Endoscopic Anatomy of the Equine Guttural Pouch: An Anatomic Observational Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
3. Results
- −
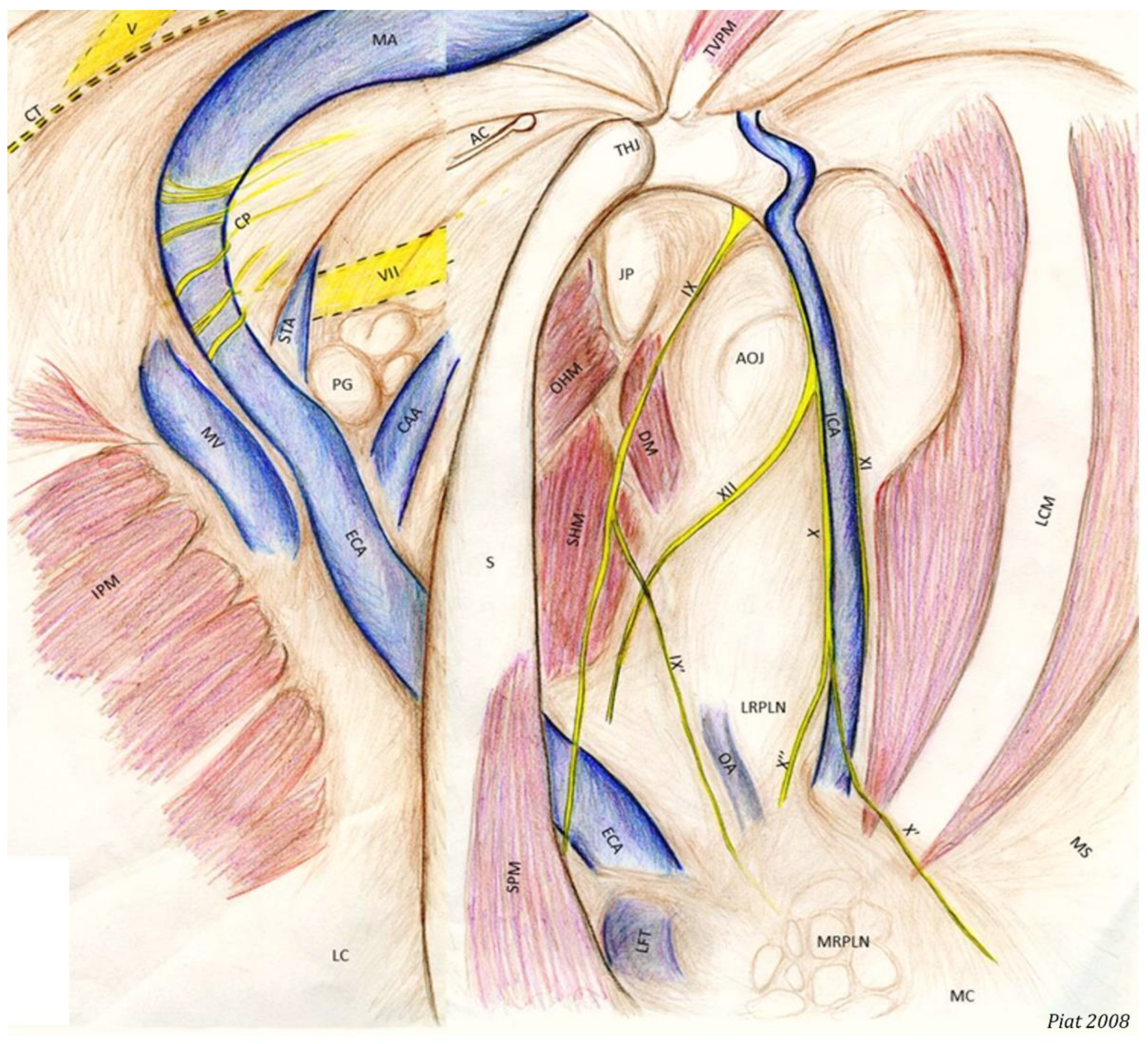
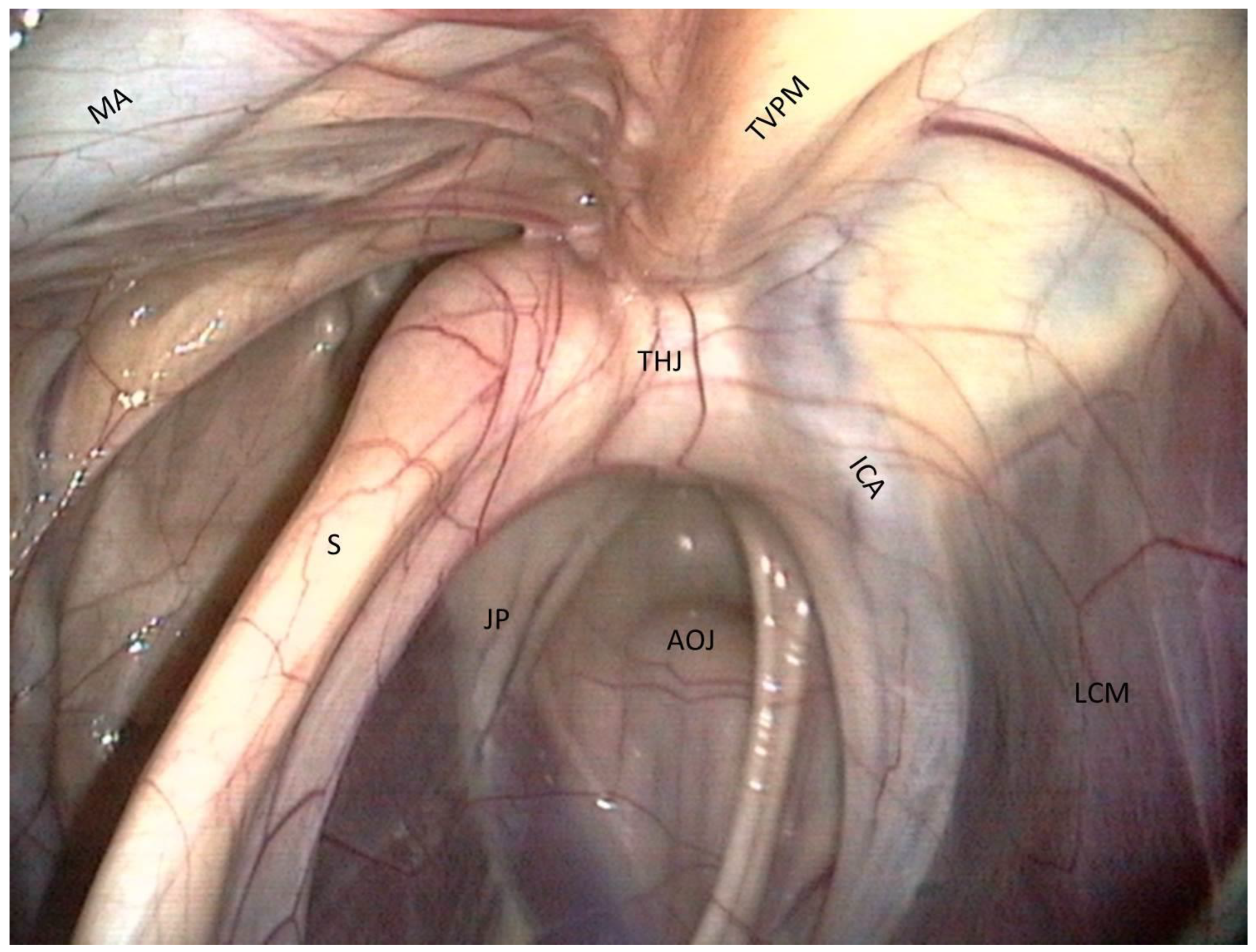
- The most clinically relevant structure in this area is the temporohyoid joint, which is a symphysis and links the stylohyoid bone and the styloid process of the temporal bone.
- −
- Just close to them lies the petrous part of the temporal bone, which we can see as a large white relief medially to the joint.
- −
- Just above the joint, the tensor veli palatini muscle is visible and runs toward the rostral part of the roof of the guttural pouch.
- −
- Medially, the internal carotid artery is visible. It forms a sigmoid inflexion before leaving contact with the pouch to join the Circle of Willis.
