Germinated Wheat as a Potential Natural Source of Antioxidants to Improve Sperm Quality: A Canary Trial
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Preparation of Wheat Germination
2.3. Determination of Antioxidant Activity of Germinated Wheat
2.4. Experimental Plan and Feeding of Canaries
2.5. Collecting Sperm from Canaries
2.6. The Evaluation of Spermatological Parameters
2.7. Statistical Analysis
3. Results
3.1. Antioxidant Activity of Germinated Wheats
3.2. Effects on the Spermatological Parameters of Germinated Wheat
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mora, A.R.; Firth, A.; Blareau, S.; Vallat, A.; Helfenstein, F. Oxidative stress affects sperm performance and ejaculate redox status in subordinate house sparrows. J. Exp. Biol. 2017, 220, 2577–2588. [Google Scholar] [PubMed]
- Bansal, A.K. Antioxidants and other potent strategies to reduce oxidative stress in semen. In Free Radicals in Human Health and Disease; Springer: New Delhi, India, 2015; pp. 381–395. [Google Scholar] [CrossRef]
- Pintus, E.; Ros-Santaella, J.L. Impact of oxidative stress on male reproduction in domestic and wild animals. Antioxidants 2021, 10, 1154. [Google Scholar] [CrossRef] [PubMed]
- Özcan, M.E.; Gulec, M.; Ozerol, E.; Polat, R.; Akyol, O. Antioxidant enzyme activities and oxidative stress in affective disorders. Int. Clin. Psychopharmacol. 2004, 19, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Sabeti, P.; Pourmasumi, S.; Rahiminia, T.; Akyash, F.; Talebi, A.R. Etiologies of sperm oxidative stress. Int. J. Reprod. Biomed. 2016, 14, 231. [Google Scholar] [CrossRef]
- Karabulut, H.; Gülay, M.Ş. Antioksidanlar. MAEU Vet. Fak. Derg. 2016, 1, 65–76. [Google Scholar] [CrossRef]
- Khan, R.U. Antioxidants and poultry semen quality. World’s Poult. Sci. J. 2011, 67, 297–308. [Google Scholar] [CrossRef]
- Banday, M.N.; Lone, F.A.; Rasool, F.; Rashid, M.; Shikari, A. Use of antioxidants reduce lipid peroxidation and improve quality of crossbred ram sperm during its cryopreservation. Cryobiology 2017, 74, 25–30. [Google Scholar] [CrossRef]
- Augustyniak, A.; Bartosz, G.; Čipak, A.; Duburs, G.; Horáková, L.U.; Łuczaj, W.; Majekova, M.; Odysseos, A.D.; Rackova, L.; Skrzydlewska, E.; et al. Natural and synthetic antioxidants: An updated overview. Free Radic. Res. 2010, 44, 1216–1262. [Google Scholar] [CrossRef]
- Atta, E.M.; Mohamed, N.H.; Abdelgawad, A.A. Antioxidants: An overview on the natural and synthetic types. Eur. Chem. Bull. 2017, 6, 365–375. [Google Scholar] [CrossRef]
