Bronchial Tree System Analysis of Live Beluga Whale (Delphinapterus leucas) Using Bronchoscopy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Sedation
| ID | Date of Birth | Sex | Examined Date | Body Length (cm) | Body Weight (kg) | Length from Blowhole to Impassable Points (cm) | Number of Observed Bronchi | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LPB | RPB | TB | LPB | RPB | TB | ||||||
| DL-1 | 1978 Expected (1) | Male | 24 August 2021 | 371 | 686 | 130 | 128 | 100 | 11 | 10 | 6 |
| 21 October | 371 | 673 | 132 | 130 | 100 | 11 | 10 | 6 | |||
| 19 November | 371 | 672 | 130 | 125 | 100 | 11 | 10 | 6 | |||
| DL-6 | 1995 Expected (2) | Female | 25 August 2021 | 407 | 808 | 125 | 130 | 100 | 10 | 11 | 6 |
| 15 November | 407 | 769 | 140 | 122 | 100 | 10 | 11 | 6 | |||
| 20 December | 407 | 788 | 135 | 135 | 95 | 10 | 11 | 6 | |||
| DL-9 | 25 July 2007 | Female | 27 October 2021 | 360 | 610 | 120 | 115 | 85 | 12 | 12 | 6 |
| 25 February 2022 | 360 | 651 | 115 | 115 | 85 | 12 | 12 | 6 | |||
| 13 June | 360 | 574 | 110 | 110 | 80 | 12 | 12 | 6 | |||
| DL-11 | 2 August 2012 | Male | 8 November 2021 | 355 | 508 | 113 | 115 | 83 | 12 | 11 | 7 |
| 26 March 2022 | 355 | 520 | 120 | 115 | 90 | 12 | 11 | 7 | |||
| 11 October | 360 | 491 | 113 | 115 | 85 | 12 | 11 | 7 | |||
| DL-12 | 2007 Expected (2) | Male | 11 December 2021 | 450 | 1179 | 155 | 150 | 110 | 12 | 12 | 9 |
| 30 May 2022 | 454 | 1255 | 145 | 145 | 105 | 12 | 12 | 9 | |||
| 30 January 2023 | 455 | 1187 | 140 | 140 | 105 | 12 | 12 | 9 | |||
2.3. Bronchoscopy
2.4. Diagrammatic Dorsal View of the Bronchial Tree
3. Results
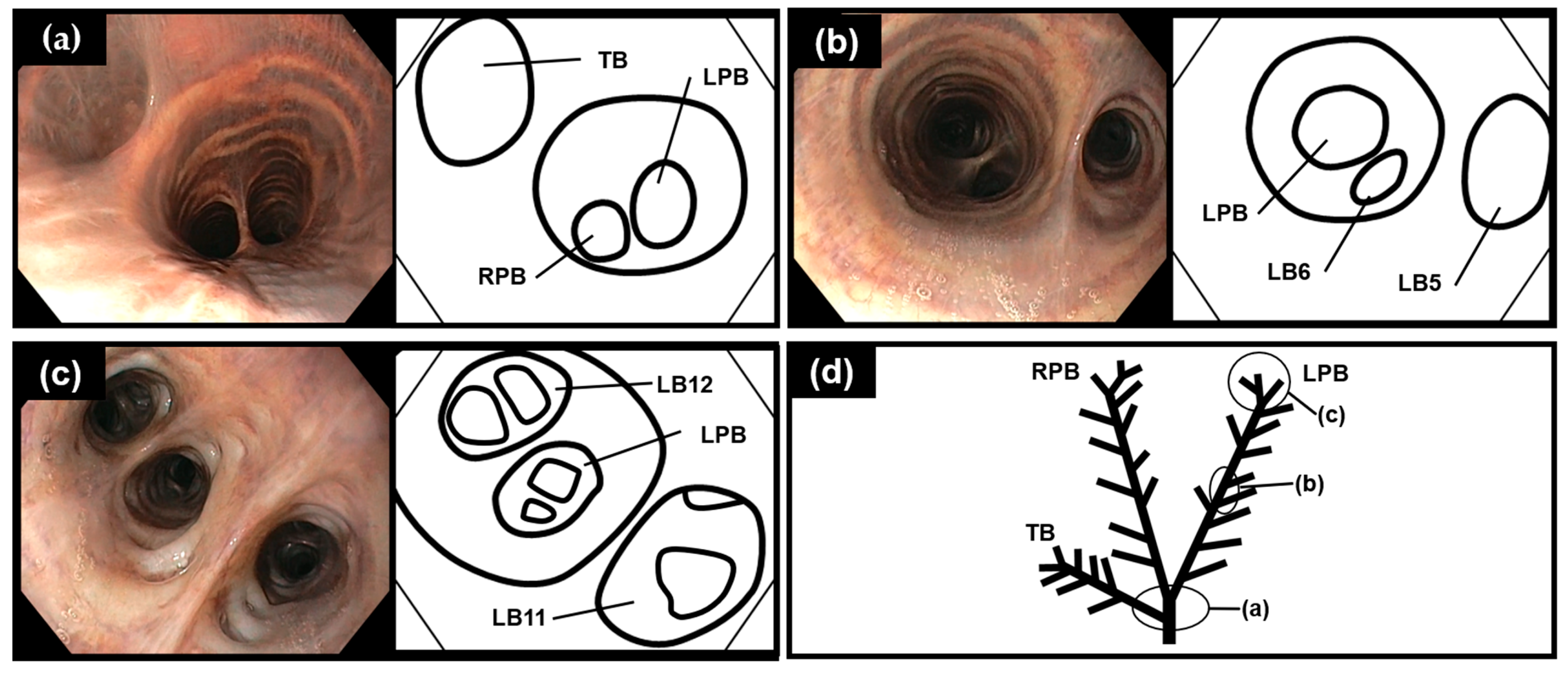
3.1. Bronchoscopy
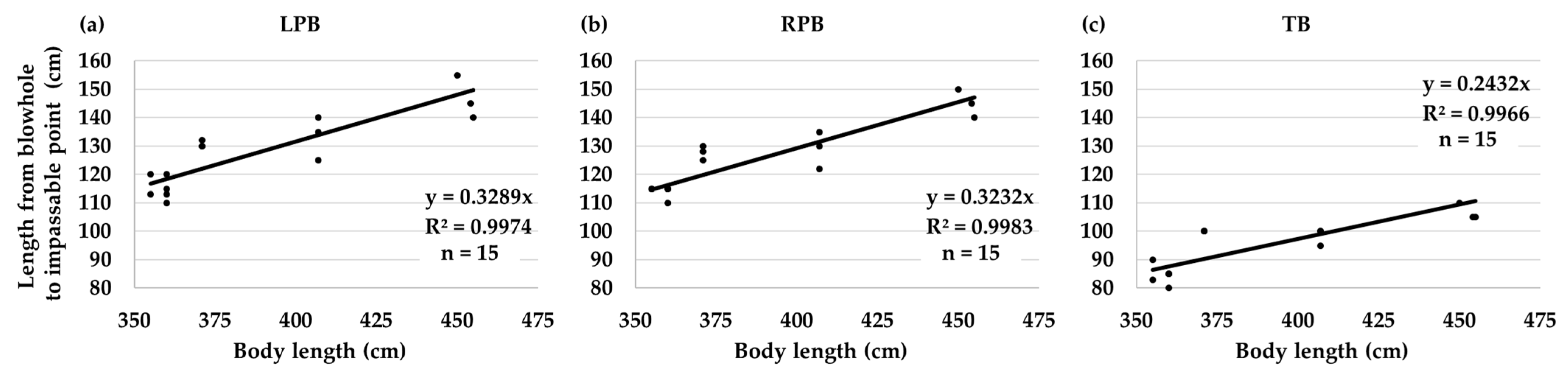
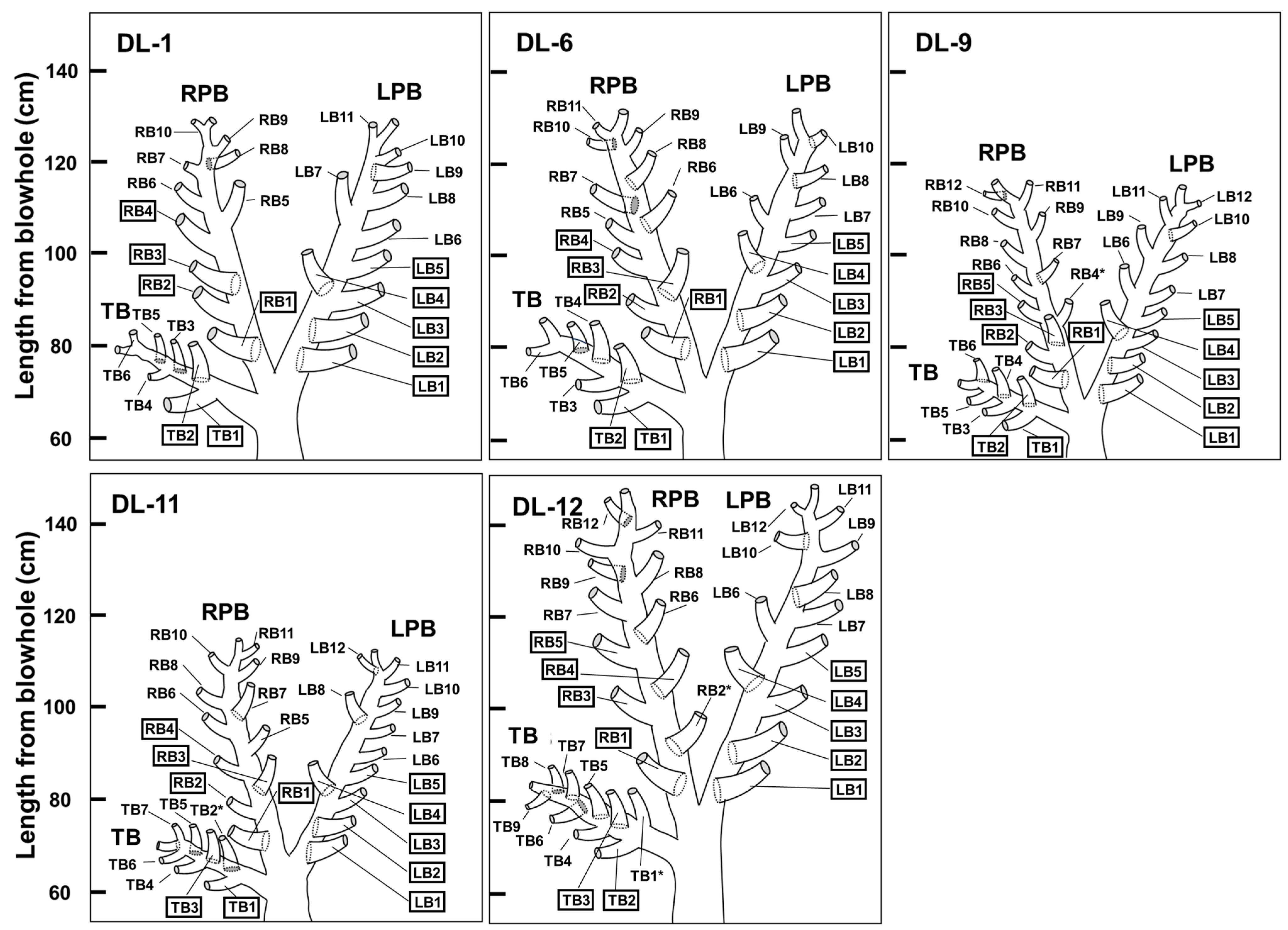
3.2. Diagrammatic Dorsal View of the Bronchial Tree Systems
