Can Productive Aptitude and Age Affect Circulating Serotonin, Total Thyroid Hormones, and Cortisol Patterns in Cows?
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Analysis of Circulating 5-HT, THs, and Cortisol Concentrations
2.3. Statistical Analyses
3. Results
4. Discussion
- (1)
- Milk production aptitude (i.e., secretion type) was characterized by significantly lower T3 concentrations than meat production aptitude (i.e., accretion type) in cows;
- (2)
- There is a significant effect of age on T4 concentration, which decreases with advancing age both in secretion and accretion types;
- (3)
- A positive and significant correlation was recorded for T4 with T3 and 5-HT in the accretion type only.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghassemi Nejad, J.; Lee, B.H.; Kim, J.Y.; Chemere, B.; Sung, K.I.; Lee, H.G. Effect of alpine grazing on plasma and hair cortisol, serotonin, and DHEA in dairy cows and its welfare impact. Domest. Anim. Endocrinol. 2021, 75, 106581. [Google Scholar] [CrossRef] [PubMed]
- Bozzo, G.; Barrasso, R.; Marchetti, P.; Roma, R.; Samoilis, G.; Tantillo, G.; Ceci, E. Analysis of Stress Indicators for Evaluation of Animal Welfare and Meat Quality in Traditional and Jewish Slaughtering. Animals 2018, 8, 43. [Google Scholar] [CrossRef]
- Escalera-Valente, F.; Alonso, M.E.; Lomillos, J.M.; Gaudioso, V.R.; Alonso Á, J.; González-Montaña, J.R. Effect of Intense Exercise on Plasma Macrominerals and Trace Elements in Lidia Bulls. Vet. Sci. 2021, 8, 97. [Google Scholar] [CrossRef] [PubMed]
- Fazio, E.; Bionda, A.; Chiofalo, V.; La Fauci, D.; Randazzo, C.; Pino, A.; Crepaldi, P.; Attard, G.; Liotta, L.; Lopreiato, V. Effects of Dietary Enrichment with Olive Cake on the Thyroid and Adrenocortical Responses in Growing Beef Calves. Animals 2023, 13, 2120. [Google Scholar] [CrossRef]
- Bertrand, P.P.; Bertrand, R.L. Serotonin release and uptake in the gastrointestinal tract. Auton. Neurosci. 2010, 153, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Bruschetta, G.; Di Pietro, P.; Sanzarello, L.; Giacoppo, E.; Ferlazzo, A.M. Plasma serotonin levels in Italian Fresian dairy cows. Vet. Res. Commun. 2010, 34 (Suppl. S1), S17–S20. [Google Scholar] [CrossRef][Green Version]
- Barrell, G.K. An Appraisal of Methods for Measuring Welfare of Grazing Ruminants. Front. Vet. Sci. 2019, 6, 289. [Google Scholar] [CrossRef]