- Yanishlieva, N.V.; Marinova, E.; Pokorný, J. Natural antioxidants from herbs and spices. Eur. J. Lipid Sci. Technol. 2006, 108, 776–793. [Google Scholar] [CrossRef]
- Kumar, Y.; Yadav, D.N.; Ahmad, T.; Narsaiah, K. Recent trends in the use of natural antioxidants for meat and meat products. Compr. Rev. Food Sci. Food Saf. 2015, 14, 796–812. [Google Scholar] [CrossRef]
- Cheng, G.; Hao, H.; Xie, S.; Wang, X.; Dai, M.; Huang, L.; Yuan, Z. Antibiotic alternatives: The substitution of antibiotics in animal husbandry? Front. Microbiol. 2014, 5, 217. [Google Scholar] [CrossRef] [PubMed]
- Ukrainets, A.I.; Pasichniy, V.M.; Zheludenko, Y.V. Antioxidant plant extracts in the meat processing industry. Biotechnol. Acta 2016, 9, 19–27. [Google Scholar] [CrossRef]
- Crozier, A.; Clifford, M.N.; Ashihara, H. Plant Secondary Metabolites: Occurrence, Structure and Role in the Human Diet; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Tiwari, R.; Rana, C.S. Plant secondary metabolites: A review. Int. J. Eng. Res. Gen. Sci. 2015, 3, 661–670. [Google Scholar] [CrossRef]
- Daghigh-Kia, H.; Olfati-Karaji, R.; Hoseinkhani, A.; Ashrafi, I. Effect of rosemary (Rosmarinus officinalis) extracts and glutathione antioxidants on bull semen quality after cryopreservation. Span. J. Agric. Res. 2014, 12, 98–105. [Google Scholar] [CrossRef]
- Mata-Campuzano, M.; Álvarez-Rodríguez, M.; Tamayo-Canul, J.; López-Urueña, E.; de Paz, P.; Anel, L.; Martínez-Pastor, F.; Álvarez, M. Refrigerated storage of ram sperm in presence of Trolox and GSH antioxidants: Effect of temperature, extender and storage time. Anim. Reprod. Sci. 2014, 151, 137–147. [Google Scholar] [CrossRef]
- Martantiningtyas, D.C.; Nurliani, A.; Rusmiati, R. Antioxidant effect of ethanol extract of bulbus dayak onion (Eleutherine americana) to the quality of spermatozoa exposed by cigarette smoke in rats (Rattus norvegicus). Indones. J. Vet. Sci. 2015, 33, 131253. [Google Scholar]
- Partyka, A.; Rodak, O.; Bajzert, J.; Kochan, J.; Niżański, W. The effect of L-carnitine, hypotaurine, and taurine supplementation on the quality of cryopreserved chicken semen. Biomed. Res. Int. 2017, 2017, 7279341. [Google Scholar] [CrossRef]
- Sarbishegi, M.; Gorgich, E.A.C.; Khajavi, O. Olive leaves extract improved sperm quality and antioxidant status in the testis of rat exposed to rotenone. Nephro-Urol. Mon. 2017, 9, e47127. [Google Scholar] [CrossRef]