- −
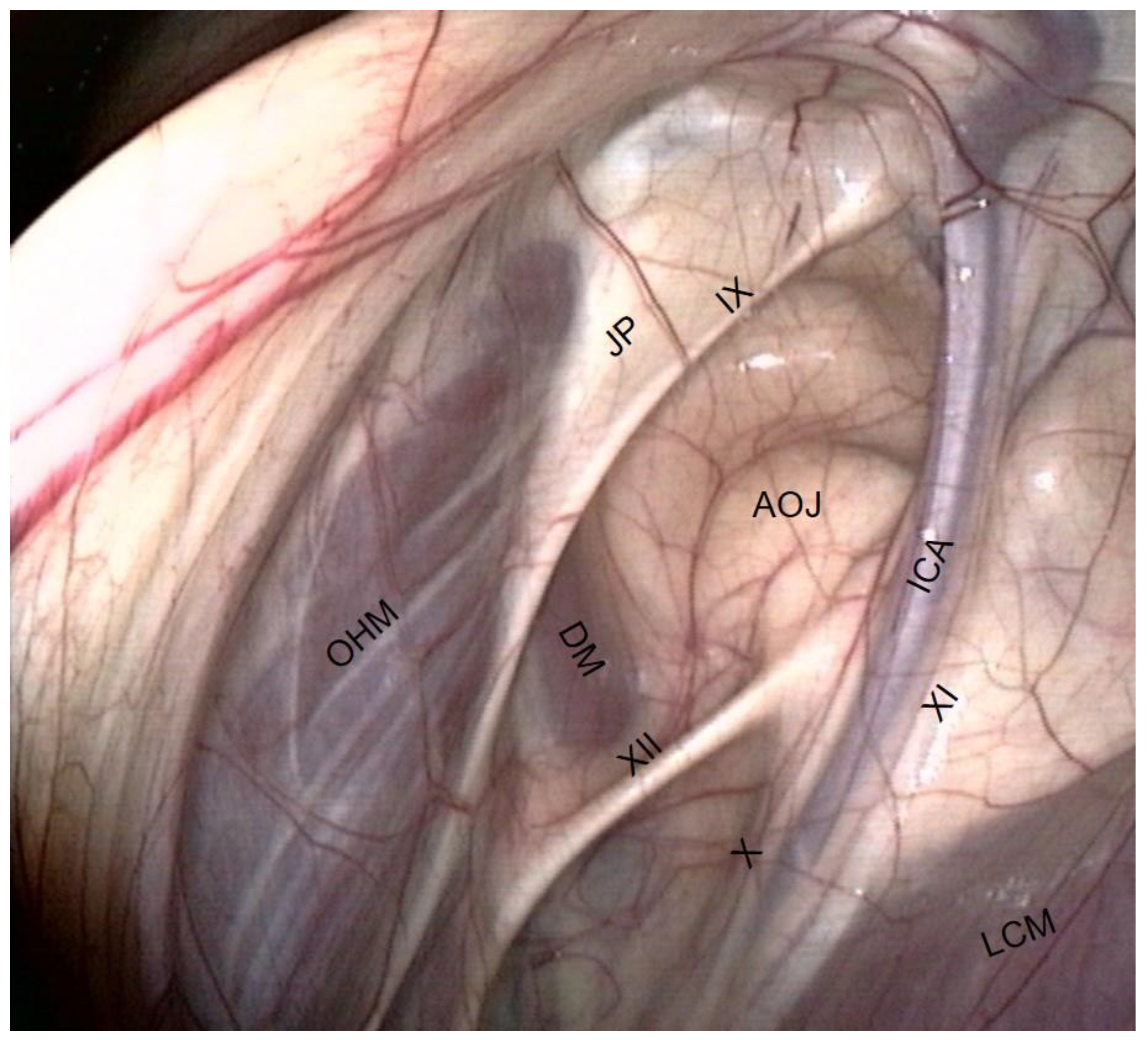
- The occipito-hyoïdeus muscle links the jugular process of the occipital bone to the stylohyoïd bone.
- −
- The caudal part of the Digastricus muscle is also inserted into the jugular process but runs ventrally and quickly loses contact with the pouch.
- −
- Finally, the stylohyoideus muscle is sometimes visible and runs along the caudal aspect of the stylohyoid bone.
- −
- The atlanto-occipital joint lies close to this mass of muscles. It looks like a large circular white surface, more or less concave. Its location is important, as fungal arthritis of this joint has been reported following guttural pouch mycosis [3].
- −
- The median septum, created by the apposition of the mucous membranes of the two pouches:
- −
- The longus capitis muscle caudally, which is a large fleshy muscular body covered by a white and shiny vertical band;
- −
- The dorsal pharyngeal recess rostro-ventrally, which is not easily visible from the pouch, but it is important to know its position, especially when performing laser surgery.
- −
- The floor lies on the Carotid trifurcation, but this major branching is never clearly visible.
- −
- Laterally, we can see the origin of the external carotid artery, which runs under the stylohyoid bone and then goes into the lateral compartment.
- −
- Occasionally, it is also possible to visualize the origin of the linguofacial trunk, running cranially, and of the occipital artery, running proximally, between the two carotids.
- −
- The stylopharyngeus muscle is particularly evident on the medial and distal aspects of the stylohyoid bone (Figure 4).