- Kanova, M.; Kohout, P. Serotonin-Its Synthesis and Roles in the Healthy and the Critically Ill. Int. J. Mol. Sci. 2021, 22, 4837. [Google Scholar] [CrossRef]
- Liu, N.; Sun, S.; Wang, P.; Sun, Y.; Hu, Q.; Wang, X. The Mechanism of Secretion and Metabolism of Gut-Derived 5-Hydroxytryptamine. Int. J. Mol. Sci. 2021, 22, 7931. [Google Scholar] [CrossRef]
- Nichols, D.E.; Nichols, C.D. Serotonin Receptors. Chem. Rev. 2008, 108, 1614–1641. [Google Scholar] [CrossRef]
- Connelly, M.K.; Weaver, S.R.; Kuehnl, J.M.; Fricke, H.P.; Klister, M.; Hernandez, L. Elevated serotonin coordinates mammary metabolism in dairy cows. Physiol. Rep. 2021, 9, e14798. [Google Scholar] [CrossRef] [PubMed]
- Kessler, E.C.; Wall, S.K.; Hernandez, L.L.; Bruckmaier, R.M.; Gross, J.J. Short communication: Circulating serotonin is related to the metabolic status and lactational performance at the onset of lactation in dairy cows. J. Dairy Sci. 2018, 101, 11455–11460. [Google Scholar] [CrossRef] [PubMed]
- Laporta, J.; Moore, S.A.; Weaver, S.R.; Cronick, C.M.; Olsen, M.; Prichard, A.P.; Schnell, B.P.; Crenshaw, T.D.; Peñagaricano, F.; Bruckmaier, R.M.; et al. Increasing serotonin concentrations alter calcium and energy metabolism in dairy cows. J. Endocrinol. 2015, 226, 43–55. [Google Scholar] [CrossRef]
- Slater, C.J.; Endres, E.L.; Weaver, S.R.; Cheng, A.A.; Lauber, M.R.; Endres, S.F.; Olstad, E.; DeBruin, A.; Crump, P.M.; Block, E.; et al. Interaction of 5-hydroxy-l-tryptophan and negative dietary cation-anion difference on calcium homeostasis in multiparous peripartum dairy cows. J. Dairy Sci. 2018, 101, 5486–5501. [Google Scholar] [CrossRef]
- Gershon, M.D.; Tack, J. The serotonin signaling system: From basic understanding to drug development for functional GI disorders. Gastroenterology 2007, 132, 397–414. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.R.; Jury, N.J.; Gregerson, K.A.; Horseman, N.D.; Hernandez, L.L. Characterization of mammary-specific disruptions for Tph1 and Lrp5 during murine lactation. Sci. Rep. 2017, 7, 15155. [Google Scholar] [CrossRef]
- Fazio, E.; Bionda, A.; Chiofalo, V.; Crepaldi, P.; Lopreiato, V.; Medica, P.; Liotta, L. Adaptive Responses of Thyroid Hormones, Insulin, and Glucose during Pregnancy and Lactation in Dairy Cows. Animals 2022, 12, 1395. [Google Scholar] [CrossRef]