- Yuniarifa, C.; Wibowo, J.W.; Nasihun, T. Effect of four antioxidants combination on number of sertoli and leydig cells, sperm quality and caspase-3 expression in rat exposed to cigarette smoke. Sains Med. 2021, 11, 81–88. [Google Scholar] [CrossRef]
- Zielinski, H.; Ciska, E.; Kozlowska, H. The cereal grains: Focus on vitamin E. Czech J. Food Sci. 2001, 19, 182–188. [Google Scholar] [CrossRef]
- Kılınçer, F.N.; Demir, M.K. Çimlendirilmiş bazı tahıl ve baklagillerin fiziksel ve kimyasal özellikleri. Gida 2019, 44, 419–429. [Google Scholar] [CrossRef]
- Kim, M.J.; Kwak, H.S.; Kim, S.S. Effects of germination on protein, γ-aminobutyric acid, phenolic acids, and antioxidant capacity in wheat. Molecules 2018, 23, 2244. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, B.; Choudhary, G.; Sodhi, N.S. A study on physicochemical, antioxidant and microbial properties of germinated wheat flour and its utilization in breads. J. Food Sci. Tech. 2020, 57, 2800–2808. [Google Scholar] [CrossRef] [PubMed]
- Miyahira, R.F.; Lopes, J.D.O.; Antunes, A.E.C. The use of sprouts to improve the nutritional value of food products: A brief review. Plant Foods Hum. Nutr. 2021, 76, 143–152. [Google Scholar] [CrossRef]
- Adibmoradi, M.; Morovvati, H.; Moradi, H.R.; Sheybani, M.T.; Amoli, J.S.; Mazaheri Nezhad Fard, R. Protective effects of wheat sprout on testicular toxicity in male rats exposed to lead. Reprod. Syst. Sex. Disord. 2015, 4, 156. [Google Scholar] [CrossRef]
- Morovvati, H.; Adibmoradi, M.; Sheybani, M.T.; Salar Amoli, J.S.A. Effects of Wheat Sprout Extract on the Quality of Sperm in Rats Exposed to Lead. Iran. Vet. J. 2016, 12, 76–85. [Google Scholar]
- Fallas-López, M.; Rodríguez-De Lara, R.; Bárcena-Gama, R.; Esqueda, M.S.T.; Hernández-Sánchez, D.; Martínez-Hernández, P.A.; Aguilar-Romero, O. Rabbit sexual behavior, semen and sperm characteristics when supplemented with sprouted wheat. Anim. Reprod. Sci. 2011, 129, 221–228. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 26, 1199–1200. [Google Scholar] [CrossRef]
- Sudha, G.; Priya, M.S.; Shree, R.I.; Vadivukkarasi, S. In vitro free radical scavenging activity of raw pepino fruit (Solanum muricatum Aiton). Int. J. Curr. Pharm. Res. 2011, 3, 137–140. [Google Scholar]
- Eser, F.; Yaglioglu, A.S.; Aktas, E.; Adem, O.; Demirtas, I. Phytochemical content of Centaurea polypodiifolia boiss. var. polypodiifolia. Int. J. Second. Metab. 