- −
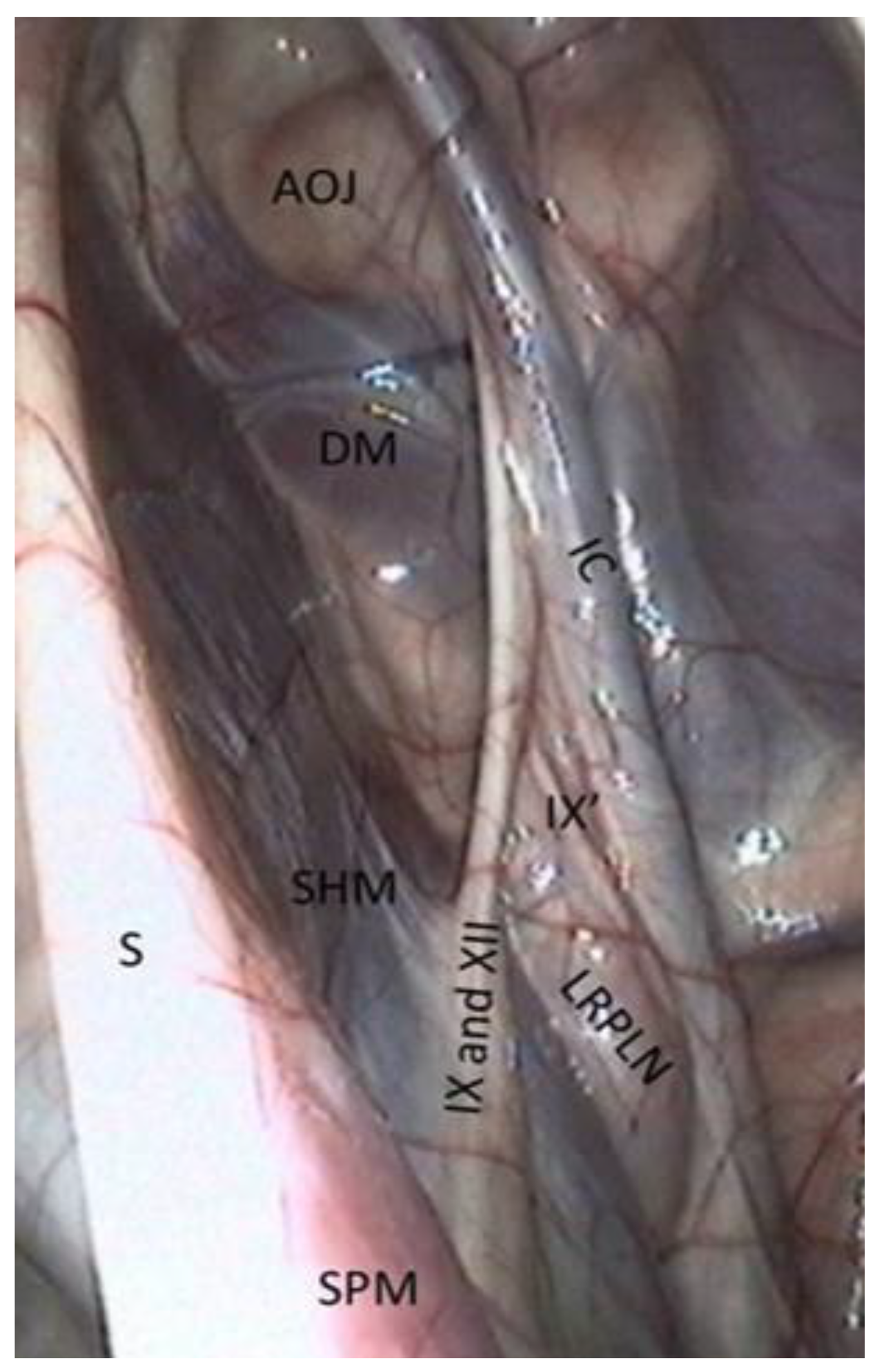
- The medial retropharyngeal lymph nodes, which can cause GP empyema, lie ventrally, under the floor of the guttural pouch.
- −
- Sometimes (as in Figure 4), it is also possible to see the lateral retropharyngeal LN, just close to the internal carotid.
