Negative Influence of Aging on Differentiation and Proliferation of CD8+ T-Cells in Dogs
Abstract
:Simple Summary
Abstract
1. Introduction
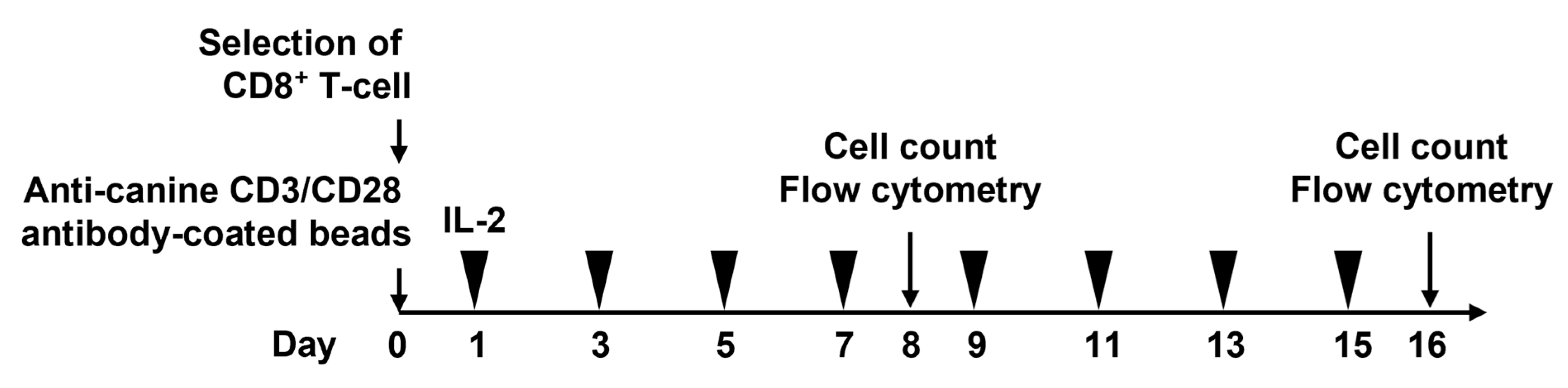
2. Materials and Methods
2.1. Animals
2.2. Cell Culture
2.3. Flow Cytometry
2.4. Statistical Analysis
3. Results
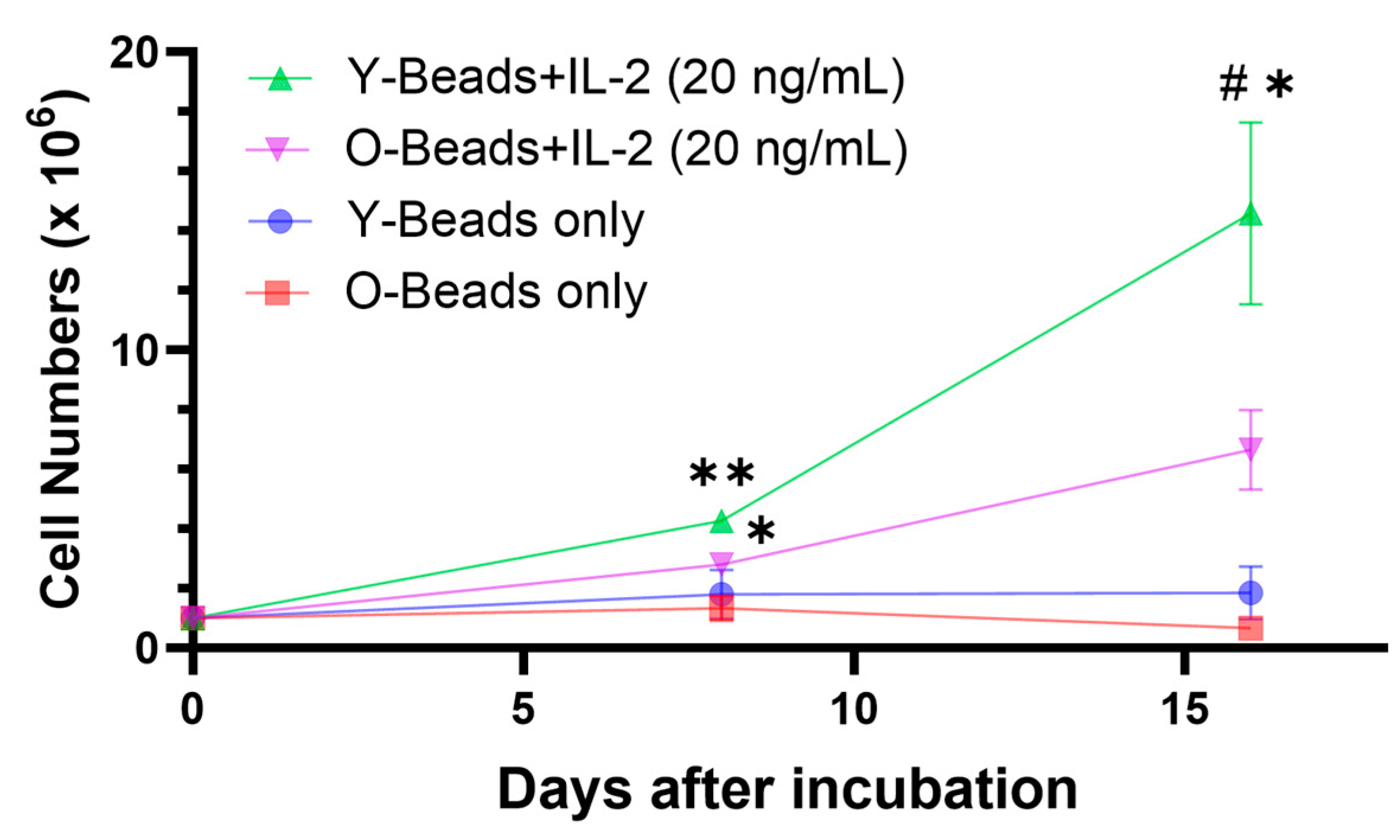
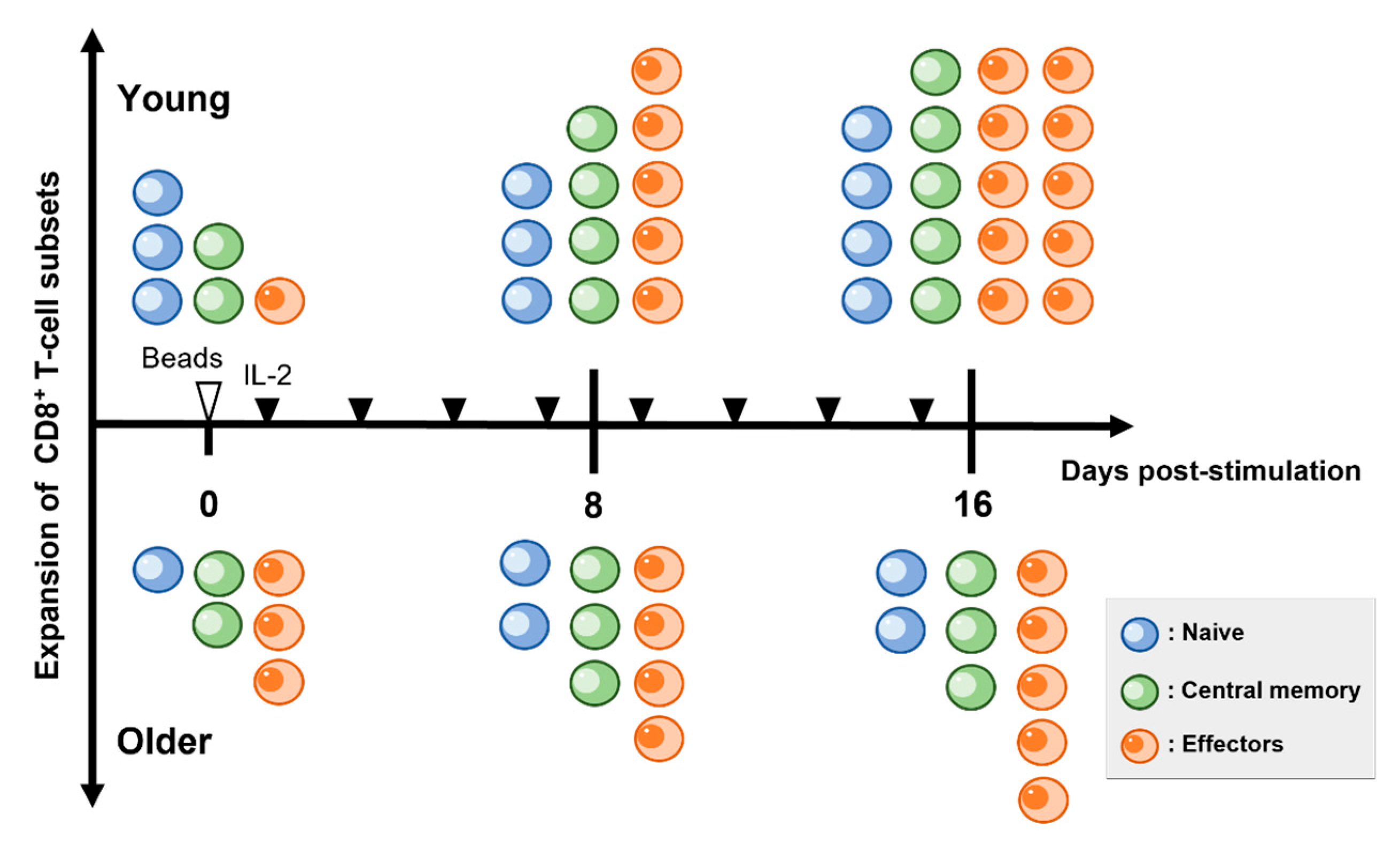
3.1. CD8+ T-Cells from Older Dogs Had Lower Proliferative Capacity than Young Dogs
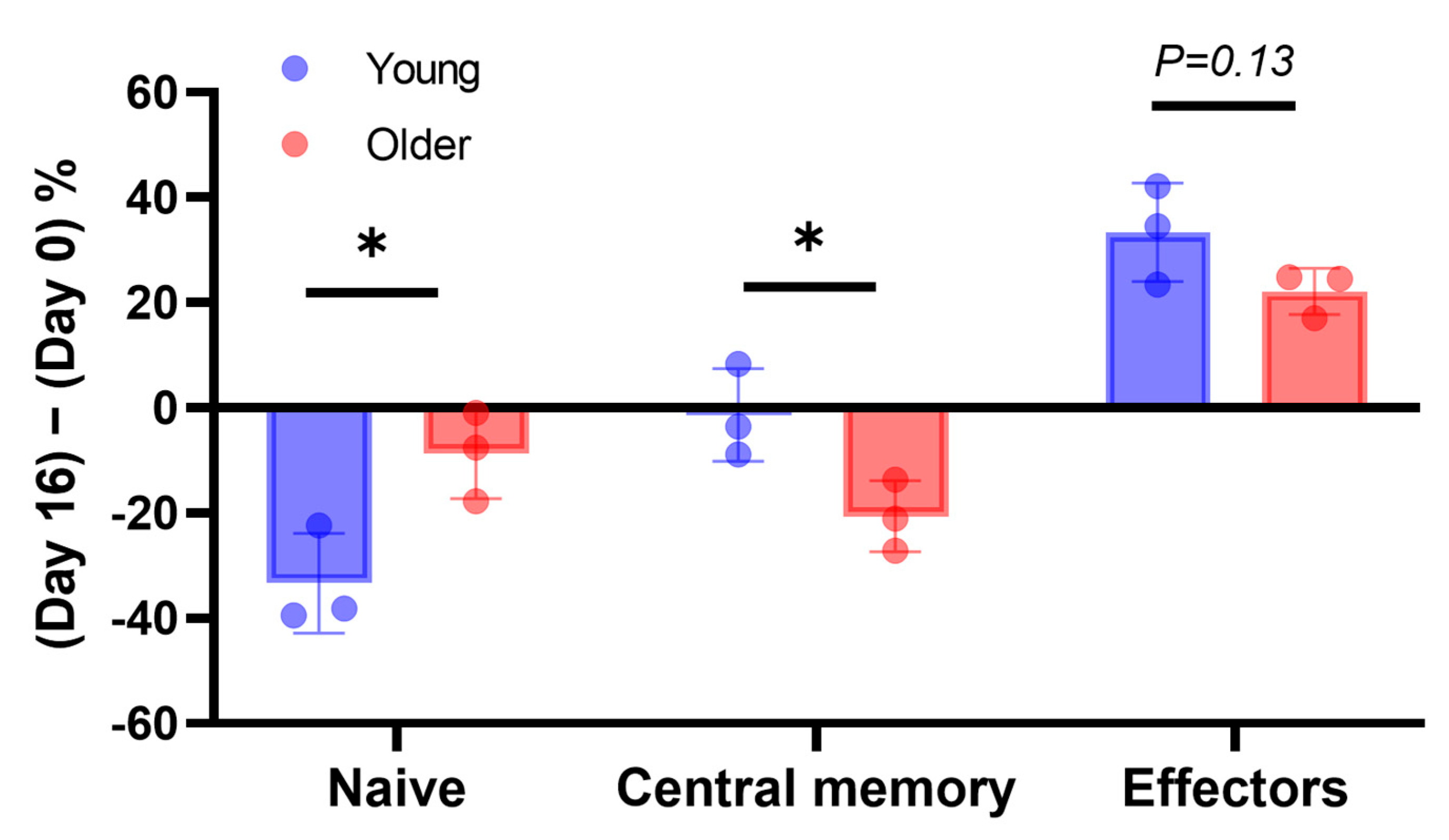
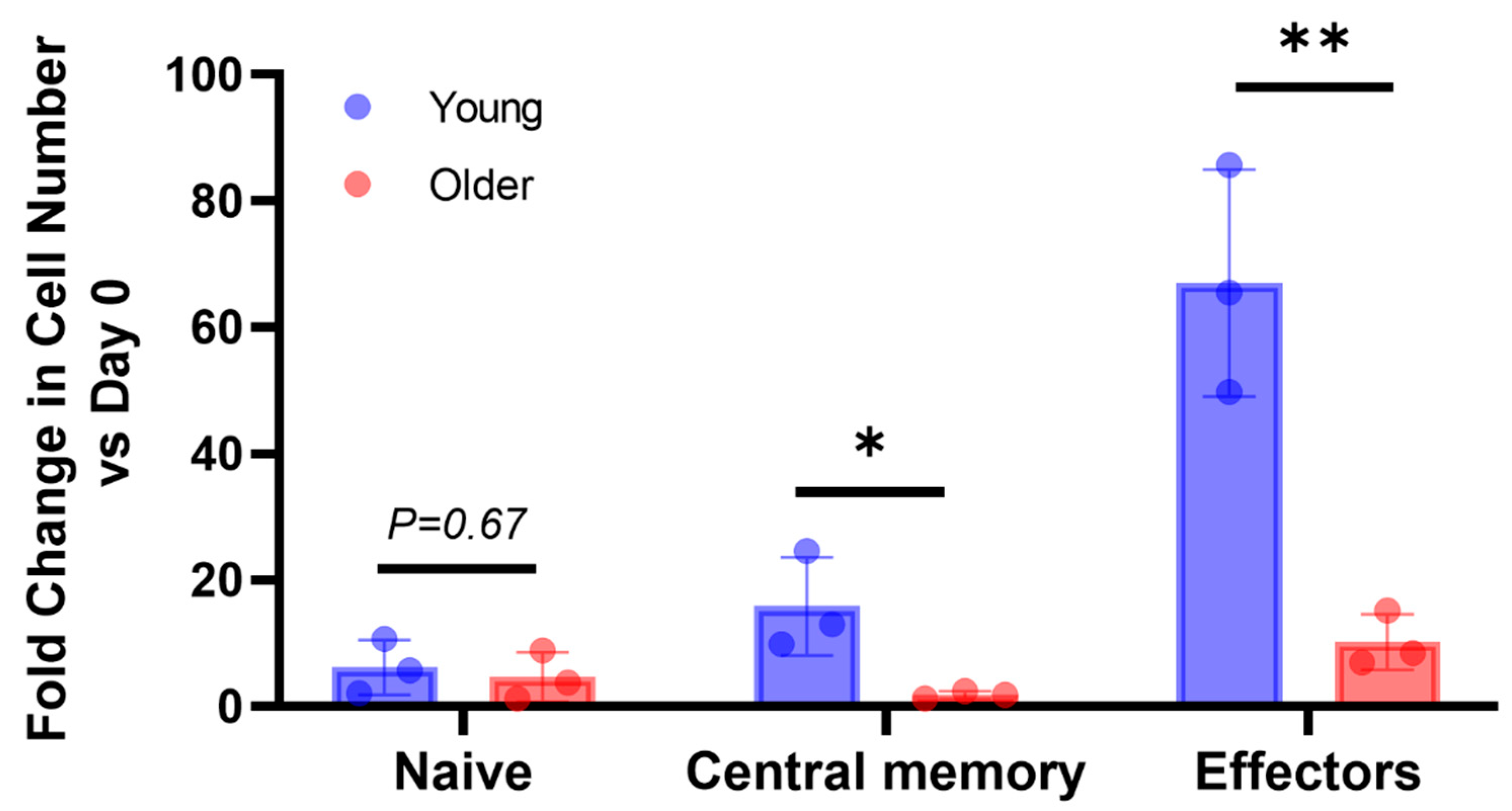
3.2. In Older Dogs, the Population of Naïve CD8+ T-Cells Was Reduced, and the Expansion of Effector and Memory Subsets Was Attenuated
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mills, G. What are the most common types of cancers in dogs? How many dogs typically get cancer? VetRecord 2021, 188, 170–171. [Google Scholar] [CrossRef]
- Demaria, O.; Cornen, S.; Daëron, M.; Morel, Y.; Medzhitov, R.; Vivier, E. Harnessing innate immunity in cancer therapy. Nature 2019, 574, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Philip, M.; Schietinger, A. CD8(+) T cell differentiation and dysfunction in cancer. Nat. Rev. Immunol. 2022, 22, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gögenur, I. Cytotoxic CD8(+) T cells in cancer and cancer immunotherapy. Br. J. Cancer 2021, 124, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Chen, X.; Li, Z.; Luo, Y.; Fang, Z.; Xu, B.; Han, M. PD-1 Antibody monotherapy for malignant melanoma: A systematic review and meta-analysis. PLoS ONE 2016, 11, e0160485. [Google Scholar] [CrossRef] [PubMed]
- Goyal, G.; Silberstein, P.T. Systemic therapy in metastatic melanoma. Fed. Pract. 2015, 32, 57s–65s. [Google Scholar]