- Pucci, E.; Chiovato, L.; Pinchera, A. Thyroid and lipid metabolism. Int. J. Obes. Relat. Metab. Disord. 2000, 24 (Suppl. S2), S109–S112. [Google Scholar] [CrossRef] [PubMed]
- Capuco, A.V.; Wood, D.L.; Elsasser, T.H.; Kahl, S.; Erdman, R.A.; Van Tassell, C.P.; Lefcourt, A.; Piperova, L.S. Effect of somatotropin on thyroid hormones and cytokines in lactating dairy cows during ad libitum and restricted feed intake. J. Dairy Sci. 2001, 84, 2430–2439. [Google Scholar] [CrossRef]
- Sinka, K.; Illek, J.; Kumprechtová, D.; Novák, P. Changes T3 and T4 plasma concentrations in dairy cows during lactation. In Proceedings of the 25th Jubilee World Buiatrics Congress, Budapest, Hungary, 11 July 2008. [Google Scholar]
- Steinhoff, L.; Jung, K.; Meyerholz, M.; Heidekorn-Dettmer, J.; Hoedemaker, M.; Schmicke, M.J.T. Thyroid hormone profiles and TSH evaluation during early pregnancy and the transition period in dairy cows. Theriogenology 2019, 129, 23–28. [Google Scholar] [CrossRef]
- Stojić, V.; Gvozdić, D.; Kirovski, D.; Nikolic-Ana, J.; Huszenicza, G.; Šamanc, H.; Ivanov, I. Serum thyroxine and triiodothyronine concentrations prior to and after delivery in primiparous Holstein cows. Acta Vet. Beogr. 2001, 51, 3–8. [Google Scholar]
- Kulcsár-Huszenicza, M.; Rudas, P. Clinical endocrinology of thyroid gland function in ruminants. Vet. Med. 2002, 47, 199–210. [Google Scholar] [CrossRef]
- Pethes, G.; Bokori, J.; Rudas, P.; Frenyó, V.L.; Fekete, S. Thyroxine, triiodothyronine, reverse-triiodothyronine, and other physiological characteristics of periparturient cows fed restricted energy. J. Dairy Sci. 1985, 68, 1148–1154. [Google Scholar] [CrossRef] [PubMed]
- Tiirats, T. Thyroxine, triiodothyronine and reverse-triiodothyronine concentrations in blood plasma in relation to lactational stage, milk yield, energy and dietary protein intake in Estonian dairy cows. Acta Vet. Scand. 1997, 38, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, S.; Salgado Nunez Del Prado, S.; Celi, F.S. Thyroid Hormone Action and Energy Expenditure. J. Endocr. Soc. 2019, 3, 1345–1356. [Google Scholar] [CrossRef]
- Waterson, M.J.; Horvath, T.L. Neuronal Regulation of Energy Homeostasis: Beyond the Hypothalamus and Feeding. Cell Metab. 2015, 22, 962–970. [Google Scholar] [CrossRef]
