Modulatory Effects of Hydatid Cyst Fluid on a Mouse Model of Experimental Autoimmune Encephalomyelitis
Abstract
Simple Summary
Abstract
1. Introduction
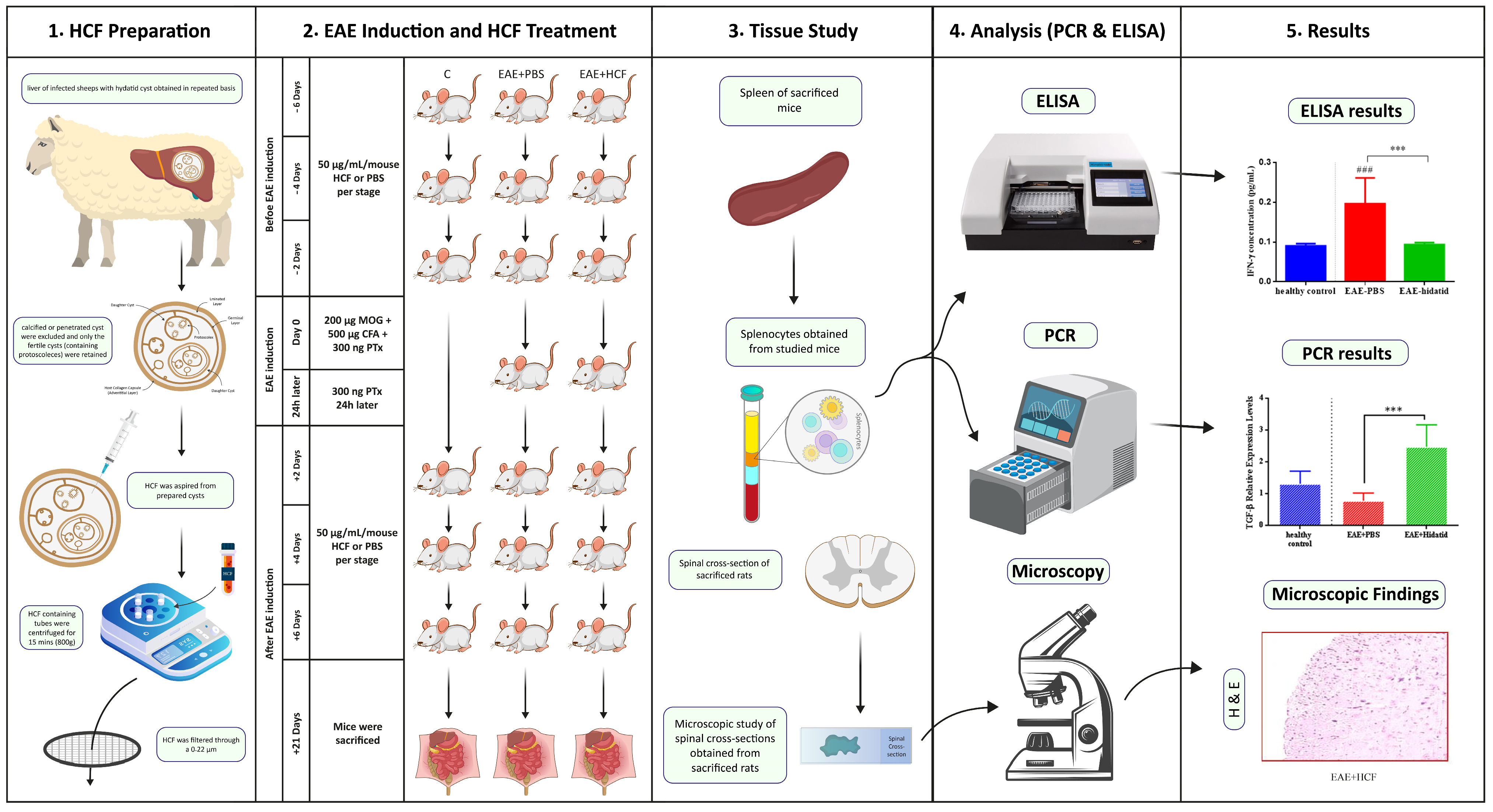
2. Materials and Methods
2.1. Animals
2.2. Preparation of HCF
2.3. EAE Induction and Intervention
2.4. Experimental Groups and Treatments
2.5. Histopathological Examination and Cell Preparation
2.6. Isolation of Splenocytes
2.7. Quantitative Real-Time Reverse Transcriptase–Polymerase Chain Reaction (qRT-PCR)
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Statistical Analysis
3. Results
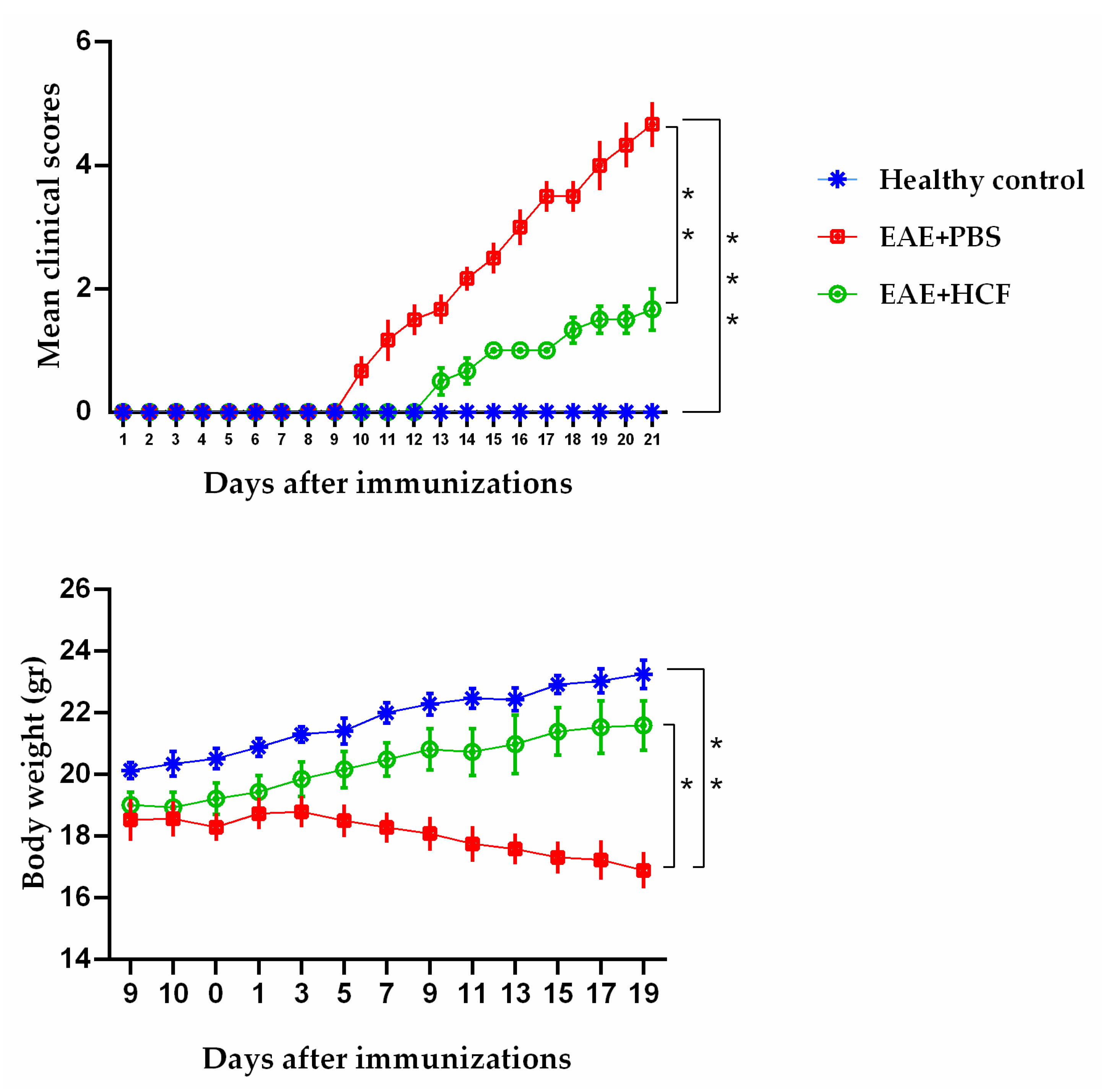
3.1. Average Clinical Scores of Mice
3.2. Weight Alterations of Mice
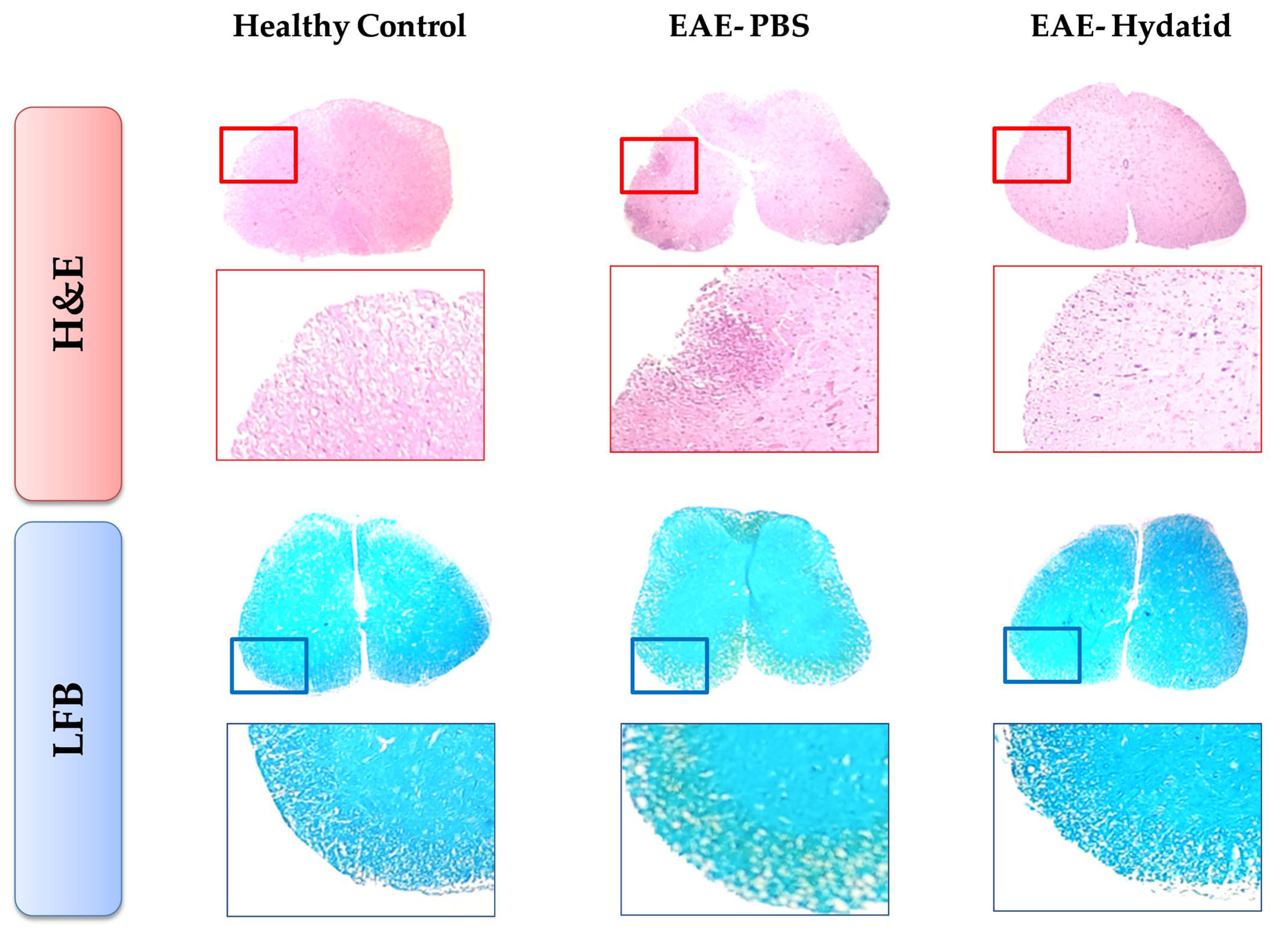
3.3. Histopathological Findings