2017, 4, 452–458. [Google Scholar] [CrossRef][Green Version]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists, AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Trivedi, A.K.; Rani, S.; Kumar, V. Control of annual reproductive cycle in the subtropical house sparrow (Passer domesticus): Evidence for conservation of photoperiodic control mechanisms in birds. Front. Zoo. 2006, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, J.R. Surgery of the avian reproductive and gastrointestinal systems. Vet. Clin. N. Am. Exot. Anim. Pract. 2000, 3, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Kucera, A.C.; Heidinger, B.J. Avian semen collection by cloacal massage and isolation of DNA from sperm. J. Vis. Exp. 2018, 132. [Google Scholar] [CrossRef]
- Laskemoen, T.; Kleven, O.; Fossøy, F.; Robertson, R.J.; Rudolfsen, G.; Lifjeld, J.T. Sperm quantity and quality effects on fertilization success in a highly promiscuous passerine, the tree swallow Tachycineta bicolor. Behav. Ecol. Sociobiol. 2010, 64, 1473–1483. [Google Scholar] [CrossRef]
- Ramu, S.; Jeyendran, R.S. The hypo-osmotic swelling test for evaluation of sperm membrane integrity. Methods Mol. Biol. 2013, 927, 21–25. [Google Scholar] [PubMed]
- Birkhead, T.R.; Immler, S.; Pellatt, E.J.; Freckleton, R. Unusual sperm morphology in the Eurasian Bullfinch (Pyrrhula pyrrhula). Auk 2006, 123, 383–392. [Google Scholar] [CrossRef][Green Version]
- Fischer, D.; Schneider, H.; Failing, K.; Meinecke-Tillmann, S.; Wehrend, A.; Lierz, M. Viability assessment of spermatozoa in large falcons (Falco spp.) using various staining protocols. Reprod. Domest. Anim. 2020, 55, 1383–1392. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows, version 20.0; IBM Corporation: Armonk, NY, USA, 2011.
- Liu, S.; Wang, W.; Lu, H.; Shu, Q.; Zhang, Y.; Chen, Q. New perspectives on physiological, biochemical and bioactive components during germination of edible seeds: A review. Trends Food Sci. Technol. 2022, 123, 187–197. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, P.; Weng, Y.; Ma, Y.; Gu, Z.; Yang, R. Comparison of phenolic profiles, antioxidant capacity and relevant enzyme activity of different Chinese wheat varieties during germination. Food Biosci. 2017, 20, 159–167. [Google Scholar] [CrossRef]
- Ohm, J.B.; Lee, C.W.; Cho, K. Germinated wheat: Phytochemical composition and mixing characteristics. Cereal Chem. 2016, 93, 612–617. [Google Scholar] [CrossRef]
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y. Optimum health management through right food intake in paramedics. J. Plant Dev. Sci. 2009, 1, 93–98. [Google Scholar]
- Ohanenye, I.C.; Tsopmo, A.; Ejike, C.E.; Udenigwe, C.C. Germination as a bioprocess for enhancing the quality and nutritional prospects of legume proteins. Trends Food Sci. Technol. 2020, 101, 213–222. [Google Scholar] [CrossRef]