- −
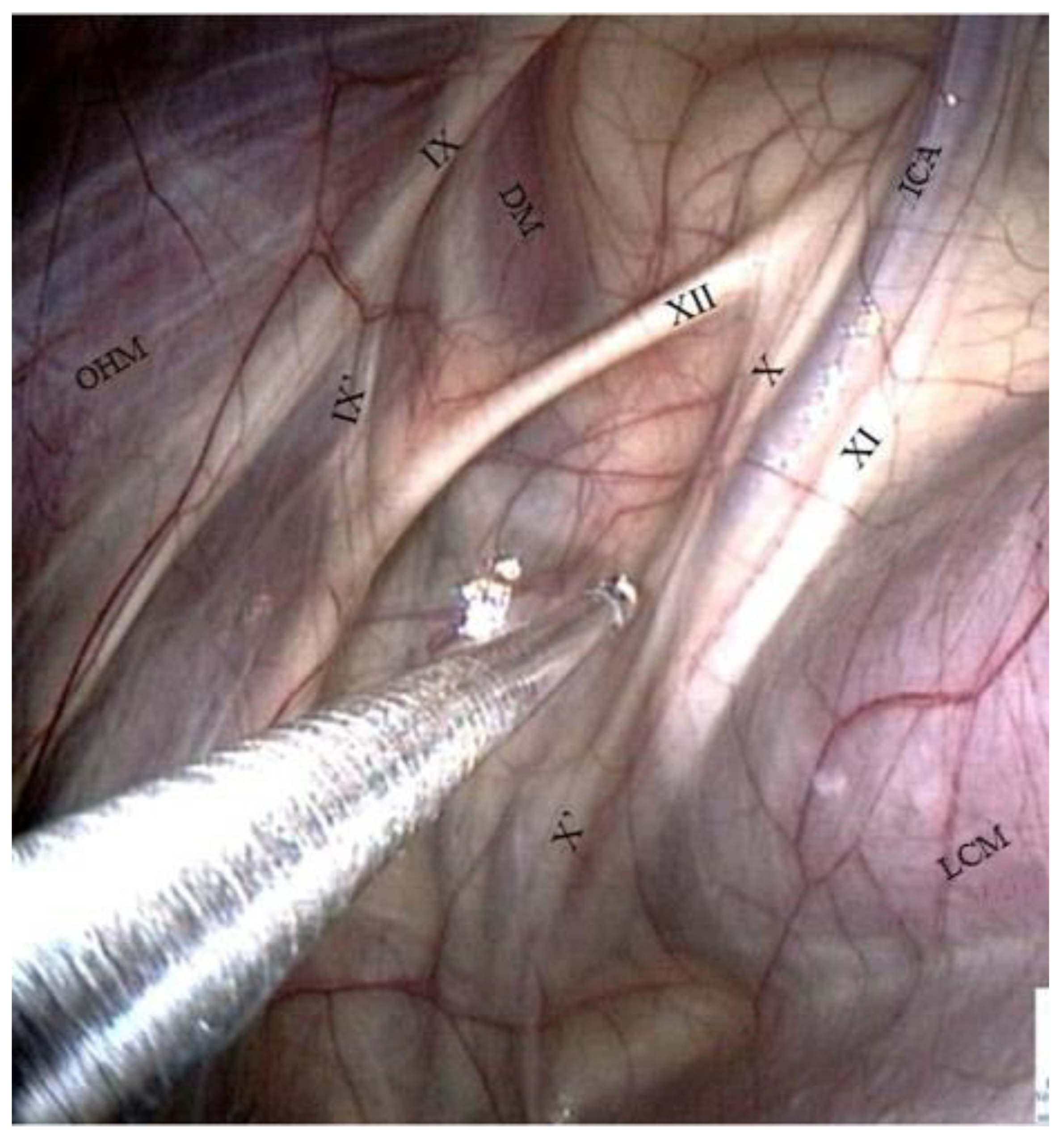
- The glossopharyngeal nerve is the most dorsal and lateral and is often thinner than its neighbor, number XII. It goes halfway through a ramification that goes down toward the carotid trifurcation: the Hering nerve, or branch of glossopharyngeal nerve, to the carotid sinus, which plays a role in the regulation of blood pressure.
- −
- The hypoglossal nerve also runs obliquely, either alone under the glossopharyngeal nerve or joined onto it (Figure 4), in the same mucosal fold. A good way to differentiate them is that distally, the hypoglossal nerve runs behind the external carotid artery, and the glossopharyngeal runs in front of it. Moreover, the hypoglossal nerve is thicker and has no ramifications.
- −
- The vagus and accessory nerves are difficult to distinguish as both of them run along or behind the internal carotid artery. A simple way to identify the vagus nerve is to identify its pharyngeal branch, which is easily visible, and go back up to its origin. This branch is the little nerve that we always see running ventrally across the longus capitis muscle, with a rostral direction toward the pharyngeal plexus. Generally, the vagus nerve is lateral to the ICA, and the accessory nerve is medial.