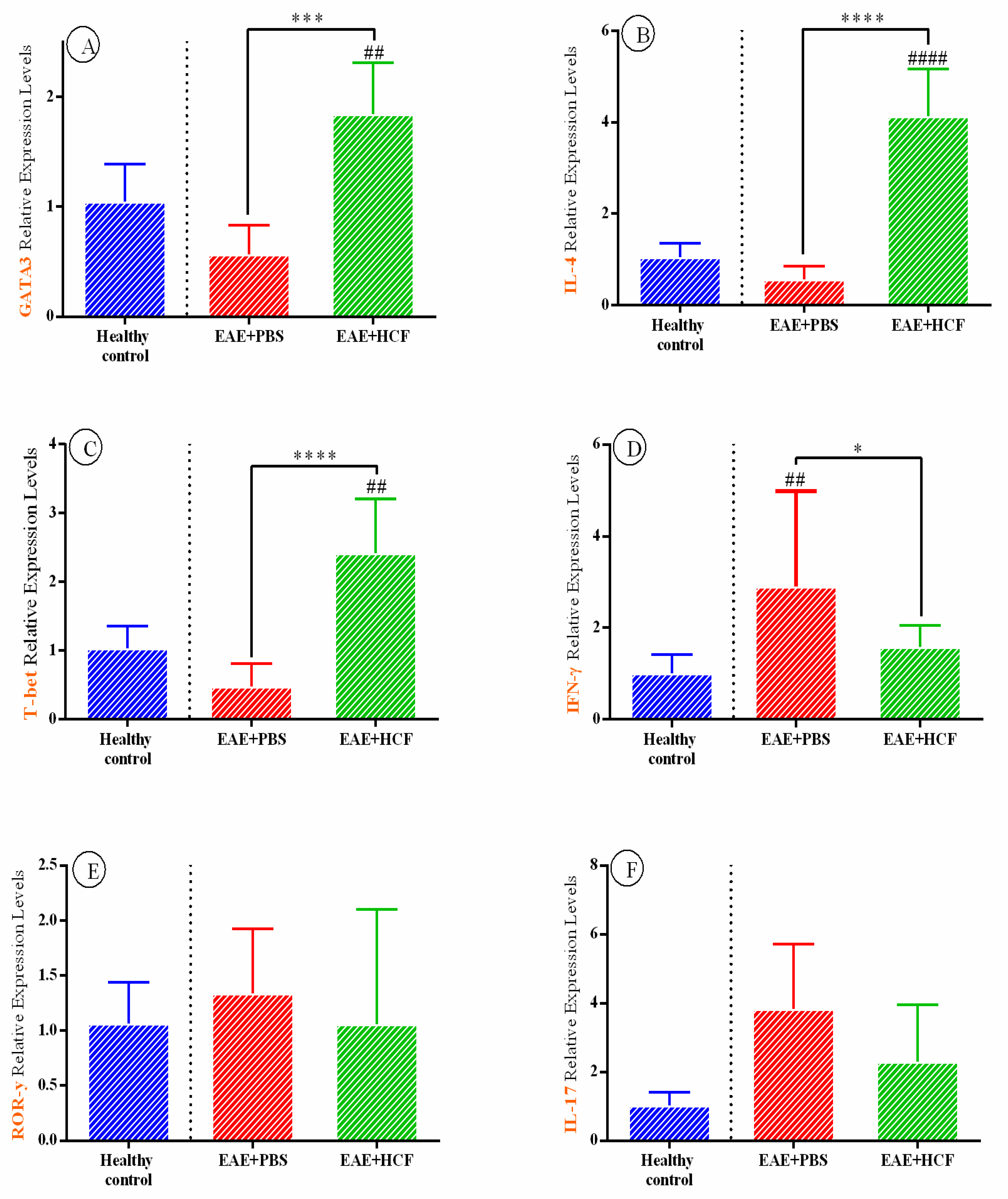
3.4. Results of Cytokine Assay Based on RT-PCR
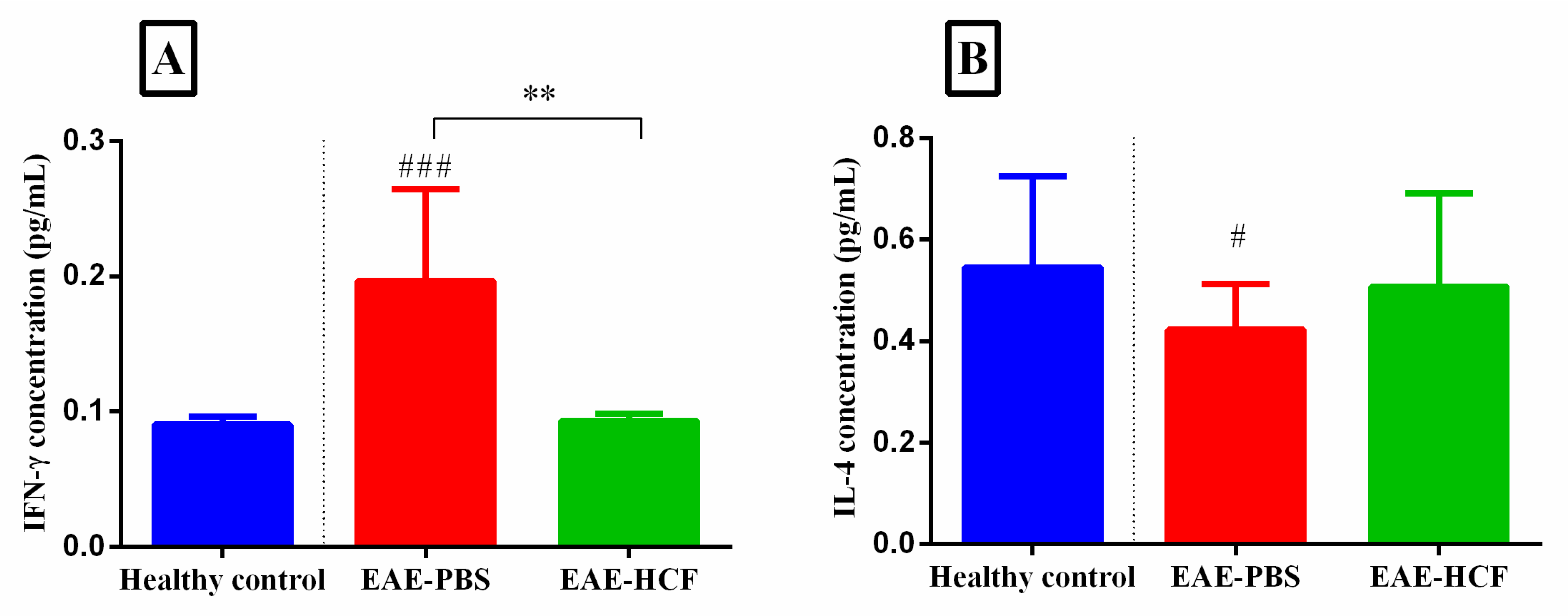
3.5. Evaluation of Cytokines Based on ELISA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [PubMed]
- Maizels, R.; McSorley, H.; Smyth, D. Helminths in the hygiene hypothesis: Sooner or later? Clin. Exp. Immunol. 2014, 177, 38–46. [Google Scholar] [CrossRef]
- Schaub, B.; Lauener, R.; von Mutius, E. The many faces of the hygiene hypothesis. J. Allergy Clin. Immunol. 2006, 117, 969–977. [Google Scholar] [CrossRef]
- Wallin, M.T.; Culpepper, W.J.; Nichols, E.; Bhutta, Z.A.; Gebrehiwot, T.T.; Hay, S.I.; Khalil, I.A.; Krohn, K.J.; Liang, X.; Naghavi, M. Global, regional, and national burden of multiple sclerosis 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 269–285. [Google Scholar] [CrossRef]