- Maekawa, N.; Konnai, S.; Takagi, S.; Kagawa, Y.; Okagawa, T.; Nishimori, A.; Ikebuchi, R.; Izumi, Y.; Deguchi, T.; Nakajima, C.; et al. A canine chimeric monoclonal antibody targeting PD-L1 and its clinical efficacy in canine oral malignant melanoma or undifferentiated sarcoma. Sci. Rep. 2017, 7, 8951. [Google Scholar] [CrossRef]
- Igase, M.; Nemoto, Y.; Itamoto, K.; Tani, K.; Nakaichi, M.; Sakurai, M.; Sakai, Y.; Noguchi, S.; Kato, M.; Tsukui, T.; et al. A pilot clinical study of the therapeutic antibody against canine PD-1 for advanced spontaneous cancers in dogs. Sci. Rep. 2020, 10, 18311. [Google Scholar] [CrossRef]
- Grover, P.; Veilleux, O.; Tian, L.; Sun, R.; Previtera, M.; Curran, E.; Muffly, L. Chimeric antigen receptor T-cell therapy in adults with B-cell acute lymphoblastic leukemia. Blood Adv. 2022, 6, 1608–1618. [Google Scholar] [CrossRef]
- Chen, Y.J.; Abila, B.; Mostafa Kamel, Y. CAR-T: What is next? Cancers 2023, 15, 663. [Google Scholar] [CrossRef]
- Panjwani, M.K.; Atherton, M.J.; MaloneyHuss, M.A.; Haran, K.P.; Xiong, A.; Gupta, M.; Kulikovsaya, I.; Lacey, S.F.; Mason, N.J. Establishing a model system for evaluating CAR T cell therapy using dogs with spontaneous diffuse large B cell lymphoma. Oncoimmunology 2020, 9, 1676615. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.M.; Vétizou, M.; Daillère, R.; Roberti, M.P.; Yamazaki, T.; Routy, B.; Lepage, P.; Boneca, I.G.; Chamaillard, M.; Kroemer, G.; et al. Resistance mechanisms to immune-checkpoint blockade in cancer: Tumor-intrinsic and -extrinsic factors. Immunity 2016, 44, 1255–1269. [Google Scholar] [CrossRef]