- −
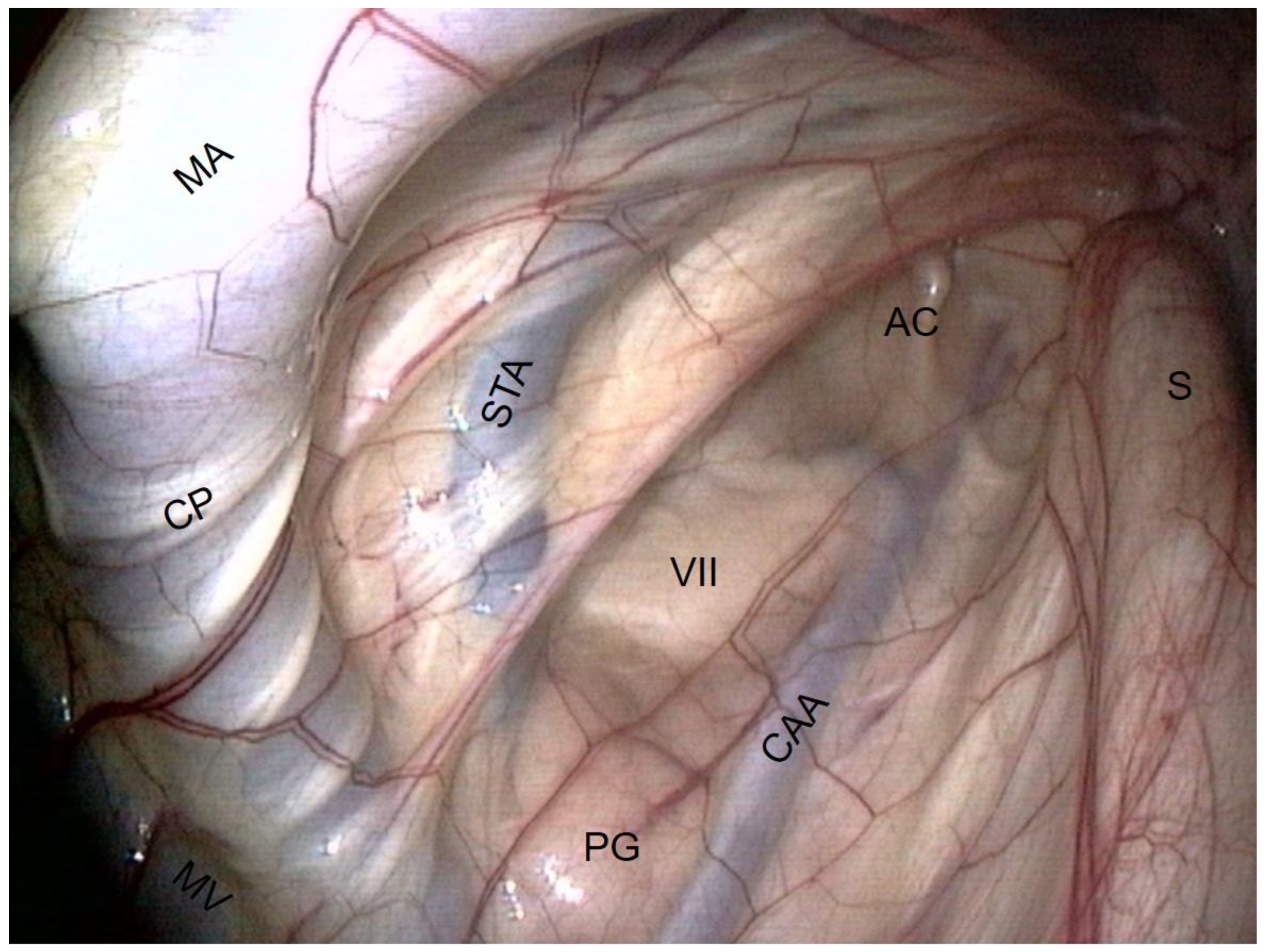
- The external carotid artery (ECA) emerges from behind the stylohyoid bone and gives rise to the others.
- −
- The first branch is the caudal auricular artery, which runs up along the stylohyoid bone.
- −
- The next branch is the superficial temporal artery.
- −
- Following this, the ECA becomes the maxillary artery, which curves and loses contact with the pouch by entering the alar foramen.
- −
- The spider web that is visible on the inflection of the maxillary artery is the carotid plexus, which innervates the external carotid and maxillary artery.
- −
- Rostrally and ventrally, the maxillary vein runs along the internal pterygoideus muscle.
- −
- Then, in the back of the compartment, it is easy to see the styloid process of the auricular cartilage. It looks like a matchstick and moves with the ear.
