Successful Treatment of Captive Common Marmosets (Callithrix jacchus) Infested with Common Cat Fleas (Ctenocephalides felis) by Using Topical Imidacloprid and Environmental Control Measures
Abstract
:Simple Summary
Abstract
1. Introduction
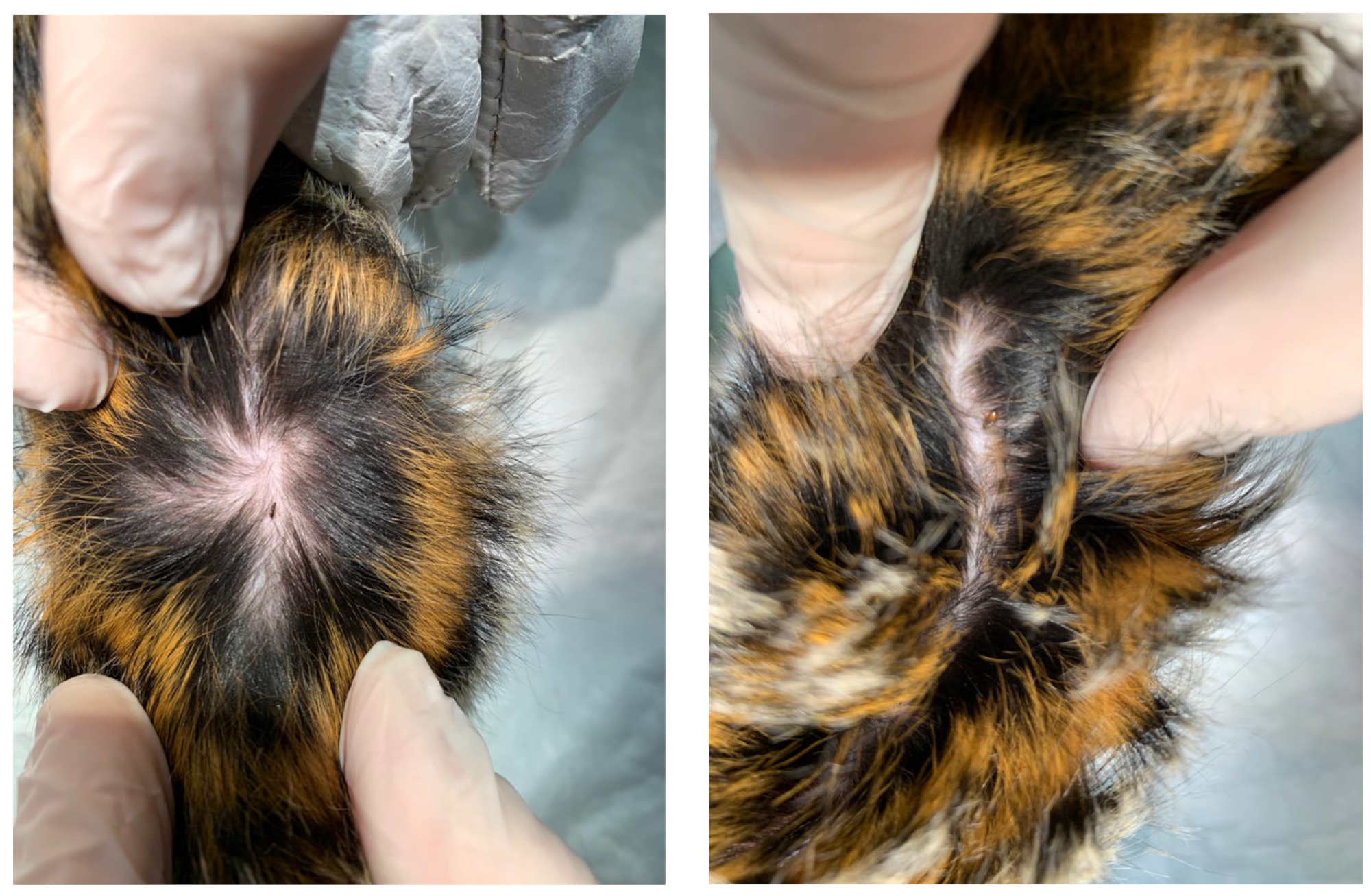
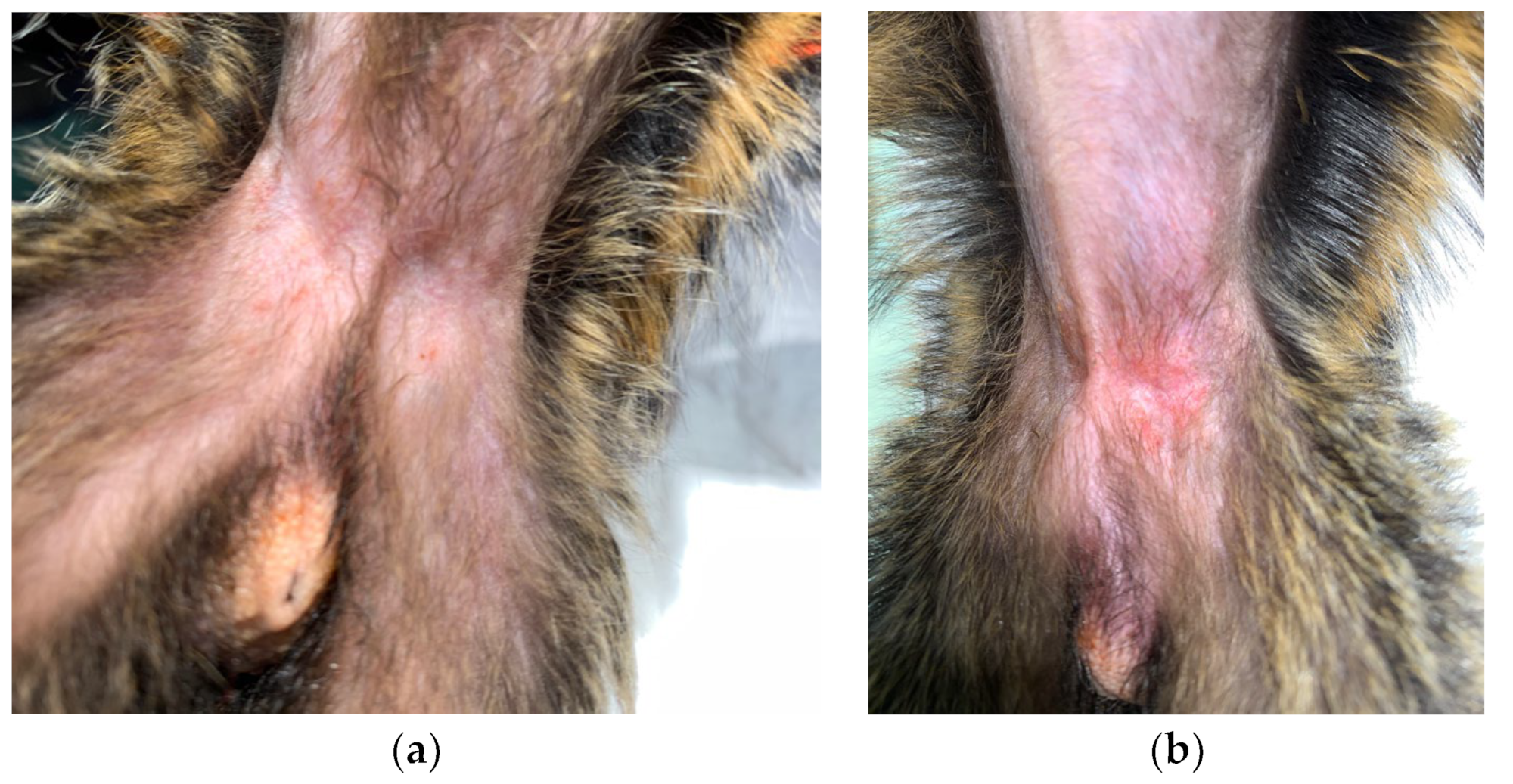
2. Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mansfield, K. Marmoset models commonly used in biomedical research. Comp. Med. 2003, 53, 383–392. [Google Scholar] [PubMed]
- Visser, M.; Rehbein, S.; Wiedemann, C. Species of flea (siphonaptera) infesting pets and hedgehogs in Germany. J. Vet. Med. B Infect. Dis. Vet. Public Health 2001, 48, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Zajac, A.M.; Conboy, G.A.; Little, S.E.; Reichard, M.V. Veterinary Clinical Parasitology, 9th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2021. [Google Scholar]
- Genchi, M.; Traldi, G.; Genchi, C.; Cervone, M. Manuel de Parasitologie Vétérinaire du Chien et du Chat, 1st ed.; Editions Med’Com: Paris, France, 2017. [Google Scholar]
- Bond, R.; Riddle, A.; Mottram, L.; Beugnet, F.; Stevenson, R. Survey of flea infestation in dogs and cats in the United Kingdom during 2005. Vet. Rec. 2007, 160, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Flynn, R.J. Parasites of Laboratory Animals, 1st ed.; Iowa State University Press: Ames, IA, USA, 1973. [Google Scholar]
- Strait, K.; Else, J.G.; Eberhard, M.L. Parasitic diseases of nonhuman primates. In Nonhuman Primates in Biomedical Research: Diseases, 2nd ed.; Abee, C.R., Mansfield, K., Tardif, S., Morris, T., Eds.; Elsevier: London, UK, 2012; Volume 2, pp. 260–264. [Google Scholar]
- Johnson-Delaney, C.A. Parasites of captive nonhuman primates. Vet. Clin. Exot. Anim. 2009, 12, 563–581. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.G. Chapter 17. Parasitic Diseases. In The Common Marmoset in Captivity and Biomedical Research; Marini, R.P., Wachtman, L.M., Tardif, S.S., Mansfield, K., Fox, J.G., Eds.; Elsevier: London, UK, 2019; p. 300. [Google Scholar]