- Skoracka, K.; Eder, P.; Łykowska-Szuber, L.; Dobrowolska, A.; Krela-Kaźmierczak, I. Diet and nutritional factors in male (in) fertility—Underestimated factors. J. Clin. Med. 2020, 9, 1400. [Google Scholar] [CrossRef] [PubMed]
- Cevallos-Casals, B.A.; Cisneros-Zevallos, L. Impact of germination on phenolic content and antioxidant activity of 13 edible seed species. Food Chem. 2010, 119, 1485–1490. [Google Scholar] [CrossRef]
- Yang, T.K.; Basu, B.; Ooraikul, F. Studies on germination conditions and antioxidant contents of wheat grain. Int. J. Food Sci. Nutr. 2001, 52, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Lintschinger, J.; Fuchs, N.; Moser, J.; Kuehnelt, D.; Goessler, W. Selenium-enriched sprouts. A raw material for fortified cereal-based diets. J. Agric. Food Chem. 2000, 48, 5362–5368. [Google Scholar] [CrossRef]
- Burk, R.F. Selenium, an antioxidant nutrient. Nutr. Clin. Care 2002, 5, 75–79. [Google Scholar] [CrossRef]
- Rengaraj, D.; Hong, Y.H. Effects of dietary vitamin E on fertility functions in poultry species. Int. J. Mol. Sci. 2015, 16, 9910–9921. [Google Scholar] [CrossRef]
- Moussavi-Nik, M.; Pearson, J.N.; Hollamby, G.J.; Graham, R.D. Dynamics of nutrient remobilization during germination and early seedling development in wheat. J. Plant Nutr. 1998, 21, 421–434. [Google Scholar] [CrossRef]
- Huang, L.; Li, X.; Wang, W.; Yang, L.; Zhu, Y. The role of zinc in poultry breeder and hen nutrition: An update. Biol. Trace Elem. Res. 2019, 192, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, M.B.; Venkatesh, S.; Kumar, R.; Gupta, N.P.; Malhotra, N.; Singh, N.; Mittal, S.; Arora, S.; Arya, D.S.; Talwar, P.; et al. Antioxidant levels in blood and seminal plasma and their impact on sperm parameters in infertile men. Indian J. Biochem. Biophys. 2010, 47, 38–43. [Google Scholar] [PubMed]
- Bansal, A.K.; Bilaspuri, G.S. Impacts of oxidative stress and antioxidants on semen functions. Vet. Med. Int. 2011, 2011, 686137. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Bashiri, R.; Ghadiri-Anari, A.; Nadjarzadeh, A. Antioxidant supplements and semen parameters: An evidence based review. Int. J. Reprod. Biomed. 2016, 14, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Falcioni, G.; Fedeli, D.; Tiano, L.; Calzuola, I.; Mancinelli, L.; Marsili, V.; Gianfranceschi, G. Antioxidant activity of wheat sprouts extract in vitro: Inhibition of DNA oxidative damage. J. Food Sci. 2002, 67, 2918–2922. [Google Scholar] [CrossRef]