- Sceneay, J.; Goreczny, G.J.; Wilson, K.; Morrow, S.; DeCristo, M.J.; Ubellacker, J.M.; Qin, Y.; Laszewski, T.; Stover, D.G.; Barrera, V.; et al. Interferon signaling is diminished with age and is associated with immune checkpoint blockade efficacy in triple-negative breast cancer. Cancer Discov. 2019, 9, 1208–1227. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, R.; Mezquita, L.; Auclin, E.; Chaput, N.; Besse, B. Immunosenescence and immunecheckpoint inhibitors in non-small cell lung cancer patients: Does age really matter? Cancer Treat. Rev. 2017, 60, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Yue, Y.; Yu, W.; Zhang, Y. Immunosenescence: A key player in cancer development. J. Hematol. Oncol. 2020, 13, 151. [Google Scholar] [CrossRef]
- Whiting, C.C.; Siebert, J.; Newman, A.M.; Du, H.W.; Alizadeh, A.A.; Goronzy, J.; Weyand, C.M.; Krishnan, E.; Fathman, C.G.; Maecker, H.T. Large-scale and comprehensive immune profiling and functional analysis of normal human aging. PLoS ONE 2015, 10, e0133627. [Google Scholar] [CrossRef]
- Czesnikiewicz-Guzik, M.; Lee, W.W.; Cui, D.; Hiruma, Y.; Lamar, D.L.; Yang, Z.Z.; Ouslander, J.G.; Weyand, C.M.; Goronzy, J.J. T cell subset-specific susceptibility to aging. Clin. Immunol. 2008, 127, 107–118. [Google Scholar] [CrossRef]
- Tedeschi, V.; Paldino, G.; Kunkl, M.; Paroli, M.; Sorrentino, R.; Tuosto, L.; Fiorillo, M.T. CD8(+) T Cell senescence: Lights and shadows in viral infections, autoimmune disorders and cancer. Int. J. Mol. Sci. 2022, 23, 3374. [Google Scholar] [CrossRef]
