Guttural Pouch Mycosis: A Three-Step Therapeutic Approach
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
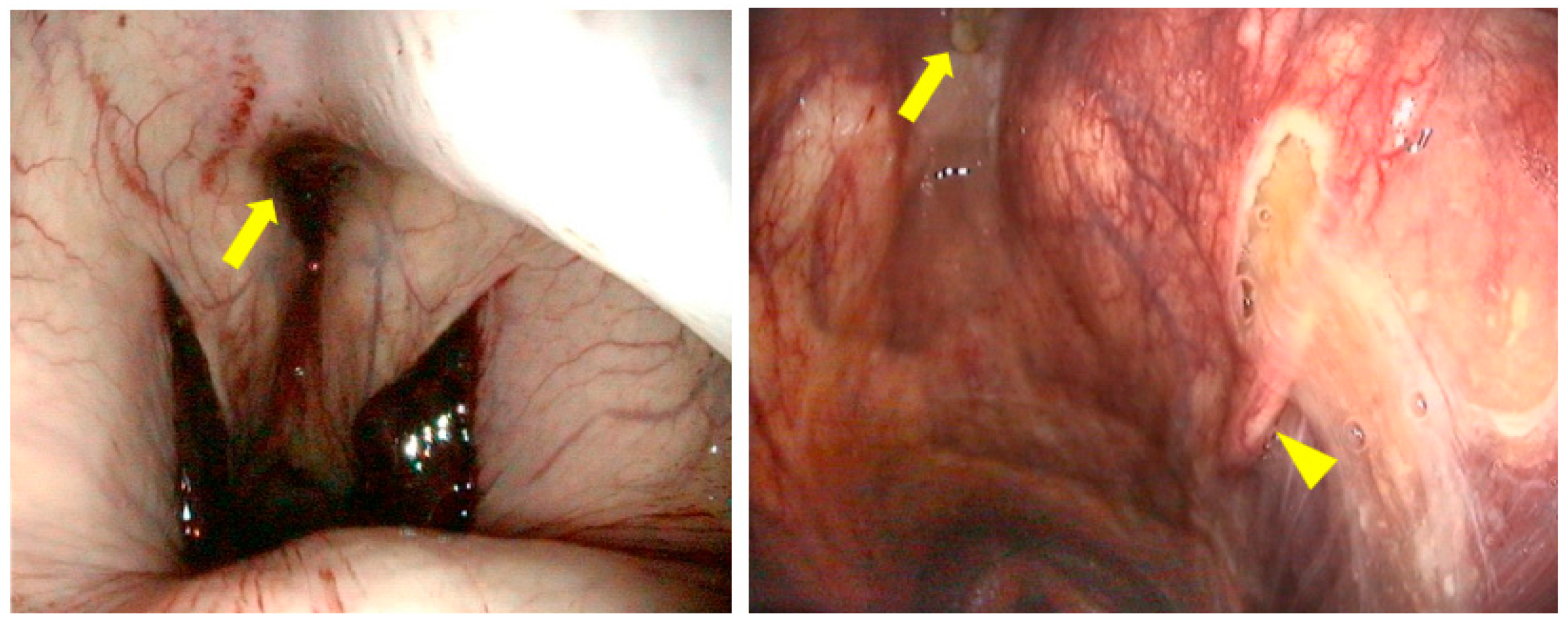