- Bellmann, O.; Wegner, J.; Rehfeldt, C.; Teuscher, F.; Schneider, F.; Voigt, J.; Derno, M.; Sauerwein, H.; Weingärtner, J.; Ender, K.J.L.P.S. Beef versus dairy cattle: A comparison of metabolically relevant hormones, enzymes, and metabolites. Livest. Prod. Sci. 2004, 89, 41–54. [Google Scholar] [CrossRef]
- Holodova, L.; Novoselova, K.; Mikhalev, E.; Onegov, A.; Chirgin, E. The effect of age on milk productivity and reproductive qualities of dairy cows. IOP Conf. Ser. Earth Environ. Sci. 2019, 315, 022087. [Google Scholar] [CrossRef]
- Bruschetta, G.; Zanghì, G.; Giunta, R.P.; Ferlazzo, A.M.; Satué, K.; D’Ascola, A.; Fazio, E. Short Road Transport and Slaughter Stress Affects the Expression Profile of Serotonin Receptors, Adrenocortical, and Hematochemical Responses in Horses. Vet. Sci. 2024, 11, 113. [Google Scholar] [CrossRef]
- Medica, P.; Giunta, R.P.; Bruschetta, G.; Ferlazzo, A.M. The Influence of Training and Simulated Race on Horse Plasma Serotonin Levels. J. Equine Vet. Sci. 2020, 84, 102818. [Google Scholar] [CrossRef]
- Kollmann, M.T.; Locher, M.; Hirche, F.; Eder, K.; Meyer, H.H.; Bruckmaier, R.M. Effects of tryptophan supplementation on plasma tryptophan and related hormone levels in heifers and dairy cows. Dom. Anim. Endocrinol. 2008, 34, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.R.; Nixon, D.A.; Akasha, M.A. Total and free thyroxine and triiodothyronine in blood serum of mammals. Comp. Biochem. Physiol. A Comp. Physiol. 1988, 89, 401–404. [Google Scholar] [CrossRef] [PubMed]
- De Moraes, G.V.; Vera-Avila, H.R.; Lewis, A.W.; Koch, J.W.; Neuendorff, D.A.; Hallford, D.M.; Reeves, J.J.; Randel, R.D. Influence of hypo- or hyperthyroidism on ovarian function in Brahman cows. J. Anim. Sci. 1998, 76, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Mutinati, M.; Rizzo, A.; Sciorsci, R.L. Cystic ovarian follicles and thyroid activity in the dairy cow. Anim. Reprod. Sci. 2013, 138, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Nixon, D.A.; Akasha, M.A.; Anderson, R.R. Free and Total Thyroid Hormones in Serum of Holstein Cows1. J. Dairy Sci. 1988, 71, 1152–1160. [Google Scholar] [CrossRef]
- Paulíková, I.; Seidel, H.; Nagy, O.; Tothova, C.; Konvičná, J.; Kadaši, M.; Kováč, G.J.F.V. Thyroid hormones, insulin, body fat, and blood biochemistry indices in dairy cows during the reproduction/production cycle. Folia Vet. 2017, 61, 43–53. [Google Scholar] [CrossRef]