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bunskoek, P.E.; Seyedmousavi, S.; Gans, S.J.M.; van Vierzen, P.B.J.; Melchers, W.J.G.; van Elk, C.E.; Mouton, J.W.; Verweij, P.E. Successful treatment of azole-resistant invasive aspergillosis in a bottlenose dolphin with high-dose posaconazole. Med. Mycol. Case Rep. 2017, 16, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Nollens, H.H.; Venn-Watson, S.; Gili, C.; McBain, J.F. Cetacean medicine. In CRC Handbook of Marine Mammal Medicine, 3rd ed.; Gulland, F.M.D., Dierauf, L.A., Whitman, K.L., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 887–907. [Google Scholar]
- Reidarson, T.H.; García-Párraga, D.; Wiederhold, N.P. Marine mammal mycoses. In CRC Handbook of Marine Mammal Medicine, 3rd ed.; Gulland, F.M.D., Dierauf, L.A., Whitman, K.L., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 389–423. [Google Scholar]
- Tryland, M.; Larsen, A.K.; Nymo, I.H. Bacterial infections and diseases. In CRC Handbook of Marine Mammal Medicine, 3rd ed.; Gulland, F.M.D., Dierauf, L.A., Whitman, K.L., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 367–388. [Google Scholar]
- Van Bonn, W.; Dover, S. Applied flexible and rigid endoscopy. In CRC Handbook of Marine Mammal Medicine, 3rd ed.; Gulland, F.M.D., Dierauf, L.A., Whitman, K.L., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 553–566. [Google Scholar]
- Harper, C.M.G.; Borkowski, R.; Hoffman, A.M.; Warner, A. Development of a standardized nomenclature for bronchoscopy of the respiratory system of harbor porpoises (Phocoena phocoena). J. Zoo Wildl. Med. 2001, 32, 190–195. [Google Scholar] [PubMed]
