadratus Lumborum Block and Sacrococcygeal Epidural in Cats Undergoing Ovariectomy. Vet. Sci. 2024, 11, 25. [Google Scholar] [CrossRef]
- Otero, P.E.; Fuensalida, S.E.; Tarragona, L.; Diaz, A.; Sanchez, M.F.; Micieli, F.; Waxman, S.; Zaccagnini, A.C.; Donati, P.A.; Portela, D.A. Ultrasound-Guided Caudal Quadratus Lumborum Block Combined with the Greater Ischiatic Notch Plane Block as Motor-Protective Analgesia for the Pelvic Limb in Dogs. Vet. Anaesth. Analg. 2024, 51, 97–106. [Google Scholar] [CrossRef]
- Otero, P.E.; Guerrero, J.A.; Tarragona, L.; Micieli, F.; Sanchez, M.F.; Donati, P.A.; Ceballos, M.R.; Portela, D.A. Ultrasound-Guided Greater Ischiatic Notch Plane Block Combined with the Caudal Quadratus Lumborum Block (GIN-TONIC Block) in Dogs Undergoing Pelvic Limb Surgery: Preliminary Results. Animals 2024, 14, 1764. [Google Scholar] [CrossRef]
- Paolini, A.; Bucci, R.; Bianchi, A.; Del Signore, F.; Parrillo, S.; Di Giosia, A.; Ristori, C.; Tamburro, R.; Robbe, D.; Carluccio, A.; et al. Use Of Caudal Quadratus Lumborum Block with Ropivacaine as Part of An Opioid-Free Analgesic Protocol in Dogs Undergoing Orchiectomy: A Randomized Trial. Animals 2024, 14, 1885. [Google Scholar] [CrossRef]
- Garbin, M.; Portela, D.A.; Bertolizio, G.; Gallastegui, A.; Otero, P.E. A Novel Ultrasound-Guided Lateral Quadratus Lumborum Block in Dogs: A Comparative Cadaveric Study of Two Approaches. Vet. Anaesth. Analg. 2020, 47, 810–818. [Google Scholar] [CrossRef]
- Kadam, V.R.; Howell, S. Ultrasound-Guided Continuous Transmuscular Quadratus Lumborum Block- L4 Or L2 Level Catheter Insertion for Analgesia in Open Abdominal Surgery: Case Series. Indian J. Anaesth. 2018, 62, 555–557. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, L.; Yang, Z.; Shen, J.; Zhu, R.; Wen, Y.; Cai, W.; Liu, L. Ultrasound guided continuous Quadratus Lumborum block hastened recovery in patients undergoing open liver resection: A randomized controlled, open-label trial. BMC Anesthesiol. 2019, 19, 23. [Google Scholar] [CrossRef]
- Pooley, R.; Veneziano, G.; Burrier, C.; Tram, N.K.; Tobias, J.D. Preliminary Experience with Quadratus Lumborum Catheters for Postoperative Pain Management in Pediatric-Aged Patients with Contraindications to Epidural Anesthesia. J. Clin. Med. Res. 2022, 14, 425–431. [Google Scholar] [CrossRef]
- Freitag, F.A.; Bozak, V.L.; Do Carmo, M.P.; Froes, T.R.; Duque, J.C. Continuous Transversus Abdominis Plane Block for Analgesia in Three Dogs with Abdominal Pain. Vet. Anaesth. Analg. 2018, 45, 581–583. [Google Scholar] [CrossRef]
- Camargo Fontanela, M.A.; Klaumann, P.R.; Piontkovsky, R.J.; Ferreira, P.S.; Montiani-Ferreira, F.; Duque Moreno, J.C. Preliminary Experience with Quadratus Lumborum Catheters for Intermittent Analgesia in Three Dogs with Acute Abdominal Pain. Vet. Anaesth, Analg. 2024, 51, 575–578. [Google Scholar] [CrossRef]