- Hung, P.V.; Maeda, T.; Yamamoto, S.; Morita, N. Effects of germination on nutritional composition of waxy wheat. J. Sci. Food Agric. 2012, 92, 667–672. [Google Scholar] [CrossRef]
- Žilić, S.; Basić, Z.; Hadži-Tašković Šukalović, V.; Maksimović, V.; Janković, M.; Filipović, M. Can the sprouting process applied to wheat improve the contents of vitamins and phenolic compounds and antioxidant capacity of the flour? Int. J. Food Sci. Technol. 2014, 49, 1040–1047. [Google Scholar] [CrossRef]
- Cakmak, I.; Strbac, D.; Marschner, H. Activities of hydrogen peroxide-scavenging enzymes in germinating wheat seeds. J. Exp. Bot. 1993, 44, 127–132. [Google Scholar] [CrossRef]
- Bréque, C.; Surai, P.; Brillard, J.P. Roles of antioxidants on prolonged storage of avian spermatozoa in vivo and in vitro. Mol. Reprod. Dev. 2003, 66, 314–323. [Google Scholar] [CrossRef]
- Partyka, A.; Łukaszewicz, E.; Niżański, W. Lipid peroxidation and antioxidant enzymes activity in avian semen. Anim. Reprod. Sci. 2012, 134, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Kelso, K.A.; Cerolini, S.; Noble, R.C.; Sparks, N.H.; Speake, B.K. The effects of dietary supplementation with docosahexaenoic acid on the phospholipid fatty acid composition of avian spermatozoa. Comp. Biochem. Physiol. 1997, 118, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Surai, P.F.; Fujihara, N.; Speake, B.K.; Brillard, J.P.; Wishart, G.J.; Sparks, N.H.C. Polyunsaturated fatty acids, lipid peroxidation and antioxidant protection in avian semen-Review. Asian-Australas. J. Anim. Sci. 2001, 14, 1024–1050. [Google Scholar] [CrossRef]
- Partyka, A.; Niżański, W. Supplementation of avian semen extenders with antioxidants to improve semen quality—Is it an effective strategy? Antioxidants 2021, 10, 1927. [Google Scholar] [CrossRef] [PubMed]
- Tufarelli, V.; Laudadio, V.; Dhama, K.; Malik, Y.S.; Prasad, M. Antioxidant activity of vitamin E and its role in avian reproduction. J. Exp. Biol. Agric. Sci. 2016, 4, 266–272. [Google Scholar] [CrossRef]
- Nazari, L.; Salehpour, S.; Hosseini, S.; Allameh, F.; Jahanmardi, F.; Azizi, E.; Ghodssi-Ghassemabadi, R.; Hashemi, T. Effect of antioxidant supplementation containing L-carnitine on semen parameters: A prospective interventional study. JBRA Assist. Reprod. 2021, 25, 76. [Google Scholar] [CrossRef] [PubMed]
- Surai, P.F.; Speake, B.K.; Sparks, N.H.C. Comparative Aspects of Lipid Peroxidation and Antioxidant Protection in Avian Semen. In Male Fertility and Lipid Metabolism; De Vriese, S., Christophe, A., Eds.; AOCS Press: Champaign, IL, USA, 2003; Chapter 15; pp. 211–249. [Google Scholar]