- Razavi, M.; Moghaddas, B.; Rakhshande, E.; Nazifi, S. Bovine Theileriosis: Effects on the Status of Thyroid Hormones, Homocystein, Serum Lipids and Lipoproteins. Res. J. Parasitol. 2015, 10, 151–159. [Google Scholar] [CrossRef][Green Version]
- Ferlazzo, A.M.; Bruschetta, G.; Di Pietro, P.; Medica, P. The influence of age on plasma serotonin levels in Thoroughbred horses. Livest. Sci. 2012, 147, 203–207. [Google Scholar] [CrossRef]
- Fink, C.; Tatar, M.; Failing, K.; Hospes, R.; Kressin, M.; Klisch, K. Serotonin-containing cells in the gastrointestinal tract of newborn foals and adult horses. Anat. Histol. Embryol. 2006, 35, 23–27. [Google Scholar] [CrossRef]
- Fazio, E.; Medica, P.; Cravana, C.; Messineo, C.; Ferlazzo, A. Total and free iodothyronine levels of growing Thoroughbred foals: Effects of weaning and gender. Livest. Sci. 2007, 110, 207–213. [Google Scholar] [CrossRef]
- De Stefano, M.A.; Ambrosio, R.; Porcelli, T.; Orlandino, G.; Salvatore, D.; Luongo, C. Thyroid Hormone Action in Muscle Atrophy. Metabolites 2021, 11, 730. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.J.; Seitz, H.J. Thyroid hormone action on intermediary metabolism. Part III. Protein metabolism in hyper- and hypothyroidism. Klin. Wochenschr. 1984, 62, 97–102. [Google Scholar] [CrossRef]
- Mooradian, A.D. Age-Related Resistance to Thyroid Hormone Action. Drugs Aging 2019, 36, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Aguayo-Mazzucato, C.; Zavacki, A.M.; Marinelarena, A.; Hollister-Lock, J.; El Khattabi, I.; Marsili, A.; Weir, G.C.; Sharma, A.; Larsen, P.R.; Bonner-Weir, S. Thyroid hormone promotes postnatal rat pancreatic β-cell development and glucose-responsive insulin secretion through MAFA. Diabetes 2013, 62, 1569–1580. [Google Scholar] [CrossRef] [PubMed]
- Bossaert, P.; Leroy, J.L.; De Vliegher, S.; Opsomer, G. Interrelations between glucose-induced insulin response, metabolic indicators, and time of first ovulation in high-yielding dairy cows. J. Dairy Sci. 2008, 91, 3363–3371. [Google Scholar] [CrossRef]
- Fowden, A.L.; Mapstone, J.; Forhead, A.J. Regulation of glucogenesis by thyroid hormones in fetal sheep during late gestation. J. Endocrinol. 2001, 170, 461–469. [Google Scholar] [CrossRef]