- Venn Watson, S.; Daniels, R.; Smith, C. Thirty year retrospective evaluation of pneumonia in a bottlenose dolphin tursiops truncatus population. Dis. Aquat. Org. 2012, 99, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Baselski, V.S.; Wunderink, R.G. Bronchoscopic diagnosis of pneumonia. Clin. Microbiol. Rev. 1994, 7, 533–558. [Google Scholar] [CrossRef] [PubMed]
- Tsang, K.W.; Kinoshita, R.; Rouke, N.; Yuen, Q.; Hu, W.; Lam, W.K. Bronchoscopy of cetaceans. J. Wildl. Dis. 2002, 38, 224–227. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dennison, S.; Saviano, P. Diagnostic imaging. In CRC Handbook of Marine Mammal Medicine, 3rd ed.; Gulland, F.M.D., Dierauf, L.A., Whitman, K.L., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 537–551. [Google Scholar]
- Piscitelli, M.A.; Raverty, S.A.; Lillie, M.A.; Shadwick, R.E. A review of cetacean lung morphology and mechanics. J. Morphol. 2013, 274, 1425–1440. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.; Moore, M.; Trumble, S.; Niemeyer, M.; Lentell, B.; McLellan, W.; Costidis, A.; Fahlman, A. A comparative analysis of marine mammal tracheas. J. Exp. Biol. 2014, 217, 1154–1166. [Google Scholar] [CrossRef] [PubMed]
- Fahlman, A.; Moore, M.J.; Garcia-Parraga, D. Respiratory function and mechanics in pinnipeds and cetaceans. J. Exp. Biol. 2017, 220, 1761–1773. [Google Scholar] [CrossRef] [PubMed]
- Huggenberger, S.; Oelschläger, H.; Cozzi, B. Atlas of the Anatomy of Dolphins and Whales; Academic Press: London, UK, 2019; pp. 5–135. [Google Scholar]
- Kida, M.Y. Morphology of the tracheobronchial tree of the Ganges river dolphin (Platanista gangetica). Okajimas Folia Anat. Jpn. 1990, 67, 289–295. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nakakuki, S. The bronchial tree and lobular division of the lung in the striped dolphin (Stenella coeruleo-albus). J. Vet. Med. Sci. 1994, 56, 1209–1211. [Google Scholar] [CrossRef] [PubMed]