- Carbeck, K.M.; DeMoranville, K.J.; D’Amelio, P.B.; Goymann, W.; Trost, L.; Pierce, B.; Bryła, A.; Dzialo, M.; Bauchinger, U.; McWilliams, S.R. Environmental cues and dietary antioxidants affect breeding behavior and testosterone of male European starlings (Sturnus vulgaris). Horm. Behav. 2018, 103, 36–44. [Google Scholar] [CrossRef]
- Castillo, A.; Lenzi, C.; Pirone, A.; Baglini, A.; Russo, C.; Soglia, D.; Schiavone, A.; Marzoni Fecia di Cossato, M. From the semen collection method to the hatchlings: The use of cryopreserved sperm from pheasants fed an antioxidant enriched diet. Animals 2021, 11, 2624. [Google Scholar] [CrossRef]
- Black, J.L.; Tredrea, A.M.; Bird, S.H.; Hughes, R.J.; Nielsen, S.G. Effects of germination on the energy value of cereal grains for livestock. Anim. Prod. Sci. 2022, 63, 256–268. [Google Scholar] [CrossRef]
- Șipoteanu, D.; Cioaric, M.; Zăbavă, I.; Gurău, M.R.; Dojană, N. Effect of long-therm vitamin A and E diet supplement on some semen traits in rooster. Vet. Med. 2023, 69, 45–54. [Google Scholar]
- Li, J.; Zhang, S.; Kuang, Y.; Bi, Y.; Wang, H. A review on losses and transformation mechanisms of common antioxidants. J. Am. Oil Chem. Soc. 2023, 100, 259–285. [Google Scholar] [CrossRef]
- Rojas Mora, L.A. Oxidative Stress in Sperm Competition Games: Experimental Tests of the Soma vs. Germline Allocation Trade-Off in Wild House Sparrows Passer Domesticus. Ph.D. Thesis, University of Neuchatel, Neuchâtel, Switzerland, 2016. [Google Scholar]
- Gafer, J.; Hassan, G.H.; Ghoneim, H.A.; Mahmoud, M.F. Amelioration of reproductive and productive performance in animal and poultry by Moringa oleifera free or nano-encapsulated. Eur. J. Veter. Med. 2023, 3, 11–18. [Google Scholar] [CrossRef]
- Khalil, H.; El-Wediny, F.G.; Abou-El-Soud, S.B.; Eid, Y. The effect of different sources of dietary zinc on egg production, egg quality, sperm quality, and immunological response in laying chickens exposed to high temperature. Egypt. J. Vet. Sci. 2024, 55, 87–94. [Google Scholar] [CrossRef]
- Shao, Y.; Li, X.; Du, S.; Sun, X.; Wang, Y.; Zhao, D.; Wang, Z. Effect of dietary supplemental zinc on laying performance, egg quality, and plasma hormone levels of breeding pigeons. Biol. Trace Elem. Res. 2023, 201, 2991–2999. [Google Scholar] [CrossRef] [PubMed]

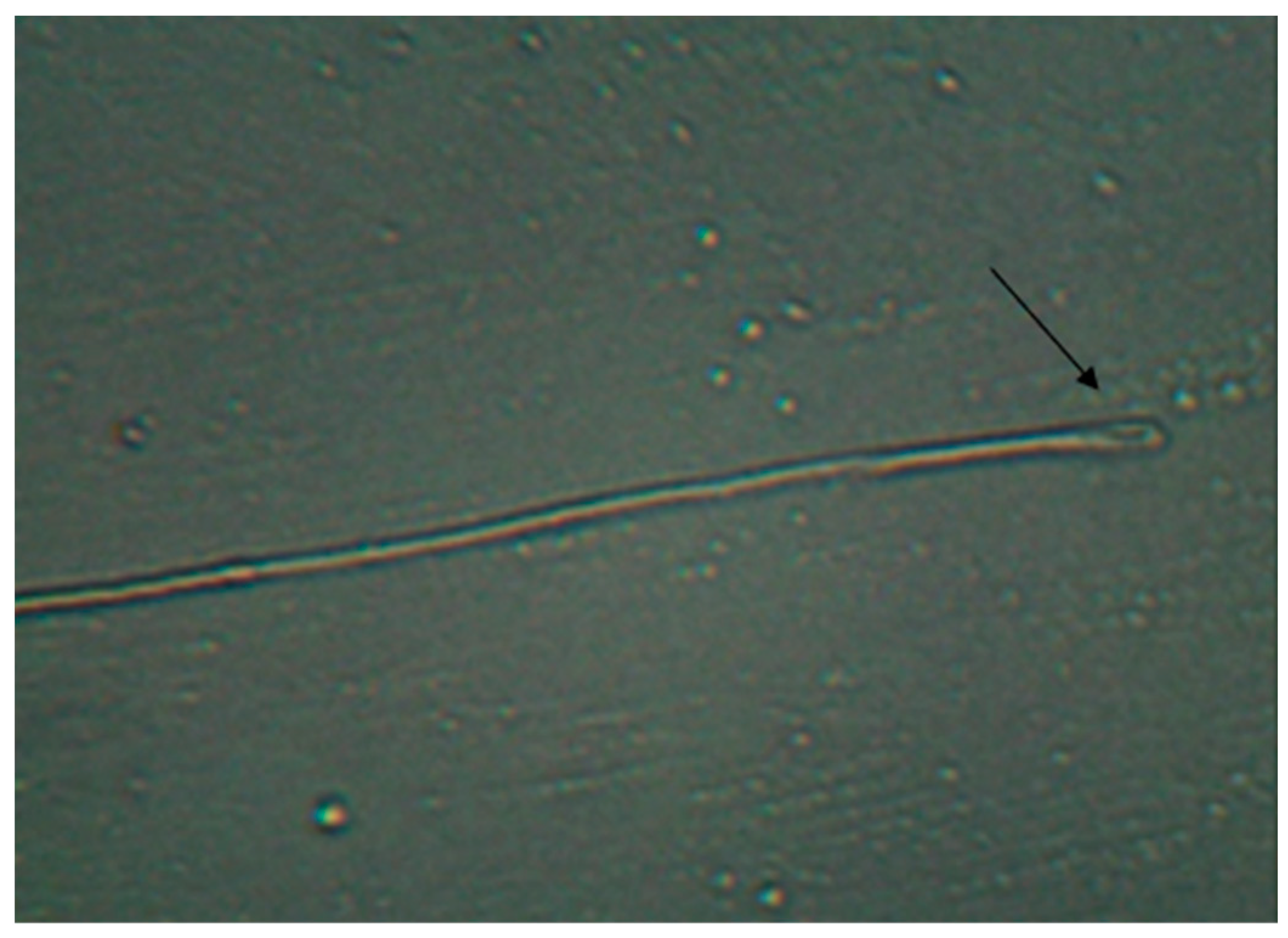
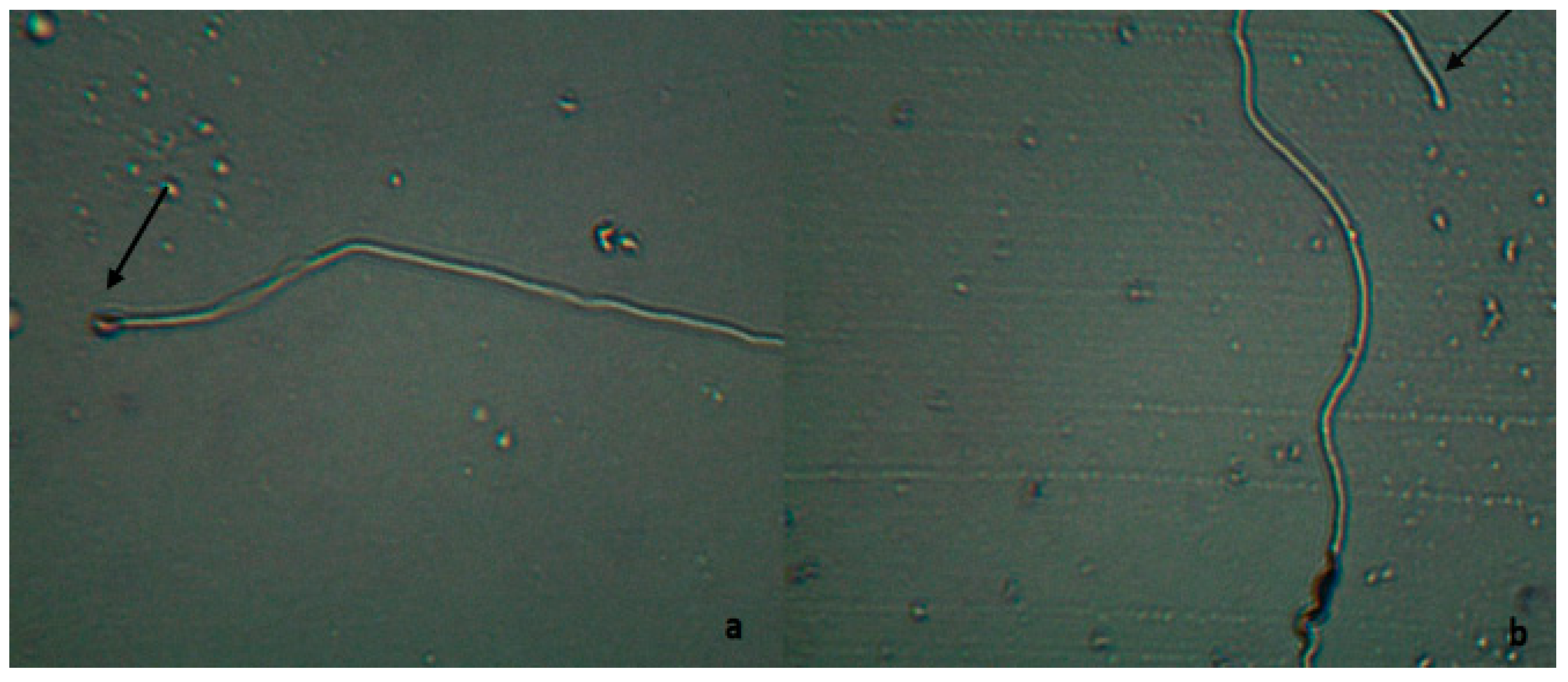
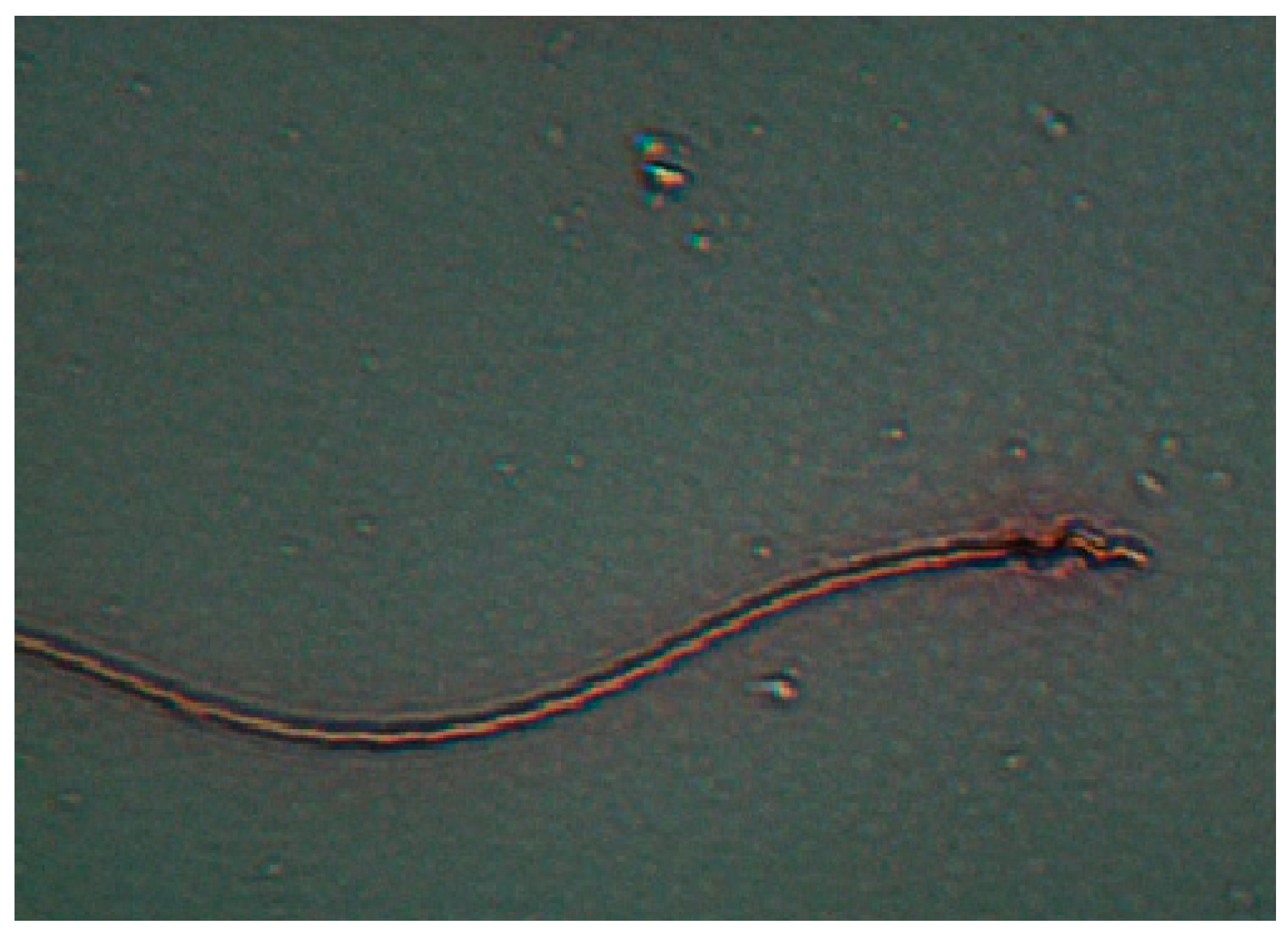