- Arcangeli, S.; Bove, C.; Mezzanotte, C.; Camisa, B.; Falcone, L.; Manfredi, F.; Bezzecchi, E.; El Khoury, R.; Norata, R.; Sanvito, F.; et al. CAR T cell manufacturing from naive/stem memory T lymphocytes enhances antitumor responses while curtailing cytokine release syndrome. J. Clin. Investig. 2022, 132, e150807. [Google Scholar] [CrossRef]
- Kaech, S.M.; Cui, W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat. Rev. Immunol. 2012, 12, 749–761. [Google Scholar] [CrossRef]
- Zhang, H.; Weyand, C.M.; Goronzy, J.J. Hallmarks of the aging T-cell system. FEBS J. 2021, 288, 7123–7142. [Google Scholar] [CrossRef] [PubMed]
- Goronzy, J.J.; Fang, F.; Cavanagh, M.M.; Qi, Q.; Weyand, C.M. Naive T cell maintenance and function in human aging. J. Immunol. 2015, 194, 4073–4080. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Kim, Y.H.; Chopra, R.K.; Proust, J.J.; Nagel, J.E.; Nordin, A.A.; Adler, W.H. Age-related effects in T cell activation and proliferation. Exp. Gerontol. 1993, 28, 313–321. [Google Scholar] [CrossRef]
- Guha, P.; Cunetta, M.; Somasundar, P.; Espat, N.J.; Junghans, R.P.; Katz, S.C. Frontline science: Functionally impaired geriatric CAR-T cells rescued by increased α5β1 integrin expression. J. Leukoc. Biol. 2017, 102, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Greeley, E.H.; Kealy, R.D.; Ballam, J.M.; Lawler, D.F.; Segre, M. The influence of age on the canine immune system. Vet. Immunol. Immunopathol. 1996, 55, 1–10. [Google Scholar] [CrossRef]
- Greeley, E.H.; Ballam, J.M.; Harrison, J.M.; Kealy, R.D.; Lawler, D.F.; Segre, M. The influence of age and gender on the immune system: A longitudinal study in Labrador Retriever dogs. Vet. Immunol. Immunopathol. 2001, 82, 57–71. [Google Scholar] [CrossRef]