- Oppenheimer, J.H.; Schwartz, H.L.; Lane, J.T.; Thompson, M.P. Functional relationship of thyroid hormone-induced lipogenesis, lipolysis, and thermogenesis in the rat. J. Clin. Investig. 1991, 87, 125–132. [Google Scholar] [CrossRef]
- Brito, M.; González, F.; Ribeiro, L.; Campos, R.; Lacerda, L.; Barbosa, P.; Bergmann, G. Blood and milk composition in dairy ewes from southern Brazil: Variations during pregnancy and lactation. Ciênc. Rural 2006, 36, 942–948. [Google Scholar] [CrossRef]
- Gerloff, B.J.; Herdt, T.H.; Wells, W.W.; Nachreiner, R.F.; Emery, R.S. Inositol and hepatic lipidosis. II. Effect of inositol supplementation and time from parturition on serum insulin, thyroxine and triiodothyronine and their relationship to serum and liver lipids in dairy cows. J. Anim. Sci. 1986, 62, 1693–1702. [Google Scholar] [CrossRef]
- Blum, J.W. Endocrinology and animal production. Schweiz. Arch. Tierheilkd. 1983, 125, 827–850. [Google Scholar]
- Gross, J.J.; Bruckmaier, R.M. Invited review: Metabolic challenges and adaptation during different functional stages of the mammary gland in dairy cows: Perspectives for sustainable milk production. J. Dairy Sci. 2019, 102, 2828–2843. [Google Scholar] [CrossRef] [PubMed]
- Fiore, E.; Piccione, G.; Gianesella, M.; Praticò, V.; Vazzana, I.; Dara, S.; Morgante, M. Serum thyroid hormone evaluation during transition periods in dairy cows. Arch. Anim. Breed. 2015, 58, 403–406. [Google Scholar] [CrossRef]
- Gueorguiev, I.P. Thyroxine and triiodothyronine concentrations during lactation in dairy cows Autore. Ann. Zootech. 1999, 48, 477–480. [Google Scholar] [CrossRef][Green Version]
- Pezzi, C.; Accorsi, P.A.; Vigo, D.; Govoni, N.; Gaiani, R. 5’-deiodinase activity and circulating thyronines in lactating cows. J. Dairy Sci. 2003, 86, 152–158. [Google Scholar] [CrossRef]
- Bauer, M.; Heinz, A.; Whybrow, P.C. Thyroid hormones, serotonin and mood: Of synergy and significance in the adult brain. Mol. Psychiatry 2002, 7, 140–156. [Google Scholar] [CrossRef]
- Hayashi, H.; Arai, C.; Ikeuchi, Y.; Yamanaka, M.; Hirayama, T. Effect of growth and parturition on hair cortisol in Holstein cattle. Anim. Sci. J. 2021, 92, e13518. [Google Scholar] [CrossRef]