- Davenport, J.; Cotter, L.; Rogan, E.; Kelliher, D.; Murphy, C. Structure, material characteristics and function of the upper respiratory tract of the pygmy sperm whale. J. Exp. Biol. 2013, 216, 4639–4646. [Google Scholar] [CrossRef] [PubMed]
- Fahlman, A.; Epple, A.; García-Párraga, D.; Robeck, T.; Haulena, M.; Piscitelli-Doshkov, M.; Brodsky, M. Characterizing respiratory capacity in belugas (Delphinapterus leucas). Respir. Physiol. Neurobiol. 2019, 260, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Fahlman, A.; Borque-Espinosa, A.; Facchin, F.; Fernandez, D.F.; Caballero, P.M.; Haulena, M.; Rocho-Levine, J. Comparative Respiratory Physiology in Cetaceans. Front. Physiol. 2020, 11, 142. [Google Scholar] [CrossRef] [PubMed]
- Haulena, M.; Schmitt, T. Anesthesia. In CRC Handbook of Marine Mammal Medicine, 3rd ed.; Gulland, F.M.D., Dierauf, L.A., Whitman, K.L., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 567–606. [Google Scholar]
- Simeone, C.A.; Stoskopf, M.K. Pharmaceuticals and formularies. In CRC Handbook of Marine Mammal Medicine, 3rd ed.; Gulland, F.M.D., Dierauf, L.A., Whitman, K.L., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 607–673. [Google Scholar]
- Kamio, T.; Odani, Y.; Ohtomo, W.; Ogushi, A.; Akune, Y.; Kurita, M.; Okada, A.; Inoshima, Y. Midazolam and butorphanol combination for sedating beluga whales (Delphinapterus leucas). J. Vet. Med. Sci. 2024, in press. [CrossRef] [PubMed]
- Vos, D.J.; Shelden, K.E.W.; Friday, N.A.; Mahoney, B.A. Age and growth analyses for the endangered belugas in Cook Inlet, Alaska. Mar. Mamm. Sci. 2020, 36, 293–304. [Google Scholar] [CrossRef]
- Luque, S.P.; Ferguson, S.H. Age structure, growth, mortality, and density of belugas (Delphinapterus leucas) in the Canadian arctic: Responses to environment? Polar Biol. 2010, 33, 163–178. [Google Scholar] [CrossRef]