- Greeley, E.H.; Spitznagel, E.; Lawler, D.F.; Kealy, R.D.; Segre, M. Modulation of canine immunosenescence by life-long caloric restriction. Vet. Immunol. Immunopathol. 2006, 111, 287–299. [Google Scholar] [CrossRef]
- Kesherwani, V.; Sodhi, A. Differential activation of macrophages in vitro by lectin concanavalin A, phytohemagglutinin and wheat germ agglutinin: Production and regulation of nitric oxide. Nitric Oxide 2007, 16, 294–305. [Google Scholar] [CrossRef]
- Lee, D.H.; Ahn, J.H.; Park, J.H.; Yan, B.C.; Cho, J.H.; Kim, I.H.; Lee, J.C.; Jang, S.H.; Lee, M.H.; Hwang, I.K.; et al. Comparison of expression of inflammatory cytokines in the spinal cord between young adult and aged beagle dogs. Cell Mol. Neurobiol. 2013, 33, 615–624. [Google Scholar] [CrossRef]
- Rotolo, A.; Atherton, M.J.; Kasper, B.T.; Haran, K.P.; Mason, N.J. Genetic re-direction of canine primary T cells for clinical trial use in pet dogs with spontaneous cancer. STAR Protoc. 2021, 2, 100905. [Google Scholar] [CrossRef]
- Appay, V.; Sauce, D. Naive T cells: The crux of cellular immune aging? Exp. Gerontol. 2014, 54, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Effros, R.B.; Dagarag, M.; Spaulding, C.; Man, J. The role of CD8+ T-cell replicative senescence in human aging. Immunol. Rev. 2005, 205, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Quinn, K.M.; Fox, A.; Harland, K.L.; Russ, B.E.; Li, J.; Nguyen, T.H.O.; Loh, L.; Olshanksy, M.; Naeem, H.; Tsyganov, K.; et al. Age-related decline in primary CD8(+) T cell responses is associated with the development of senescence in virtual memory CD8(+) T cells. Cell Rep. 2018, 23, 3512–3524. [Google Scholar] [CrossRef] [PubMed]