- Mehlhorn, H.; Hansen, O.; Mencke, N. Comparative study on the effects of three insecticides (fipronil, imidacloprid, selamectin) on developmental stages of the cat flea (Ctenocephalides felis Bouché 1835): A light and electron microscopic analysis of in vivo and in vitro experiments. Parasitol. Res. 2001, 87, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Cadiergues, M.C.; Deloffre, P.; Franc, M. Species of fleas found on cats in France. Rev. Med. Vet. 2000, 151, 447–450. [Google Scholar]
- Schnieder, T.; Wolken, S.; Mencke, N. Comparative efficacy of imidacloprid, selamectin, fipronil-(S)-methoprene, and metaflumizone against cats experimentally infested with Ctenocephalides felis. Vet. Ther. 2008, 9, 176–183. [Google Scholar] [PubMed]
- Dryden, M.W.; Denenberg, T.M.; Bunch, S. Control of fleas on naturally infested dogs and cats in private residences with topical spot applications of fipronil or imidacloprid. Vet. Parasitol. 2000, 93, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Rust, M.K.; Hemsarth, W.L.H. Synergism of Adulticides and Insect Growth Regulators Against Larval Cat Fleas (Siphonaptera: Pulicidae). J. Med. Entomol. 2019, 56, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Beck, W.; Stickel, M. Migrationsverhalten von Ctenocephalides felis auf Katzen und in deren Lagerstätten. Wien. Klin. Wochenschr. 2008, 120, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Rust, M.K. The Biology and Ecology of Cat Fleas and Advancements in Their Pest Management: A Review. Insects 2017, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Rust, M.K.; Dryden, M.W. The biology, ecology, and management of the cat flea. Annu. Rev. Entomol. 1997, 42, 451–473. [Google Scholar] [CrossRef] [PubMed]
- Rust, M.K. Interhost movement of adult cat fleas. J. Med. Entomol. 1994, 31, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Franc, M.; Bouhsira, É.; Beugnet, F. Direct transmission of the cat flea (Ctenocephalides felis) between cats exhibiting social behaviour. Parasite 2013, 20, 49. [Google Scholar] [CrossRef] [PubMed]