- LaFlamme, D.P. Development and validation of a Body Condition Score system for dogs. Canine Pract. 1997, 22, 10–15. [Google Scholar]
- Reid, J.; Nolan, A.; Hughes, J.; Lascelles, D.; Pawson, P.; Scott, E. Development of the short-form Glasgow Composite Measure Pain Scale (CMPS-SF) and Derivation of an Analgesic Intervention Score. Anim. Welf. 2007, 16, 97–104. [Google Scholar] [CrossRef]
- Garbin, M.; Portela, D.A.; Bertolizio, G.; Garcia-Pereira, F.; Gallastegui, A.; Otero, P.E. Description of ultrasound-guided quadratus lumborum block technique and evaluation of injectate spread in canine cadavers. Vet. Anaesth. Analg. 2020, 47, 249–258. [Google Scholar] [CrossRef]
- Marchina-Gonçalves, A.; Laredo, F.G.; Gil, F.; Soler, M.; Agut, A.; Redondo, J.I.; Belda, E. An Ultrasound-Guided Latero-Ventral Approach to Perform the Quadratus Lumborum Block in Dog Cadavers. Animals 2023, 13, 2214. [Google Scholar] [CrossRef]
- Alaman, M.; Bonastre, C.; de Blas, I.; Gomez-Alvarez, C.M.; Laborda, A. Description of a Novel Ultrasound-Guided Approach for a Dorsal Quadratus Lumborum Block: A Canine Cadaver Study. Vet. Anaesth. Analg. 2022, 49, 118–125. [Google Scholar] [CrossRef]
- Viscasillas, J.; Terrado, J.; Marti-Scharfhausen, R.; Castiñeiras, D.; Esteve, V.; Clancy, N.; Redondo, J.I. A Modified Approach for the Ultrasound-Guided Quadratus Lumborum Block in Dogs: A Cadaveric Study. Animals 2021, 11, 2945. [Google Scholar] [CrossRef]
- Marchina-Gonçalves, A.; Gil, F.; Laredo, F.G.; Soler, M.; Agut, A.; Belda, E. Evaluation of High-Volume Injections Using a Modified Dorsal Quadratus Lumborum Block Approach in Canine Cadavers. Animals 2022, 12, 18. [Google Scholar] [CrossRef]
- Dadure, C.; Bringuier, S.; Raux, O.; Rochette, A.; Troncin, R.; Canaud, N.; Lubrano-Lavadera, J.-F.; Capdevila, X. Continuous Peripheral Nerve Blocks for Postoperative Analgesia in Children: Feasibility and Side Effects in A Cohort Study of 339 catheters. Can. J. Anesth. 2009, 56, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Hattammaru, Y.; Mio, Y.; Hascilowicz, T.; Utsumi, I.; Murakami, Y.; Omi, S. Reduction of Leakage from Insertion Site During Continuous Femoral Nerve Block with Catheter-Through-Needle versus Catheter-Over-Needle Technique for Postoperative Analgesia after Total Knee Arthroplasty: A Randomized Controlled Trial. BMC Anesth. 2022, 22, 11. [Google Scholar] [CrossRef] [PubMed]
- Suksompong, S.; von Bormann, B.; Cheungpasitporn, W.; Thongprayoon, C.; Mao, M.A.; Phatharacharukul, S.; Aeddula, N.R. Regional Catheters for Postoperative Pain Control: Review and Observational Data. Anesth. Pain Med. 2020, 10, e99745. [Google Scholar] [CrossRef] [PubMed]