- Withers, S.S.; Moore, P.F.; Chang, H.; Choi, J.W.; McSorley, S.J.; Kent, M.S.; Monjazeb, A.M.; Canter, R.J.; Murphy, W.J.; Sparger, E.E.; et al. Multi-color flow cytometry for evaluating age-related changes in memory lymphocyte subsets in dogs. Dev. Comp. Immunol. 2018, 87, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Golubovskaya, V.; Wu, L. Different subsets of T cells, memory, effector functions, and CAR-T immunotherapy. Cancers 2016, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Ballegeer, M.; Libert, C. Different cell types involved in mediating concanavalin A induced liver injury: A comprehensive overview. J. Gastroenterol. Hepatol. Rev. 2016, 1, 1. [Google Scholar] [CrossRef]
- Möller, S.A.; Danielsson, L.; Borrebaeck, C.A. Concanavalin A-induced B-cell proliferation mediated by allogeneically derived helper factors. Immunology 1986, 57, 387–393. [Google Scholar]
- Langsdorf, C.L.; Liu, J.; Bradford, J.; Buller, G. Abstract 1943: A comparison of three techniques to induce efficient ex vivo T-cell expansion. Cancer Res. 2010, 70, 1943. [Google Scholar] [CrossRef]
- Chen, C.C.; Chang, Z.Y.; Tsai, F.J.; Chen, S.Y. Resveratrol pretreatment ameliorates concanavalin A-induced advanced renal glomerulosclerosis in aged mice through upregulation of sirtuin 1-mediated klotho expression. Int. J. Mol. Sci. 2020, 21, 6766. [Google Scholar] [CrossRef]
- Liu, Y.; Hao, H.; Hou, T. Concanavalin A-induced autoimmune hepatitis model in mice: Mechanisms and future outlook. Open Life Sci. 2022, 17, 91–101. [Google Scholar] [CrossRef]
- Tiegs, G.; Hentschel, J.; Wendel, A. A T cell-dependent experimental liver injury in mice inducible by concanavalin A. J. Clin. Investig. 1992, 90, 196–203. [Google Scholar] [CrossRef]