- −
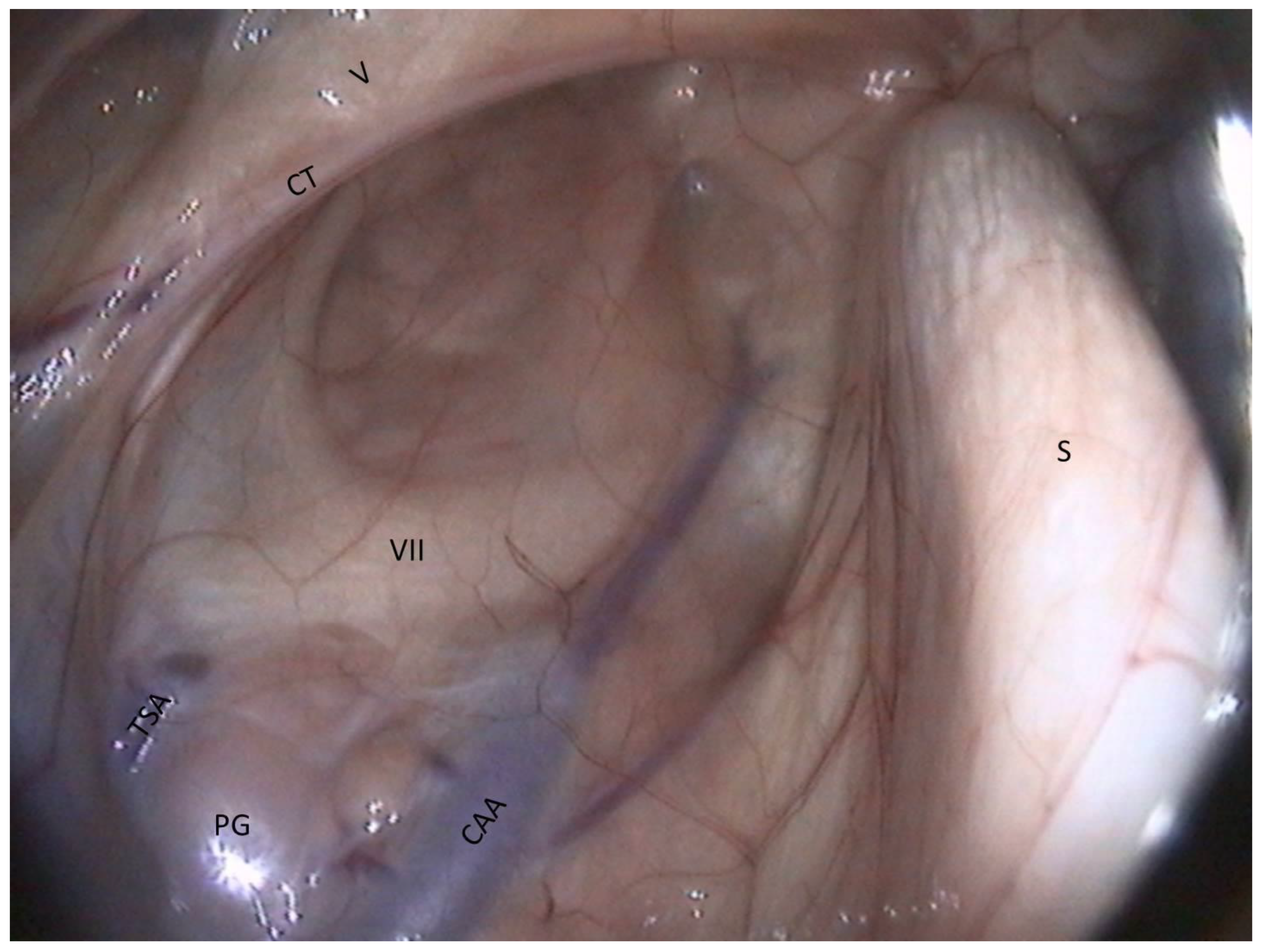
- The facial nerve lies ventrally to this cartilage. It consists of a large horizontal white band that crosses the compartment between the two arteries. It is the largest nerve visible in the guttural pouch.
- −
- Just in the triangle formed by the facial nerve and the two arteries, we observe some pink globular structures, which are part of the parotid gland.
- −
- Very rarely, it is possible to see the chorda tympani and the maxillary nerve, as in Figure 8.