- Bach, J.-F. The effect of infections on susceptibility to autoimmune and allergic diseases. N. Engl. J. Med. 2002, 347, 911–920. [Google Scholar] [CrossRef]
- Mahad, D.H.; Trapp, B.D.; Lassmann, H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015, 14, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 2011, 476, 214–219. [CrossRef]
- Maghzi, A.-H.; Minagar, A.; Waubant, E. Neuroprotection in multiple sclerosis: A therapeutic approach. CNS Drugs 2013, 27, 799–815. [Google Scholar] [CrossRef]
- Conlon, P.; Oksenberg, J.R.; Zhang, J.; Steinman, L. The immunobiology of multiple sclerosis: An autoimmune disease of the central nervous system. Neurobiol. Dis. 1999, 6, 149–166. [Google Scholar] [CrossRef]
- Camelo, S.; Iglesias, A.H.; Hwang, D.; Due, B.; Ryu, H.; Smith, K.; Gray, S.G.; Imitola, J.; Duran, G.; Assaf, B. Transcriptional therapy with the histone deacetylase inhibitor trichostatin A ameliorates experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2005, 164, 10–21. [Google Scholar] [CrossRef]
- Baecher-Allan, C.; Kaskow, B.J.; Weiner, H.L. Multiple sclerosis: Mechanisms and immunotherapy. Neuron 2018, 97, 742–768. [Google Scholar] [CrossRef]
- Jäger, A.; Dardalhon, V.; Sobel, R.A.; Bettelli, E.; Kuchroo, V.K. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J. Immunol. 2009, 183, 7169–7177. [Google Scholar] [CrossRef]
- Correale, J.; Gaitán, M.I.; Ysrraelit, M.C.; Fiol, M.P. Progressive multiple sclerosis: From pathogenic mechanisms to treatment. Brain 2017, 140, 527–546. [Google Scholar] [CrossRef] [PubMed]
- Myhr, K.M. Diagnosis and treatment of multiple sclerosis. Acta Neurol. Scand. 2008, 117, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Krupp, L.B. Fatigue in multiple sclerosis: Definition, pathophysiology and treatment. CNS Drugs 2003, 17, 225–234. [Google Scholar] [CrossRef] [PubMed]
- McManus, D.P.; Zhang, W.; Li, J.; Bartley, P.B. Echinococcosis. Lancet 2003, 362, 1295–1304. [Google Scholar] [CrossRef]
- Moro, P.; Schantz, P.M. Echinococcosis: A review. Int. J. Infect. Dis. 2009, 13, 125–133. [Google Scholar] [CrossRef]
- World Health Organization. Charateristics and Details of Echinococcosis; World Health Organization: Geneva, Switzerland, 2021.