- Szopa, I.M.; Granica, M.; Bujak, J.K.; Łabędź, A.; Błaszczyk, M.; Paulos, C.M.; Majchrzak-Kuligowska, K. Effective activation and expansion of canine lymphocytes using a novel nano-sized magnetic beads approach. Front. Immunol. 2021, 12, 604066. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Rivière, I. Clinical manufacturing of CAR T cells: Foundation of a promising therapy. Mol. Ther. Oncolytics 2016, 3, 16015. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tsao, S.T.; Gu, M.; Fu, C.; He, F.; Li, X.; Zhang, M.; Li, N.; Hu, H.M. A simple and effective method to purify and activate T cells for successful generation of chimeric antigen receptor T (CAR-T) cells from patients with high monocyte count. J. Transl. Med. 2022, 20, 608. [Google Scholar] [CrossRef] [PubMed]
- Ukrainskaya, V.; Rubtsov, Y.; Pershin, D.; Podoplelova, N.; Terekhov, S.; Yaroshevich, I.; Sokolova, A.; Bagrov, D.; Kulakovskaya, E.; Shipunova, V.; et al. Antigen-specific stimulation and expansion of CAR-T cells using membrane vesicles as target cell surrogates. Small 2021, 17, e2102643. [Google Scholar] [CrossRef]
- Panjwani, M.K.; Smith, J.B.; Schutsky, K.; Gnanandarajah, J.; O’Connor, C.M.; Powell, D.J., Jr.; Mason, N.J. Feasibility and safety of RNA-transfected CD20-specific chimeric antigen receptor T cells in dogs with spontaneous B cell lymphoma. Mol. Ther. 2016, 24, 1602–1614. [Google Scholar] [CrossRef]
- Herndler-Brandstetter, D.; Schwaiger, S.; Veel, E.; Fehrer, C.; Cioca, D.P.; Almanzar, G.; Keller, M.; Pfister, G.; Parson, W.; Würzner, R.; et al. CD25-expressing CD8+ T cells are potent memory cells in old age. J. Immunol. 2005, 175, 1566–1574. [Google Scholar] [CrossRef]