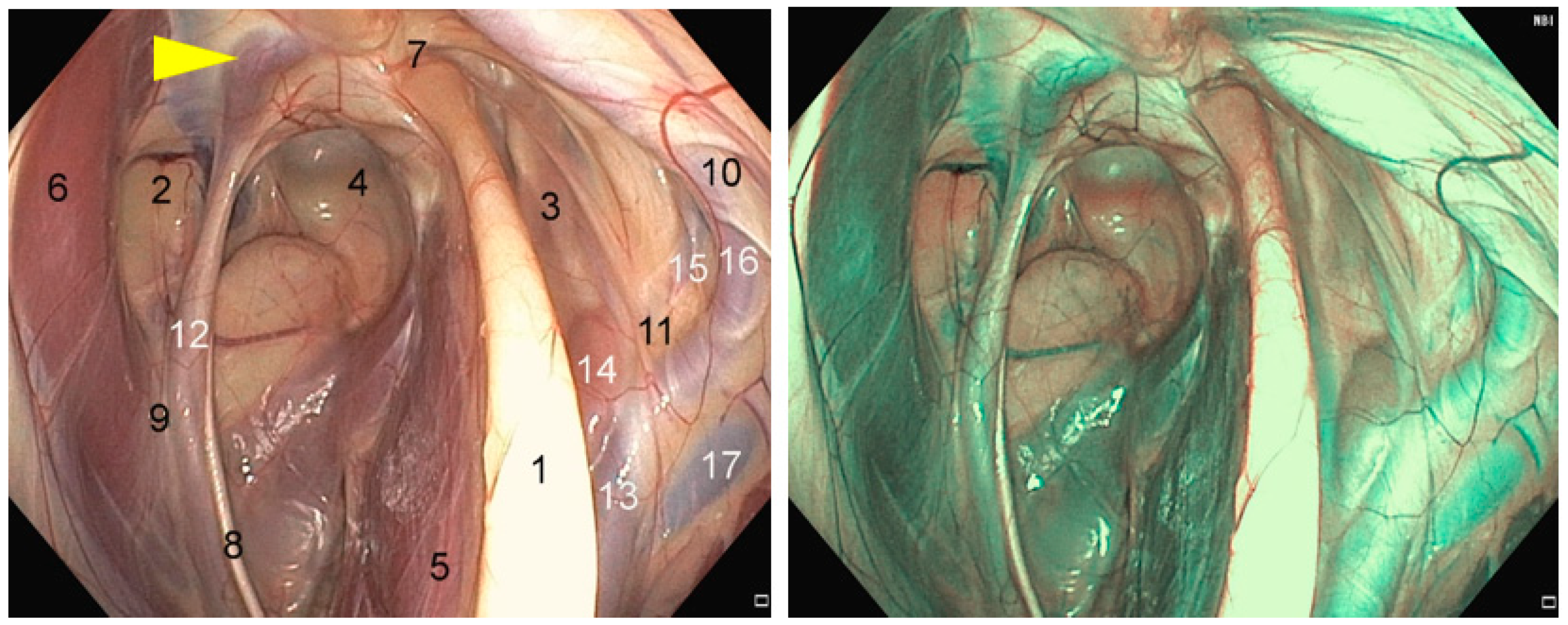
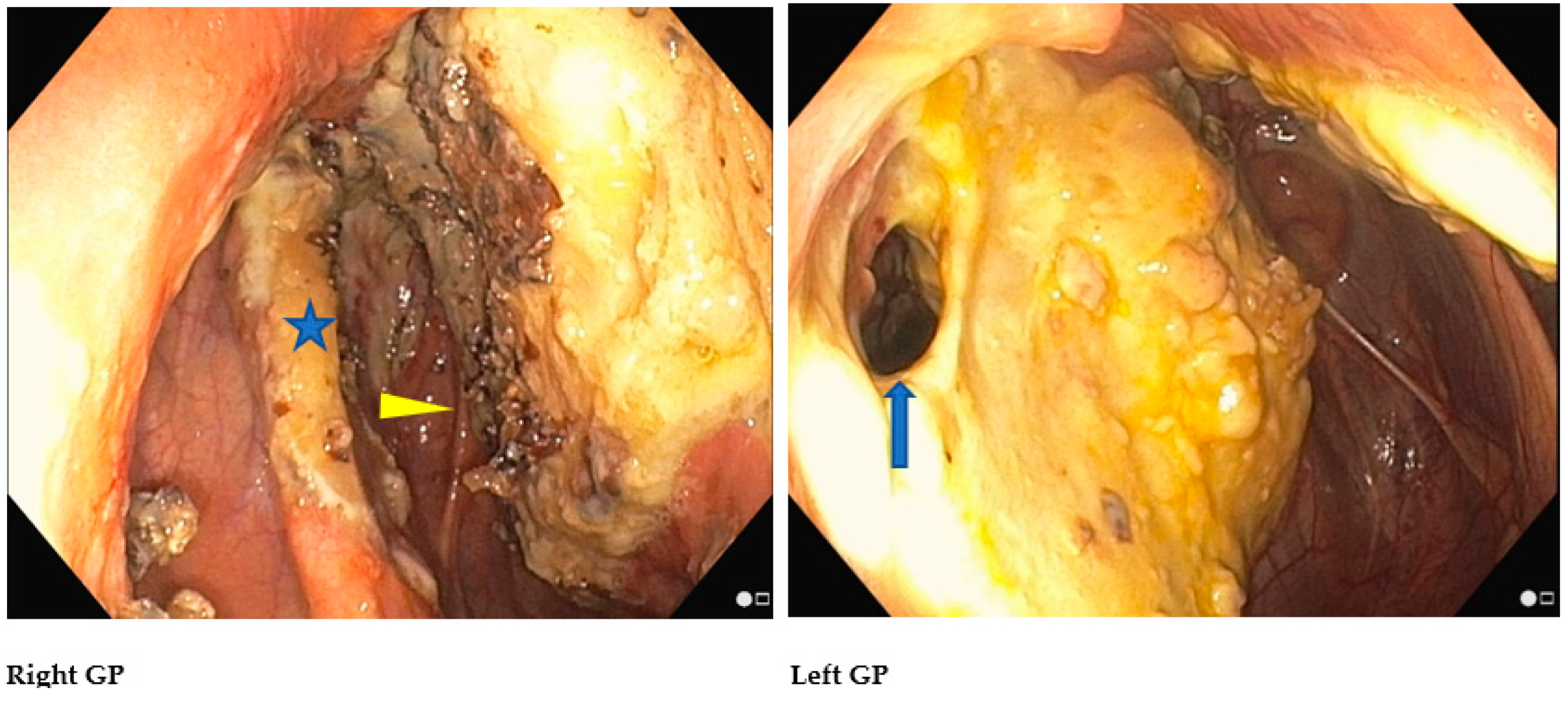