- Ito, A.; Budke, C.M. The echinococcoses in Asia: The present situation. Acta Trop. 2017, 176, 11–21. [Google Scholar] [CrossRef]
- Mahmoudi, S.; Mamishi, S.; Banar, M.; Pourakbari, B.; Keshavarz, H. Epidemiology of echinococcosis in Iran: A systematic review and meta-analysis. BMC Infect. Dis. 2019, 19, 929. [Google Scholar] [CrossRef]
- Díaz, Á. Immunology of cystic echinococcosis (hydatid disease). Br. Med. Bull. 2017, 19, 1–13. [Google Scholar] [CrossRef]
- Díaz, A.; Casaravilla, C.; Allen, J.E.; Sim, R.B.; Ferreira, A.M. Understanding the laminated layer of larval Echinococcus II: Immunology. Trends Parasitol. 2011, 27, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Charabati, M.; Donkers, S.J.; Kirkland, M.C.; Osborne, L.C. A critical analysis of helminth immunotherapy in multiple sclerosis. Mult. Scler. J. 2020, 26, 1448–1458. [Google Scholar] [CrossRef] [PubMed]
- Smallwood, T.B.; Giacomin, P.R.; Loukas, A.; Mulvenna, J.P.; Clark, R.J.; Miles, J.J. Helminth immunomodulation in autoimmune disease. Front. Immunol. 2017, 8, 453. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Li, J.; Wu, X.; Zhang, S.; Niu, J.; Chen, X.; Yao, J.; Sun, H. Detection of Osteopontin in the pericyst of human hepatic Echinococcus granulosus. Acta Trop. 2006, 100, 163–171. [Google Scholar] [CrossRef]
- Vatankhah, A.; Halász, J.; Piurko, V.; Barbai, T.; Rásó, E.; Tímár, J. Characterization of the inflammatory cell infiltrate and expression of costimulatory molecules in chronic Echinococcus granulosus infection of the human liver. BMC Infect. Dis. 2015, 15, 530. [Google Scholar] [CrossRef] [PubMed]
- Gottstein, B.; Soboslay, P.; Ortona, E.; Wang, J.; Siracusano, A.; Vuitton, D. Immunology of alveolar and cystic echinococcosis (AE and CE). Adv. Parasitol. 2017, 96, 1–54. [Google Scholar] [PubMed]
- Petrone, L.; Vanini, V.; Petruccioli, E.; Ettorre, G.M.; Schinina, V.; Busi Rizzi, E.; Ludovisi, A.; Corpolongo, A.; Ippolito, G.; Pozio, E. Polyfunctional specific response to Echinococcus granulosus associates to the biological activity of the cysts. PLoS Neglected Trop. Dis. 2015, 9, e0004209. [Google Scholar] [CrossRef]
- Pang, N.; Zhang, F.; Ma, X.; Zhang, Z.; Zhao, H.; Xin, Y.; Wang, S.; Zhu, Y.; Wen, H.; Ding, J. Th9/IL-9 profile in human echinococcosis: Their involvement in immune response during infection by Echinococcus granulosus. Mediat. Inflamm. 2014, 2014, 781649. [Google Scholar] [CrossRef]
- Ayatollahi, A.M.; Haji Molla Hoseini, M.; Ghanadian, S.M.; Kosari-Nasab, M.; Mami, F.; Yazdiniapoure, Z.; Zolfaghari, B.; Salari, A.-A. TAMEC: A new analogue of cyclomyrsinol diterpenes decreases anxiety-and depression-like behaviors in a mouse model of multiple sclerosis. Neurol. Res. 2017, 39, 1056–1065. [Google Scholar] [CrossRef]
- Hajizadeh, M.; Saboor-Yaraghi, A.A.; Meamar, A.R.; Khoshmirsafa, M.; Razmjou, E.; Sadeghipour, A.; Bagheri, Y.; Sadeghi, F.; Jalallou, N.; Kazemi, M.H. The fatty acid-binding protein (FABP) decreases the clinical signs and modulates immune responses in a mouse model of experimental autoimmune encephalomyelitis (EAE). Int. Immunopharmacol. 2021, 96, 107756. [Google Scholar] [CrossRef]
- Hernández, A.; O’Connor, J.E.; Mir, A. Phenotypic analysis of peripheral lymphocyte subpopulations in hydatid patients. Parasitol. Res. 1999, 85, 948–950. [Google Scholar] [CrossRef]