| Groups | Number of Replicates | Germinated Wheat |
|---|---|---|
| C | 6 | − |
| GW | 6 | + |
| Proximate Composition | Day 1 | Day 3 | Day 5 |
|---|---|---|---|
| Dry matter (%) | 91.91 | 92.99 | 92.50 |
| Crude protein (%) | 14.03 | 14.40 | 14.82 |
| Ether extract (%) | 1.60 | 1.82 | 1.80 |
| Crude ash (%) | 1.58 | 1.70 | 1.89 |
| Crude fibre (%) | 3.17 | 3.21 | 3.23 |
| Germination Time (day) | DPPH (%) | Total Phenolic Content mg GAE/g | |
|---|---|---|---|
| 1 | 63.09 c | 157.00 c | |
| 3 | 71.63 a | 236.58 b | |
| 5 | 69.23 b | 218.42 a | |
| p values | <0.001 | <0.001 | |
| F values | 698.65 | 12,737.07 | |
| Solvent rate (%) | |||
| 30 | 67.04 b | 196.17 c | |
| 60 | 58.12 c | 243.42 a | |
| 90 | 78.79 a | 235.42 b | |
| p values | <0.001 | <0.001 | |
| F values | 3865.75 | 2051.53 | |
| Germination time × Solvent rate (day/%) | |||
| 1 | 30 | 64.42 ± 0.37 e | 149.75 ± 2.22 g |
| 60 | 56.89 ± 0.61 f | 136.25 ± 1.71 h | |
| 90 | 67.95 ± 0.52 d | 185.00 ± 1.16 f | |
| 3 | 30 | 69.39 ± 0.81 c | 203.75 ± 1.71 e |
| 60 | 63.14 ± 0.37 e | 301.00 ± 1.41 b | |
| 90 | 82.37 ± 0.37 b | 205.00 ± 1.41 e | |
| 5 | 30 | 67.31 ± 0.74 d | 235.00 ± 2.45 d |
| 60 | 54.33 ± 0.61 g | 293.00 ± 2.94 c | |
| 90 | 86.06 ± 0.61 a | 316.25 ± 1.71 a | |
| p values | <0.001 | <0.001 | |
| F values | 359.93 | 1913.28 | |
| Groups | Motility (%) | Abnormal Sperm (%) | HOST (%) | Viability Rate (%) |
|---|---|---|---|---|
| C | 42.50 ± 3.59 | 25.50 ± 2.41 | 75.00 ± 2.52 | 46.83 ± 4.53 |
| GW | 55.83 ± 3.96 | 26.67 ± 2.84 | 79.67 ± 2.42 | 59.17 ± 2.80 |
| p values | 0.032 | 0.760 | 0.211 | 0.043 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özkök, A.O.; Kilinç, G. Germinated Wheat as a Potential Natural Source of Antioxidants to Improve Sperm Quality: A Canary Trial. Vet. Sci. 2024, 11, 4. https://doi.org/10.3390/vetsci11010004
Özkök AO, Kilinç G. Germinated Wheat as a Potential Natural Source of Antioxidants to Improve Sperm Quality: A Canary Trial. Veterinary Sciences. 2024; 11(1):4. https://doi.org/10.3390/vetsci11010004
Chicago/Turabian StyleÖzkök, Arda Onur, and Gözde Kilinç. 2024. "Germinated Wheat as a Potential Natural Source of Antioxidants to Improve Sperm Quality: A Canary Trial" Veterinary Sciences 11, no. 1: 4. https://doi.org/10.3390/vetsci11010004
APA StyleÖzkök, A. O., & Kilinç, G. (2024). Germinated Wheat as a Potential Natural Source of Antioxidants to Improve Sperm Quality: A Canary Trial. Veterinary Sciences, 11(1), 4. https://doi.org/10.3390/vetsci11010004