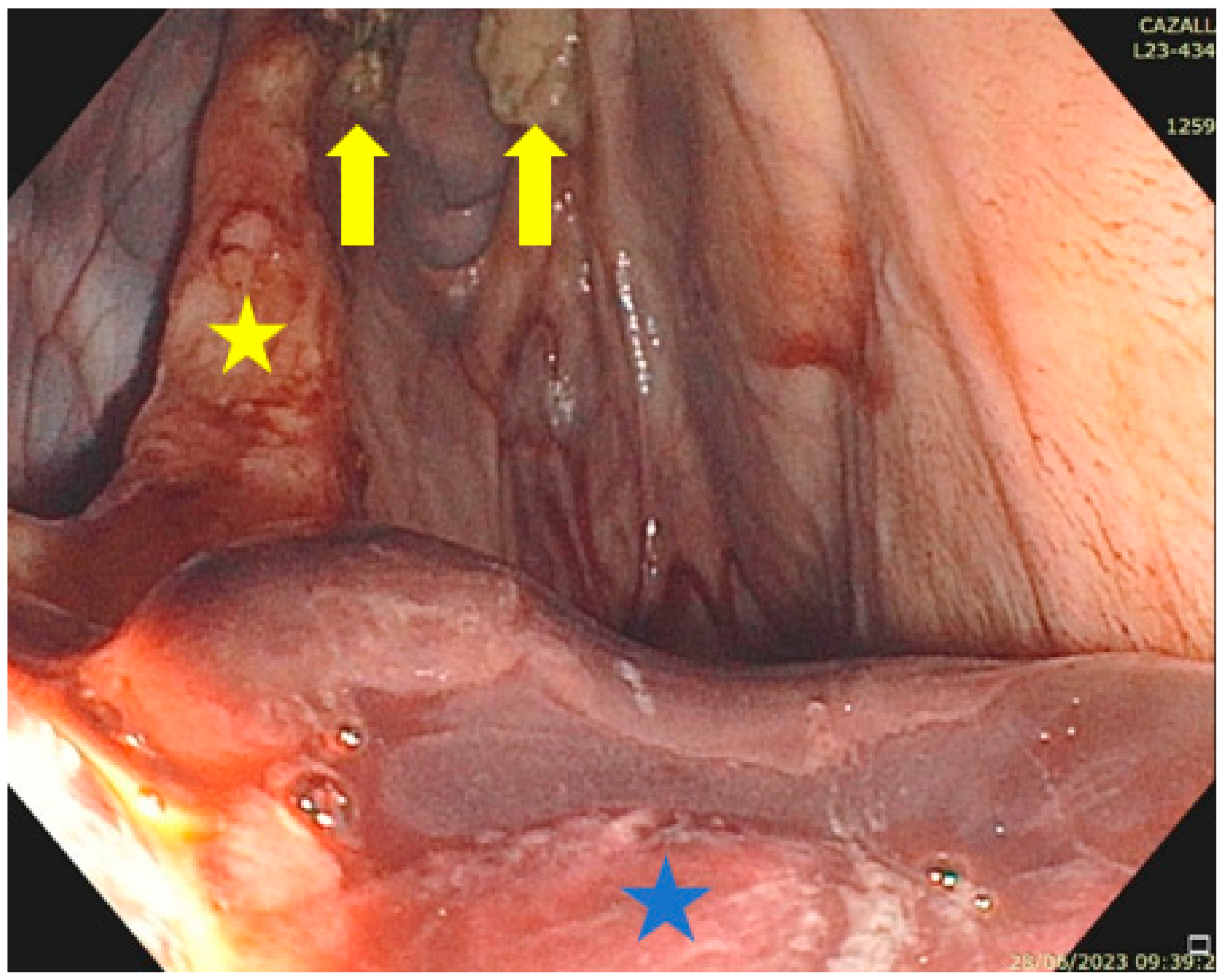
2.1. Anatomical