- Etesam, Z.; Nemati, M.; Ebrahimizadeh, M.-A.; Ebrahimi, H.-A.; Hajghani, H.; Khalili, T.; Jafarzadeh, A. Different expressions of specific transcription factors of Th1 (T-bet) and Th2 cells (GATA-3) by peripheral blood mononuclear cells from patients with multiple sclerosis. Basic Clin. Neurosci. 2018, 9, 458. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wang, Q.; Mao, G.; Dowling, C.A.; Lundy, S.K.; Mao-Draayer, Y. Dimethyl fumarate selectively reduces memory T cells and shifts the balance between Th1/Th17 and Th2 in multiple sclerosis patients. J. Immunol. 2017, 198, 3069–3080. [Google Scholar] [CrossRef] [PubMed]
- Cunill, V.; Massot, M.; Clemente, A.; Calles, C.; Andreu, V.; Núñez, V.; López-Gómez, A.; Díaz, R.M.; Jiménez, M.d.l.R.; Pons, J. relapsing–remitting Multiple sclerosis is characterized by a T Follicular cell Pro-inflammatory shift, reverted by Dimethyl Fumarate Treatment. Front. Immunol. 2018, 9, 1097. [Google Scholar] [CrossRef]
- Kihara, Y.; Groves, A.; Rivera, R.R.; Chun, J. Dimethyl fumarate inhibits integrin α4 expression in multiple sclerosis models. Ann. Clin. Transl. Neurol. 2015, 2, 978–983. [Google Scholar] [CrossRef] [PubMed]
- Spencer, C.M.; Crabtree-Hartman, E.C.; Lehmann-Horn, K.; Cree, B.A.; Zamvil, S.S. Reduction of CD8+ T lymphocytes in multiple sclerosis patients treated with dimethyl fumarate. Neurol.-Neuroimmunol. Neuroinflamm. 2015, 2. [Google Scholar] [CrossRef]
- Dendrou, C.A.; Fugger, L. Immunomodulation in multiple sclerosis: Promises and pitfalls. Curr. Opin. Immunol. 2017, 49, 37–43. [Google Scholar] [CrossRef]
- Wu, Z.; Nagano, I.; Asano, K.; Takahashi, Y. Infection of non-encapsulated species of Trichinella ameliorates experimental autoimmune encephalomyelitis involving suppression of Th17 and Th1 response. Parasitol. Res. 2010, 107, 1173–1188. [Google Scholar] [CrossRef]
- Donskow-Łysoniewska, K.; Krawczak, K.; Doligalska, M. Heligmosomoides polygyrus: EAE remission is correlated with different systemic cytokine profiles provoked by L4 and adult nematodes. Exp. Parasitol. 2012, 132, 243–248. [Google Scholar] [CrossRef]
- Mariki, A.; Barzin, Z.; Fasihi-Harandi, M.; Karbasi-Ravari, K.; Davoodi, M.; Mousavi, S.M.; Rezakhani, S.; Nazeri, M.; Shabani, M. Antigen B modulates anti-inflammatory cytokines in the EAE model of multiple sclerosis. Brain Behav. 2023, 13, e2874. [Google Scholar] [CrossRef]
- Sewell, D.; Qing, Z.; Reinke, E.; Elliot, D.; Weinstock, J.; Sandor, M.; Fabry, Z. Immunomodulation of experimental autoimmune encephalomyelitis by helminth ova immunization. Int. Immunol. 2003, 15, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Terrazas, C.; de Dios Ruiz-Rosado, J.; Amici, S.A.; Jablonski, K.A.; Martinez-Saucedo, D.; Webb, L.M.; Cortado, H.; Robledo-Avila, F.; Oghumu, S.; Satoskar, A.R. Helminth-induced Ly6Chi monocyte-derived alternatively activated macrophages suppress experimental autoimmune encephalomyelitis. Sci. Rep. 2017, 7, 40814. [Google Scholar] [CrossRef] [PubMed]
- Finlay, C.M.; Stefanska, A.M.; Walsh, K.P.; Kelly, P.J.; Boon, L.; Lavelle, E.C.; Walsh, P.T.; Mills, K.H. Helminth products protect against autoimmunity via innate type 2 cytokines IL-5 and IL-33, which promote eosinophilia. J. Immunol. 2016, 196, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.L.; Espinoza-Jiménez, A.F.; González, M.I.; Verdin, L.; Terrazas, L.I. Taenia crassiceps infection abrogates experimental autoimmune encephalomyelitis. Cell. Immunol. 2011, 267, 77–87. [Google Scholar] [CrossRef]