- Romano, M. Ultrasound-Guided Transversus Abdominis Plane (TAP) Block. In Small Animal Regional Anesthesia and Analgesia, 2nd ed.; Reid, M., Campoy, L., Fischer, B., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2024; pp. 189–202. [Google Scholar]
- Sambugaro, B.; De Gennaro, C.; Hattersley, R.D.; Vettorato, E. Extradural Anaesthesia-Analgesia in Dogs Undergoing Cholecystectomy: A Single Centre Retrospective Study. Front. Vet. Sci. 2022, 9, 966183. [Google Scholar] [CrossRef]
- Campoy, L. Development of Enhanced Recovery after Surgery (ERAS) Protocols in Veterinary Medicine through a One-Health Approach: The Role of Anesthesia and Locoregional Techniques. J. Am. Vet. Med. Assoc. 2022, 260, 1751–1759. [Google Scholar] [CrossRef]
- Merlin, T.; Veres-Nyéki, K. Anaesthetic Management and Complications of Canine Adrenalectomies: 41 cases (2007–2017). Acta Vet. Hung. 2019, 67, 282–295. [Google Scholar] [CrossRef]
- Martínez, I.; Ferré, B.; Bellido, V.M.; Viilmann, I.; Vettorato, E. Retrospective comparison between low-volume high-concentration and high-volume low-concentration levobupivacaine for bilateral erector spinae plane block in dogs undergoing hemilaminectomy. Vet. Anaes Analg. 2024, 51, 362–371. [Google Scholar] [CrossRef]

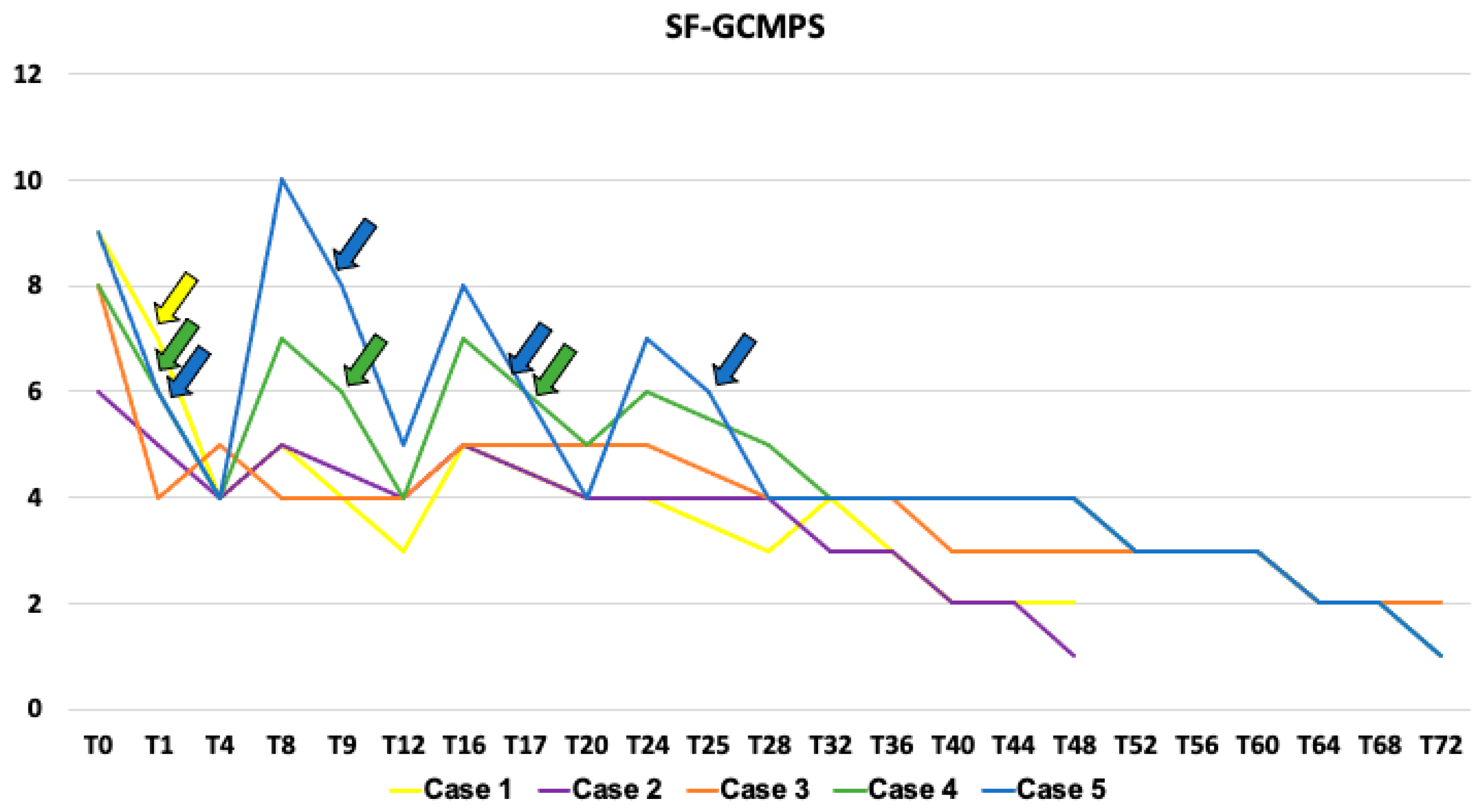
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | |
|---|---|---|---|---|---|
| Breed | Siberian Husky | Beagle | Chihuahua | Brittany Spaniel | Mixed breed |
| Sex | F | M | M | MC | MC |
| Age (years) | 6 | 8 | 11 | 9 | 11 |
| Weight (kg) | 23.5 | 29 | 4.6 | 18.3 | 13 |
| BCS | 5/9 | 6/9 | 5/9 | 5/9 | 4/9 |
| ASA status | 3 | 2 | 4 | 3 | 4 |
| Condition | Right-sided cortisol-secreting adenocarcinoma | Left-sided non-secreting adrenal mass | Emphysematous cholecystitis, pancreatitis | Right-sided phaeochromocytoma | Gallbladder mucocele, pancreatitis |
| Breed | Sex | Weight | BCS (Out of 9) |
|---|---|---|---|
| English Setter | Male castred | 23 | 5 |
| Border Collie | Female | 17 | 4 |
| Mixed breed | Male castred | 22 | 6 |
| Mixed breed | Female | 13 | 5 |
| American Amstaff | Male castred | 22 | 5 |
| Border Collie | Male castred | 25 | 6 |
| Jack Russell | Male castred | 6 | 4 |
| Siberian husky | Female | 25 | 5 |
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | |
|---|---|---|---|---|---|
| Surgery | Right-sided adrenalectomy | Left-sided adrenalectomy | Cholecystectomy | Right-sided adrenalectomy | Cholecystectomy |
| Premedication | Dexmedetomidine 1 μg kg−1, methadone 0.2 mg kg−1 IV | Dexmedetomidine CRI 1 μg kg−1 h−1, methadone 0.2 mg kg−1 IV | Maropitant 1 mg kg−1, methadone 0.2 mg kg−1 IV | Acepromazine 10 mcg kg−1 IM, methadone 0.2 mg kg−1 IV | Maropitant 1 mg kg−1, methadone 0.2 mg kg−1 IV |