- Kovaleva, J.; Peters, F.T.M.; van der Mei, H.C.; Degener, J.E. Transmission of infection by flexible gastrointestinal endoscopy and bronchoscopy. Clin. Microbiol. Rev. 2013, 26, 231–254. [Google Scholar] [CrossRef] [PubMed]
- Amis, T.C.; McKiernan, B.C. Systematic identification of endobronchial anatomy during bronchoscopy in the dog. Am. J. Vet. Res. 1986, 47, 2649–2657. [Google Scholar] [PubMed]
- Wada, R.; Aida, H.; Kaneko, M.; Oikawa, M.; Yoshihara, T.; Tomioka, Y.; Nitta, M. Identification of the bronchi for bronchoscopy in the horse and segmentation of the horse lung. Jpn. J. Equine Sci. 1992, 3, 37–43. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamio, T.; Odani, Y.; Ohtomo, W.; Ogushi, A.; Akune, Y.; Kurita, M.; Okada, A.; Inoshima, Y. Bronchial Tree System Analysis of Live Beluga Whale (Delphinapterus leucas) Using Bronchoscopy. Vet. Sci. 2024, 11, 33. https://doi.org/10.3390/vetsci11010033
Kamio T, Odani Y, Ohtomo W, Ogushi A, Akune Y, Kurita M, Okada A, Inoshima Y. Bronchial Tree System Analysis of Live Beluga Whale (Delphinapterus leucas) Using Bronchoscopy. Veterinary Sciences. 2024; 11(1):33. https://doi.org/10.3390/vetsci11010033
Chicago/Turabian StyleKamio, Takashi, Yukako Odani, Wataru Ohtomo, Akira Ogushi, Yuichiro Akune, Masanori Kurita, Ayaka Okada, and Yasuo Inoshima. 2024. "Bronchial Tree System Analysis of Live Beluga Whale (Delphinapterus leucas) Using Bronchoscopy" Veterinary Sciences 11, no. 1: 33. https://doi.org/10.3390/vetsci11010033
APA StyleKamio, T., Odani, Y., Ohtomo, W., Ogushi, A., Akune, Y., Kurita, M., Okada, A., & Inoshima, Y. (2024). Bronchial Tree System Analysis of Live Beluga Whale (Delphinapterus leucas) Using Bronchoscopy. Veterinary Sciences, 11(1), 33. https://doi.org/10.3390/vetsci11010033