- Rust, M.K. Recent advancements in the control of cat fleas. Insect 2020, 11, 668. [Google Scholar] [CrossRef] [PubMed]
- Heymann, E.W. Scent marking strategies of New World primates. Am. J. Primatol. 2006, 68, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Lazaro-Perea, C.; Snowdon, C.; de Fátima Arruda, M. Scent-marking behavior in wild groups of common marmosets (Callithrix jacchus). Behav. Ecol. Sociobiol. 1999, 46, 313–324. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cermolacce, A.; Lacoste, R.; Moulin, V.; Briand, A.; Bakker, J. Successful Treatment of Captive Common Marmosets (Callithrix jacchus) Infested with Common Cat Fleas (Ctenocephalides felis) by Using Topical Imidacloprid and Environmental Control Measures. Vet. Sci. 2023, 10, 580. https://doi.org/10.3390/vetsci10090580
Cermolacce A, Lacoste R, Moulin V, Briand A, Bakker J. Successful Treatment of Captive Common Marmosets (Callithrix jacchus) Infested with Common Cat Fleas (Ctenocephalides felis) by Using Topical Imidacloprid and Environmental Control Measures. Veterinary Sciences. 2023; 10(9):580. https://doi.org/10.3390/vetsci10090580
Chicago/Turabian StyleCermolacce, Alexia, Romain Lacoste, Valérie Moulin, Amaury Briand, and Jaco Bakker. 2023. "Successful Treatment of Captive Common Marmosets (Callithrix jacchus) Infested with Common Cat Fleas (Ctenocephalides felis) by Using Topical Imidacloprid and Environmental Control Measures" Veterinary Sciences 10, no. 9: 580. https://doi.org/10.3390/vetsci10090580
APA StyleCermolacce, A., Lacoste, R., Moulin, V., Briand, A., & Bakker, J. (2023). Successful Treatment of Captive Common Marmosets (Callithrix jacchus) Infested with Common Cat Fleas (Ctenocephalides felis) by Using Topical Imidacloprid and Environmental Control Measures. Veterinary Sciences, 10(9), 580. https://doi.org/10.3390/vetsci10090580