| Induction | Propofol 2 mg kg−1 | Propofol 2 mg kg−1 IV | Ketamine 1 mg kg−1, propofol 1 mg kg−1 IV | Propofol 2 mg kg−1 IV | Midazolam 0.2 mg kg−1 Propofol 1.5 mg kg−1 IV |
| Maintenance | Isoflurane in an oxygen/air mixture | Isoflurane in an oxygen/air mixture | Isoflurane in an oxygen/air mixture | Isoflurane in an oxygen/air mixture | Isoflurane in an oxygen/air mixture |
| Intraoperative medication | Dexmedetomidine CRI 1 μg kg−1 h−1 IV | - | Fentanyl infusion 5–10 μg kg−1 h−1 IV | Fentanyl infusion 2–5 μg kg−1 h−1 IV | Ketamine 1 mg kg−1 IV |
| Postoperative medication | Prednisolone 0.5 mg kg−1 IV BID, cefazoline 20 mg kg−1 IV TID, and trazodone 4 mg kg−1 PO TID | Meloxicam 0.2 mg kg−1 IV SID, cefazoline 20 mg kg−1 IV TID | Meloxicam 0.2 mg kg−1 IV SID, amoxicilline clavulanic acid 20 mg kg−1 TID maropitant 1 mg kg−1 SID, ondasetron 0.5 mg kg−1 PO BID, pimobendan 0.5 mg kg−1 PO BID | Meloxicam 0.2 mg kg−1 IV SID, cefazoline 20 mg kg−1 IV TID, trazodone 4 mg kg−1 TID | Meloxicam 0.2 mg kg−1 IV SID, amoxicilline clavulanic acid 20 mg kg−1 TID, maropitant 1 mg kg−1 SID |
| Duration of anesthesia (minutes) | 360 | 255 | 90 | 240 | 120 |
| Duration of surgery (minutes) | 300 | 210 | 60 | 180 | 65 |
| TE − T0 (minutes) | 60 | 180 | 300 | 180 | 300 |
| Hospitalization time (hours) | 48 | 48 | 72 | 72 | 72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Degani, M.; Bolen, G.; Talarico, C.; Noël, S.; Gommeren, K.; Di Franco, C.; Sandersen, C. Description and Outcomes of an Ultrasound-Guided Technique for Catheter Placement in the Canine Quadratus Lumborum Plane: A Cadaveric Tomographic Study and Clinical Case Series. Vet. Sci. 2024, 11, 472. https://doi.org/10.3390/vetsci11100472
Degani M, Bolen G, Talarico C, Noël S, Gommeren K, Di Franco C, Sandersen C. Description and Outcomes of an Ultrasound-Guided Technique for Catheter Placement in the Canine Quadratus Lumborum Plane: A Cadaveric Tomographic Study and Clinical Case Series. Veterinary Sciences. 2024; 11(10):472. https://doi.org/10.3390/vetsci11100472
Chicago/Turabian StyleDegani, Massimiliano, Géraldine Bolen, Chiara Talarico, Stéphanie Noël, Kris Gommeren, Chiara Di Franco, and Charlotte Sandersen. 2024. "Description and Outcomes of an Ultrasound-Guided Technique for Catheter Placement in the Canine Quadratus Lumborum Plane: A Cadaveric Tomographic Study and Clinical Case Series" Veterinary Sciences 11, no. 10: 472. https://doi.org/10.3390/vetsci11100472
APA StyleDegani, M., Bolen, G., Talarico, C., Noël, S., Gommeren, K., Di Franco, C., & Sandersen, C. (2024). Description and Outcomes of an Ultrasound-Guided Technique for Catheter Placement in the Canine Quadratus Lumborum Plane: A Cadaveric Tomographic Study and Clinical Case Series. Veterinary Sciences, 11(10), 472. https://doi.org/10.3390/vetsci11100472