Structures of Interest
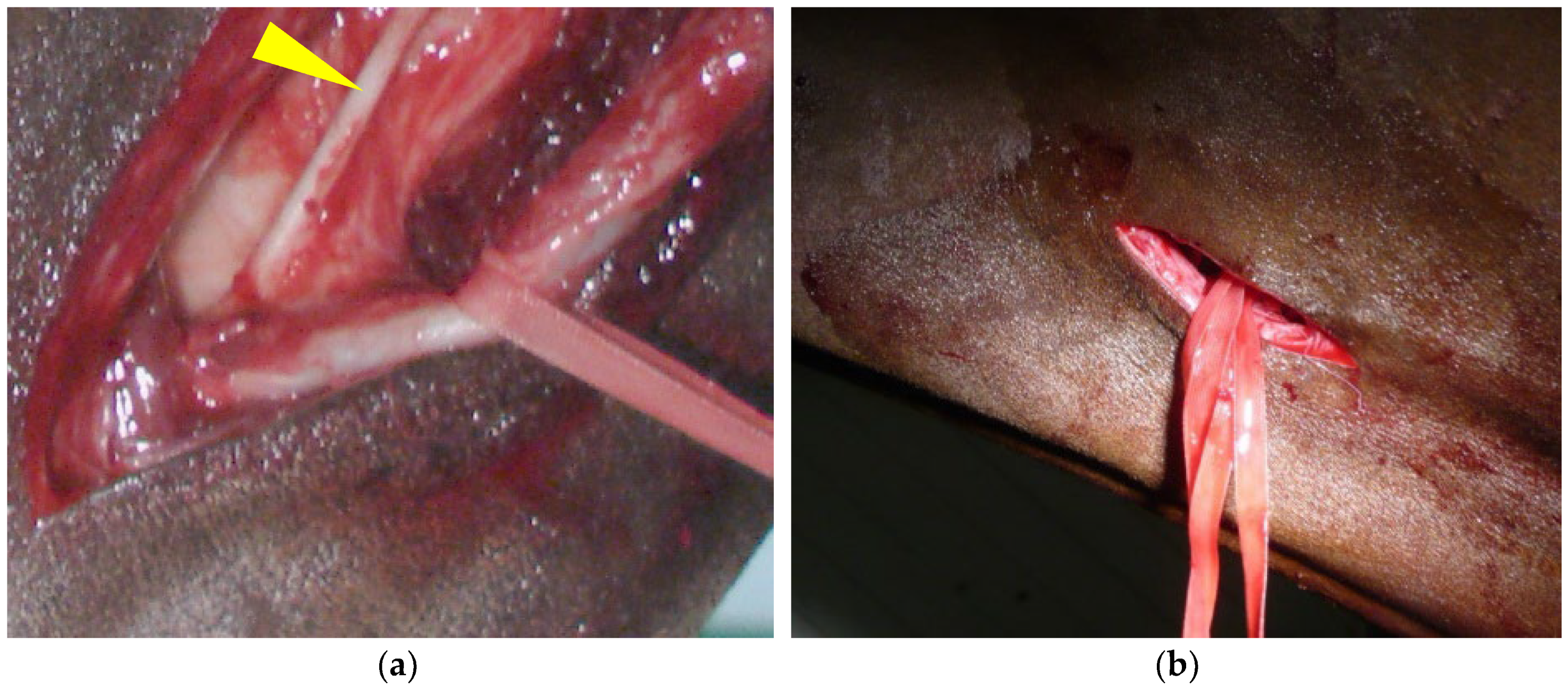