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LC | Lateral compartment |
| MC | Medial compartment |
| MS | Median septum |
| Arteries | |
| ECA | External carotid a. |
| ICA | Internal carotid a. |
| OA | Occipital a. |
| LFT | Linguofacial trunk |
| CAA | Caudal auricular a. |
| STA | Superficial temporal a. |
| MA | Maxillary a. |
| Veins | |
| MV | Maxillary v. |
| Muscles | |
| DM | Digastric m. |
| LCM | Longus capitis m. |
| OHM | Occipitohyoideus m. |
| IPM | Internal pterygoideus m. |
| SHM | Stylohyoideus m. |
| SPM | Stylopharyngeus m. |
| TVPM | Tensor veli palatini m. |
| Nerves | |
| V | Mandibular n. |
| VII | Facial n. |
| CT | Chorda tympani n. |
| IX | Glossopharyngeal n. |
| IX’ | Hering n. |
| X | Vagus n. |
| X’ | Pharyngeal branch of X |
| X’’ | Cranial laryngeal n. |
| XI | Accessory n. |
| XII | Hypoglossal n. |
| CP | Carotid plexus |
| Bones and cartilage | |
| AOJ | Atlanto-occipital joint |
| THJ | Temporohyoid joint |
| JP | Jugular process of occipital bone |
| S | Stylohyoid bone |
| AC | Auricular cartilage |
| Glands and lymph nodes | |
| PG | Parotid gland |
| MRPLN | Medial retropharyngeal lymph nodes |
| LRPLN | Lateral retropharyngeal lymph nodes |
References
- Barone, R.; Dannacher, G. Observations anatomiques sur les poches gutturales des Equidés. Bull. Soc. Sci. Vet. Lyon 1955, 6, 229–238. [Google Scholar]
- Muylle, S.; Van Loon, G.; Deprez, P.; Simoens, P. Luchtzak-endoscopie bij het paard: Anatomische interpretatie van de beelden. Vlaams Diergeneeskd. Tijdschr. 1997, 66, 75–80. [Google Scholar]
- Dixon, P.M.; Rowlands, A.C. Atlanto-occipital joint infection associated with guttural pouch mycosis in a horse. Equine Vet. J. 1981, 13, 260–262. [Google Scholar] [CrossRef] [PubMed]
- Genton, M.; Farfan, M.; Tesson, C.; Laclaire, A.L.; Rossignol, F.; Mespoulhes-Rivière, C. Balloon catheter occlusion of the maxillary, internal, and external carotid arteries in standing horses. Vet. Surg. 2021, 50, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Boyle, A.G.; Timoney, J.F.; Newton, J.R.; Hines, M.T.; Waller, A.S.; Buchanan, B.R. Streptococcus equi Infections in Horses: Guidelines for Treatment, Control, and Prevention of Strangles-Revised Consensus Statement. J. Vet. Intern. Med. 2018, 32, 633–647. [Google Scholar] [CrossRef] [PubMed]
- Tate, L.P.; Blikslager, A.T.; Little, E.D.E. Transendoscopic laser treatment of guttural pouch tympanites in eight foals. Vet. Surg. 1995, 24, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Schambourg, M.A.; Marcoux, M.; Céleste, C. Salpingoscopy for the treatment of recurrent guttural pouch tympany in a filly. Equine Vet. Educ. 2006, 18, 231–237. [Google Scholar] [CrossRef]
- Bentz, B.G.; Dowd, A.L.; Freeman, D.E. Treatment of guttural pouch empyema with acetylcysteine irrigation. Equine Pract. 1996, 18, 33–35. [Google Scholar]
- Seahorn, T.L.; Schumacher, J. Nonsurgical removal of chondroid masses from the guttural pouches of two horses. J. Am. Vet. Med. Assoc. 1991, 199, 368–369. [Google Scholar] [PubMed]
- Gehlen, H.; Ohnesorge, B. Laser fenestration of the medial septum for treatment of guttural pouch chondroids in a pony. Vet. Surg. 2005, 34, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Munoz, J.A.; Stephen, J.; Baptiste, K.E.; Lepage, O.M. A surgical approach to the lateral compartment of the equine guttural pouch in the standing horse: Modification of the forgotten “Garm technique”. Vet. J. 2007, 177, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Watkins, A.R.; Parente, E.J. Salpingopharyngeal fistula as a treatment for guttural pouch mycosis in seven horses. Equine Vet. J. 2018, 50, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Koch, D.W.; Ericksen, K.A.; Easley, J.T.; Hackett, E.S. Clinical outcome of horses with guttural pouch infection following transpharyngeal fenestration. J. Am. Vet. Med. Assoc. 2022, 260, 1211–1215. [Google Scholar] [CrossRef] [PubMed]
- Denoix, J.-M. Réflexion anatomique sur les diverses techniques de paracenthèse de la poche gutturale. Entret. Bourgelat 1982, tome II, 347–366. [Google Scholar]
- Freeman, D.E. Complications of surgery for diseases of the guttural pouch. Vet. Clin. N. Am. Equine Pract. 2008, 24, 485–497. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piat, P.; Cadoré, J.-L. Endoscopic Anatomy of the Equine Guttural Pouch: An Anatomic Observational Study. Vet. Sci. 2023, 10, 542. https://doi.org/10.3390/vetsci10090542
Piat P, Cadoré J-L. Endoscopic Anatomy of the Equine Guttural Pouch: An Anatomic Observational Study. Veterinary Sciences. 2023; 10(9):542. https://doi.org/10.3390/vetsci10090542
Chicago/Turabian StylePiat, Perrine, and Jean-Luc Cadoré. 2023. "Endoscopic Anatomy of the Equine Guttural Pouch: An Anatomic Observational Study" Veterinary Sciences 10, no. 9: 542. https://doi.org/10.3390/vetsci10090542
APA StylePiat, P., & Cadoré, J.-L. (2023). Endoscopic Anatomy of the Equine Guttural Pouch: An Anatomic Observational Study. Veterinary Sciences, 10(9), 542. https://doi.org/10.3390/vetsci10090542