- Dennett, N.S.; Barcia, R.N.; McLeod, J.D. Age associated decline in CD25 and CD28 expression correlate with an increased susceptibility to CD95 mediated apoptosis in T cells. Exp. Gerontol. 2002, 37, 271–283. [Google Scholar] [CrossRef]
- Kotani, H.; Gongbo, L.; Jiqiang, Y.; Tania, E.M.; Jon, C.; Justin, C.B.; Sean, J.Y.; Jing, Z.; Marco, L.D. Aged CAR T cells exhibit enhanced cytotoxicity and effector function but shorter persistence and less memory-like phenotypes. Blood 2018, 132, 2047. [Google Scholar] [CrossRef]
- Gardner, H.L.; Fenger, J.M.; London, C.A. Dogs as a model for cancer. Annu. Rev. Anim. Biosci. 2016, 4, 199–222. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamauchi, A.; Yoshimoto, S.; Kudo, A.; Takagi, S. Negative Influence of Aging on Differentiation and Proliferation of CD8+ T-Cells in Dogs. Vet. Sci. 2023, 10, 541. https://doi.org/10.3390/vetsci10090541
Yamauchi A, Yoshimoto S, Kudo A, Takagi S. Negative Influence of Aging on Differentiation and Proliferation of CD8+ T-Cells in Dogs. Veterinary Sciences. 2023; 10(9):541. https://doi.org/10.3390/vetsci10090541
Chicago/Turabian StyleYamauchi, Akinori, Sho Yoshimoto, Ayano Kudo, and Satoshi Takagi. 2023. "Negative Influence of Aging on Differentiation and Proliferation of CD8+ T-Cells in Dogs" Veterinary Sciences 10, no. 9: 541. https://doi.org/10.3390/vetsci10090541
APA StyleYamauchi, A., Yoshimoto, S., Kudo, A., & Takagi, S. (2023). Negative Influence of Aging on Differentiation and Proliferation of CD8+ T-Cells in Dogs. Veterinary Sciences, 10(9), 541. https://doi.org/10.3390/vetsci10090541