- Walsh, K.P.; Brady, M.T.; Finlay, C.M.; Boon, L.; Mills, K.H. Infection with a helminth parasite attenuates autoimmunity through TGF-β-mediated suppression of Th17 and Th1 responses. J. Immunol. 2009, 183, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- Peón, A.N.; Ledesma-Soto, Y.; Olguín, J.E.; Bautista-Donis, M.; Sciutto, E.; Terrazas, L.I. Helminth products potently modulate experimental autoimmune encephalomyelitis by downregulating neuroinflammation and promoting a suppressive microenvironment. Mediat. Inflamm. 2017, 2017, 8494572. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.S.; Hasseldam, H.; Bacher, I.H.; Thamsborg, S.M.; Johansen, F.F.; Kringel, H. Trichuris suis secrete products that reduce disease severity in a multiple sclerosis model. Acta Parasitol. 2017, 62, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Sofronic-Milosavljevic, L.; Radovic, I.; Ilic, N.; Majstorovic, I.; Cvetkovic, J.; Gruden-Movsesijan, A. Application of dendritic cells stimulated with Trichinella spiralis excretory–secretory antigens alleviates experimental autoimmune encephalomyelitis. Med. Microbiol. Immunol. 2013, 202, 239–249. [Google Scholar] [CrossRef]
- Talbot, S.R.; Biernot, S.; Bleich, A.; van Dijk, R.M.; Ernst, L.; Häger, C.; Helgers, S.O.A.; Koegel, B.; Koska, I.; Kuhla, A. Defining body-weight reduction as a humane endpoint: A critical appraisal. Lab. Anim. 2020, 54, 99–110. [Google Scholar] [CrossRef]
- Correale, J.; Farez, M.F. The impact of parasite infections on the course of multiple sclerosis. J. Neuroimmunol. 2011, 233, 6–11. [Google Scholar] [CrossRef]
- Correale, J.; Farez, M. Association between parasite infection and immune responses in multiple sclerosis. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 2007, 61, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Rostami-Rad, S.; Jafari, R.; Darani, H.Y. Th1/Th2-type cytokine profile in C57 black mice inoculated with live Echinococcus granulosus protoscolices. J. Infect. Public Health 2018, 11, 834–839. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajizadeh, M.; Jabbari, A.; Spotin, A.; Hejazian, S.S.; Mikaeili Galeh, T.; Hassannia, H.; Sahlolbei, M.; Pagheh, A.S.; Ahmadpour, E. Modulatory Effects of Hydatid Cyst Fluid on a Mouse Model of Experimental Autoimmune Encephalomyelitis. Vet. Sci. 2024, 11, 34. https://doi.org/10.3390/vetsci11010034
Hajizadeh M, Jabbari A, Spotin A, Hejazian SS, Mikaeili Galeh T, Hassannia H, Sahlolbei M, Pagheh AS, Ahmadpour E. Modulatory Effects of Hydatid Cyst Fluid on a Mouse Model of Experimental Autoimmune Encephalomyelitis. Veterinary Sciences. 2024; 11(1):34. https://doi.org/10.3390/vetsci11010034
Chicago/Turabian StyleHajizadeh, Maryam, Aynaz Jabbari, Adel Spotin, Seyyed Sina Hejazian, Tahereh Mikaeili Galeh, Hadi Hassannia, Maryam Sahlolbei, Abdol Sattar Pagheh, and Ehsan Ahmadpour. 2024. "Modulatory Effects of Hydatid Cyst Fluid on a Mouse Model of Experimental Autoimmune Encephalomyelitis" Veterinary Sciences 11, no. 1: 34. https://doi.org/10.3390/vetsci11010034
APA StyleHajizadeh, M., Jabbari, A., Spotin, A., Hejazian, S. S., Mikaeili Galeh, T., Hassannia, H., Sahlolbei, M., Pagheh, A. S., & Ahmadpour, E. (2024). Modulatory Effects of Hydatid Cyst Fluid on a Mouse Model of Experimental Autoimmune Encephalomyelitis. Veterinary Sciences, 11(1), 34. https://doi.org/10.3390/vetsci11010034