- Svennersten-Sjaunja, K.; Olsson, K. Endocrinology of milk production. Domest. Anim. Endocrinol. 2005, 29, 241–258. [Google Scholar] [CrossRef]
- Braun, T.P.; Marks, D.L. The regulation of muscle mass by endogenous glucocorticoids. Front. Physiol. 2015, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Peters, R.R.; Kohn, R.A. Effect of a Transition Diet on Production Performance and Metabolism in Periparturient Dairy Cows. J. Dairy Sci. 2007, 90, 5247–5258. [Google Scholar] [CrossRef]
- Gladden, N.; McKeegan, D.; Viora, L.; Ellis, K.A. Postpartum ketoprofen treatment does not alter stress biomarkers in cows and calves experiencing assisted and unassisted parturition: A randomised controlled trial. Vet. Rec. 2018, 183, 414. [Google Scholar] [CrossRef]
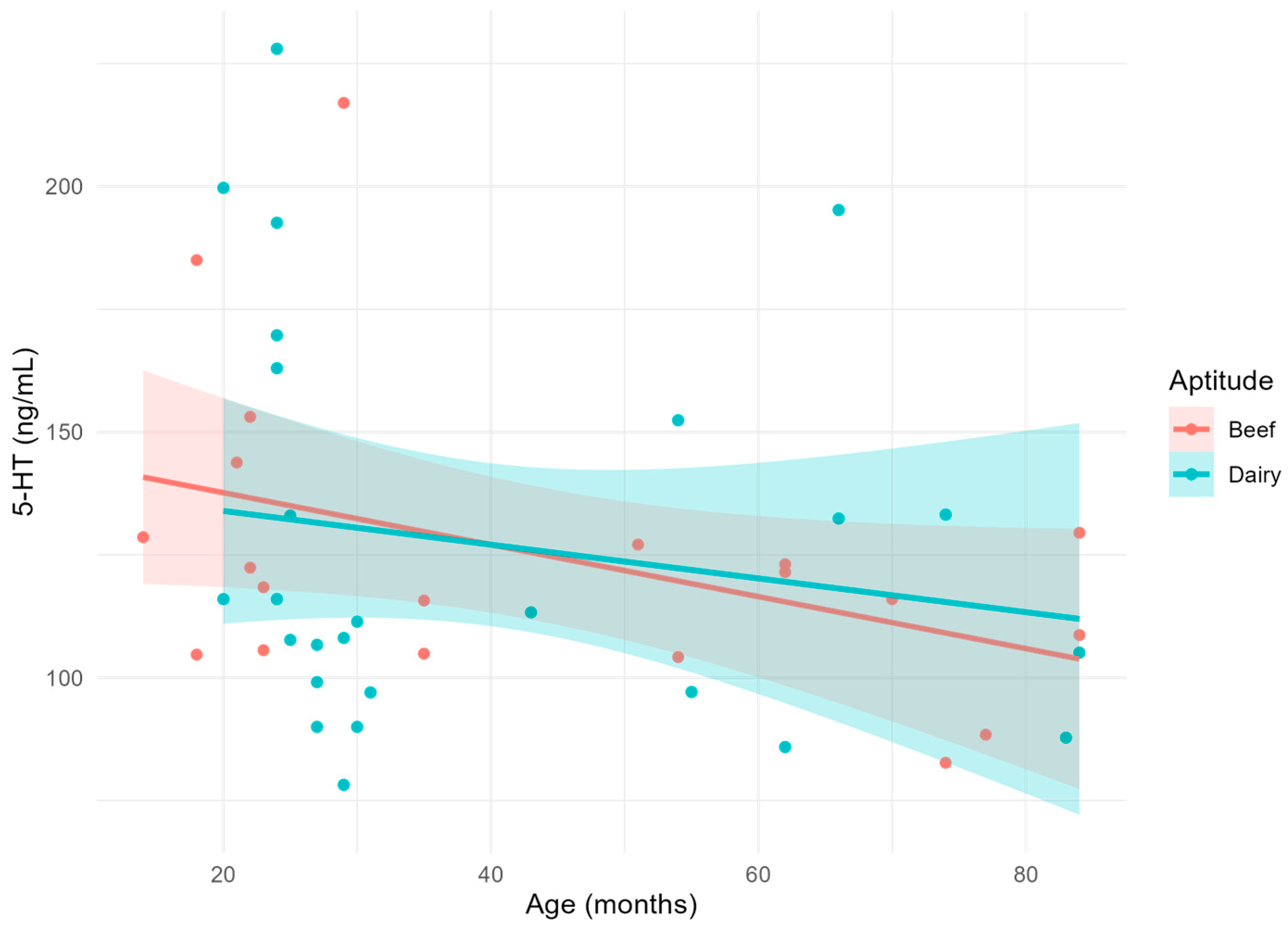


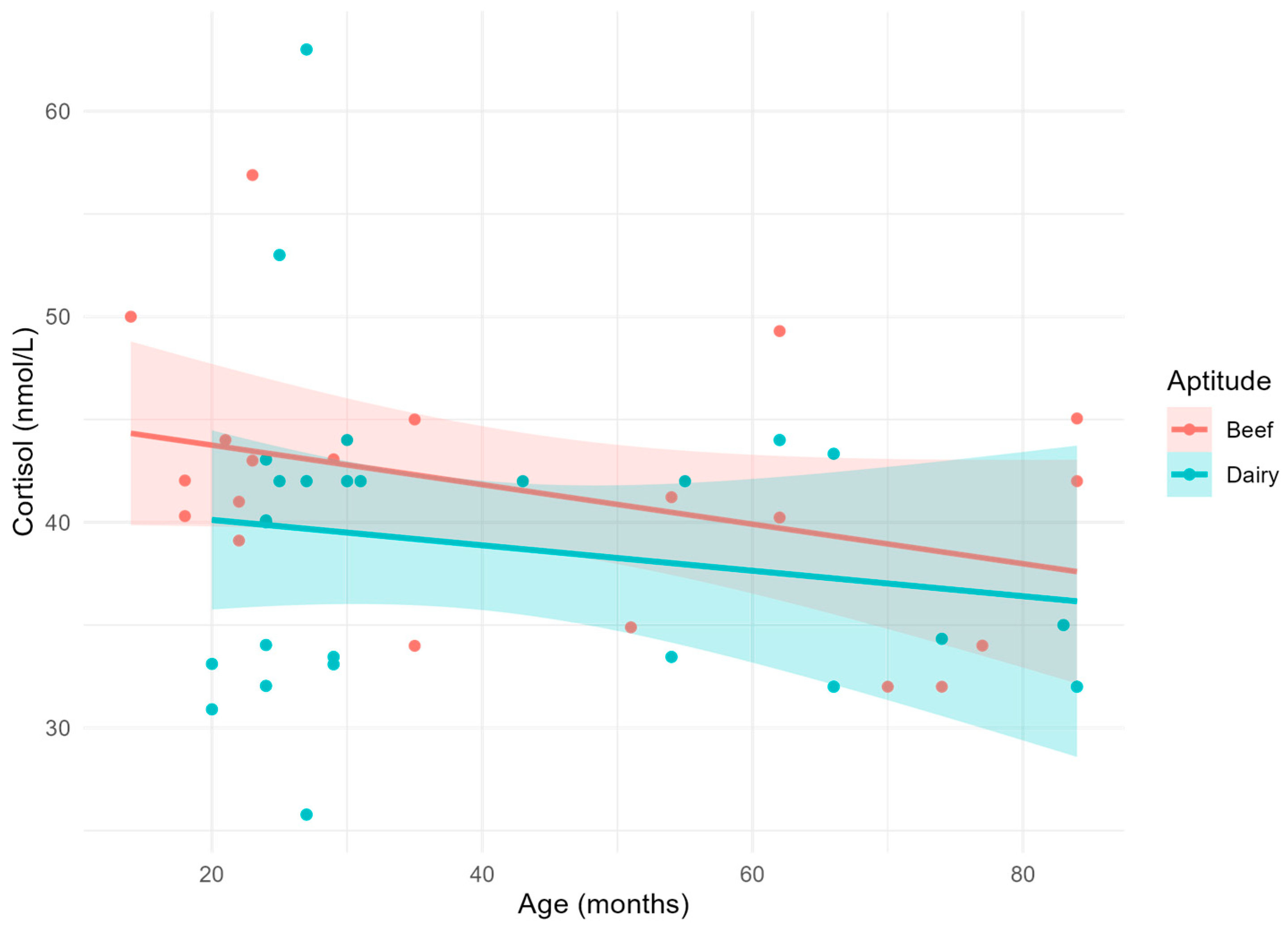
| Parameter | Age (months) | Aptitude | Age × Aptitude | R2 |
|---|---|---|---|---|
| 5-HT (ng/mL) | 0.0724 | 0.8434 | 0.6348 | 0.081 |
| T3 (nmol/L) | 0.1199 | 0.0421 | 0.9946 | 0.131 |
| T4 (nmol/L) | 0.0008 | 0.1095 | 0.6823 | 0.262 |
| Cortisol (nmol/L) | 0.1114 | 0.1324 | 0.6463 | 0.103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruschetta, G.; Bionda, A.; Giunta, R.P.; Costa, G.L.; Fazio, E.; Licata, P.; Bruno, F. Can Productive Aptitude and Age Affect Circulating Serotonin, Total Thyroid Hormones, and Cortisol Patterns in Cows? Vet. Sci. 2024, 11, 471. https://doi.org/10.3390/vetsci11100471
Bruschetta G, Bionda A, Giunta RP, Costa GL, Fazio E, Licata P, Bruno F. Can Productive Aptitude and Age Affect Circulating Serotonin, Total Thyroid Hormones, and Cortisol Patterns in Cows? Veterinary Sciences. 2024; 11(10):471. https://doi.org/10.3390/vetsci11100471
Chicago/Turabian StyleBruschetta, Giuseppe, Arianna Bionda, Renato Paolo Giunta, Giovanna Lucrezia Costa, Esterina Fazio, Patrizia Licata, and Fabio Bruno. 2024. "Can Productive Aptitude and Age Affect Circulating Serotonin, Total Thyroid Hormones, and Cortisol Patterns in Cows?" Veterinary Sciences 11, no. 10: 471. https://doi.org/10.3390/vetsci11100471
APA StyleBruschetta, G., Bionda, A., Giunta, R. P., Costa, G. L., Fazio, E., Licata, P., & Bruno, F. (2024). Can Productive Aptitude and Age Affect Circulating Serotonin, Total Thyroid Hormones, and Cortisol Patterns in Cows? Veterinary Sciences, 11(10), 471. https://doi.org/10.3390/vetsci11100471