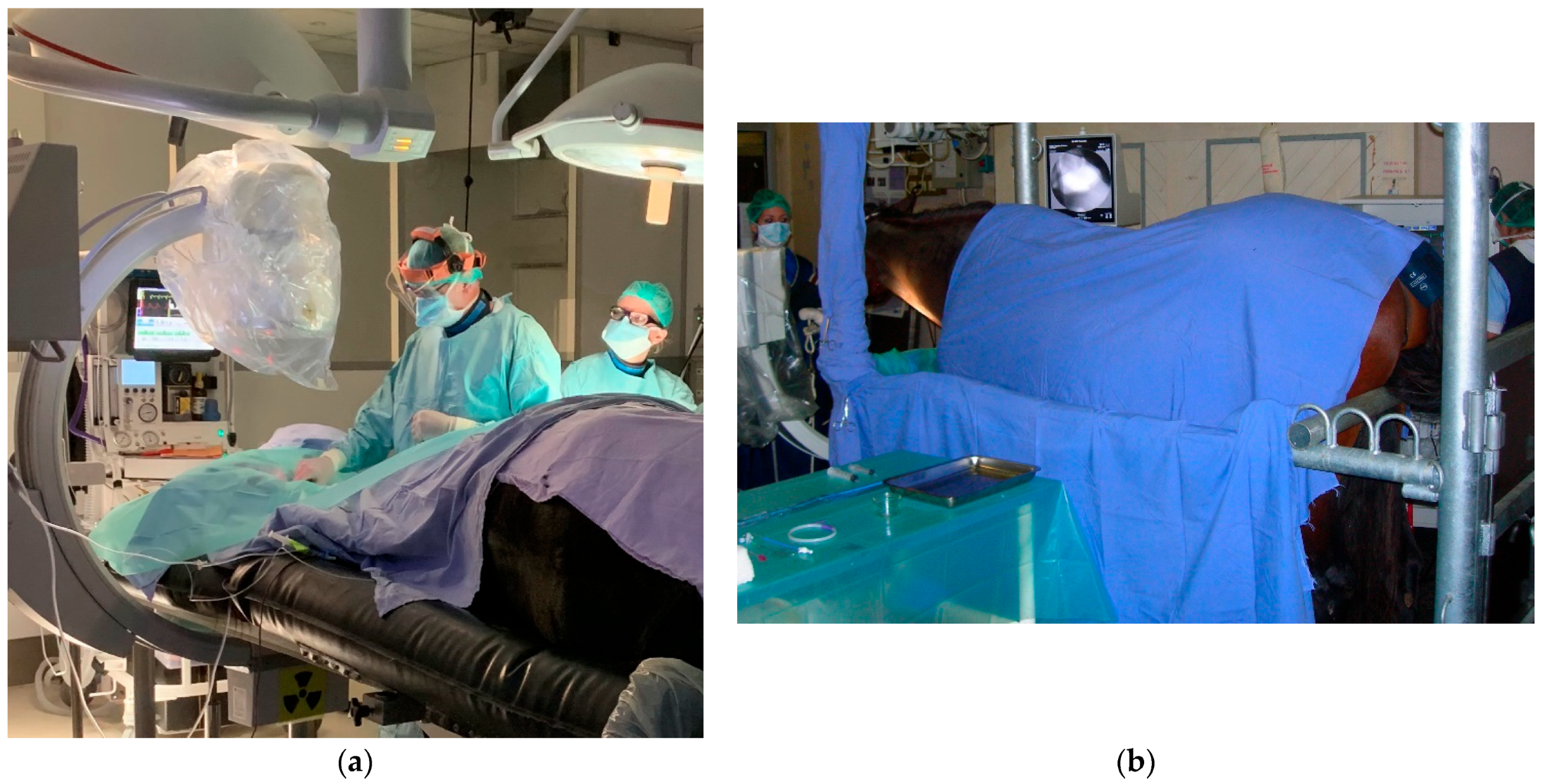
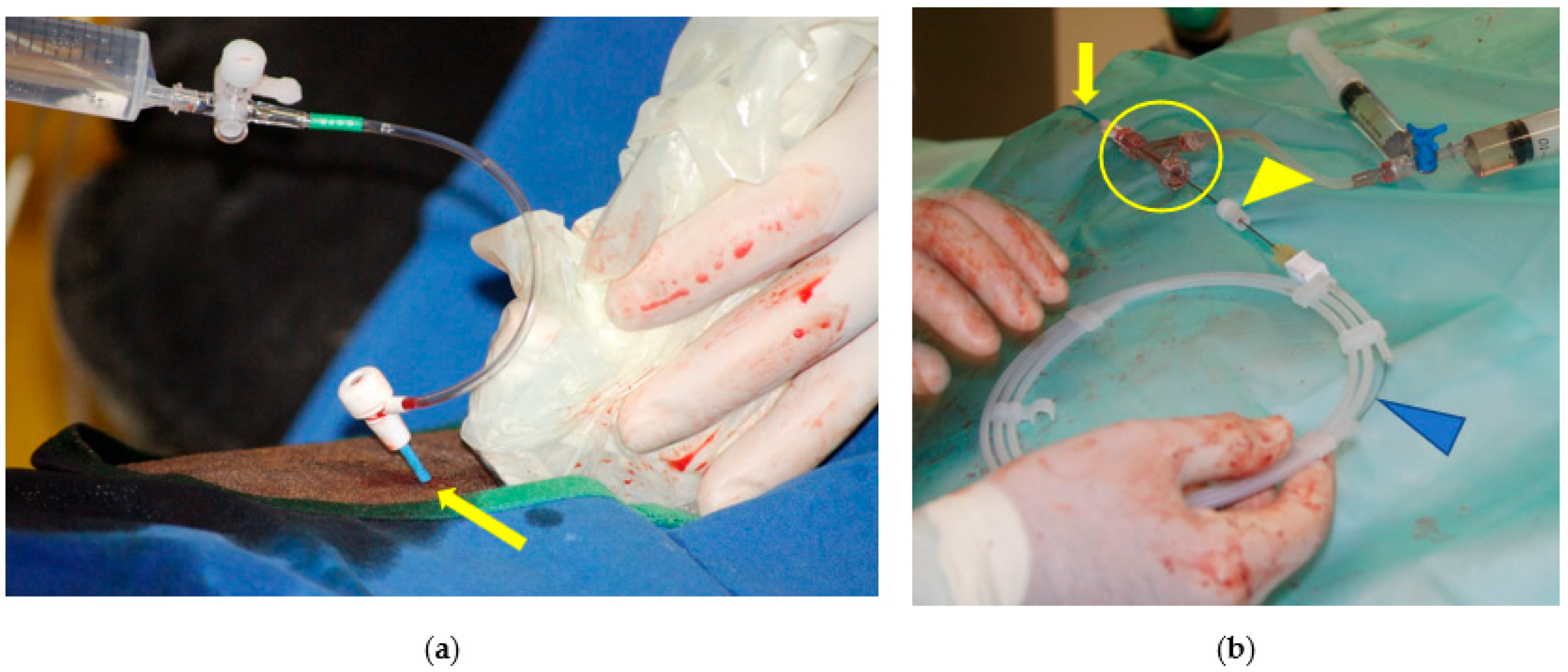
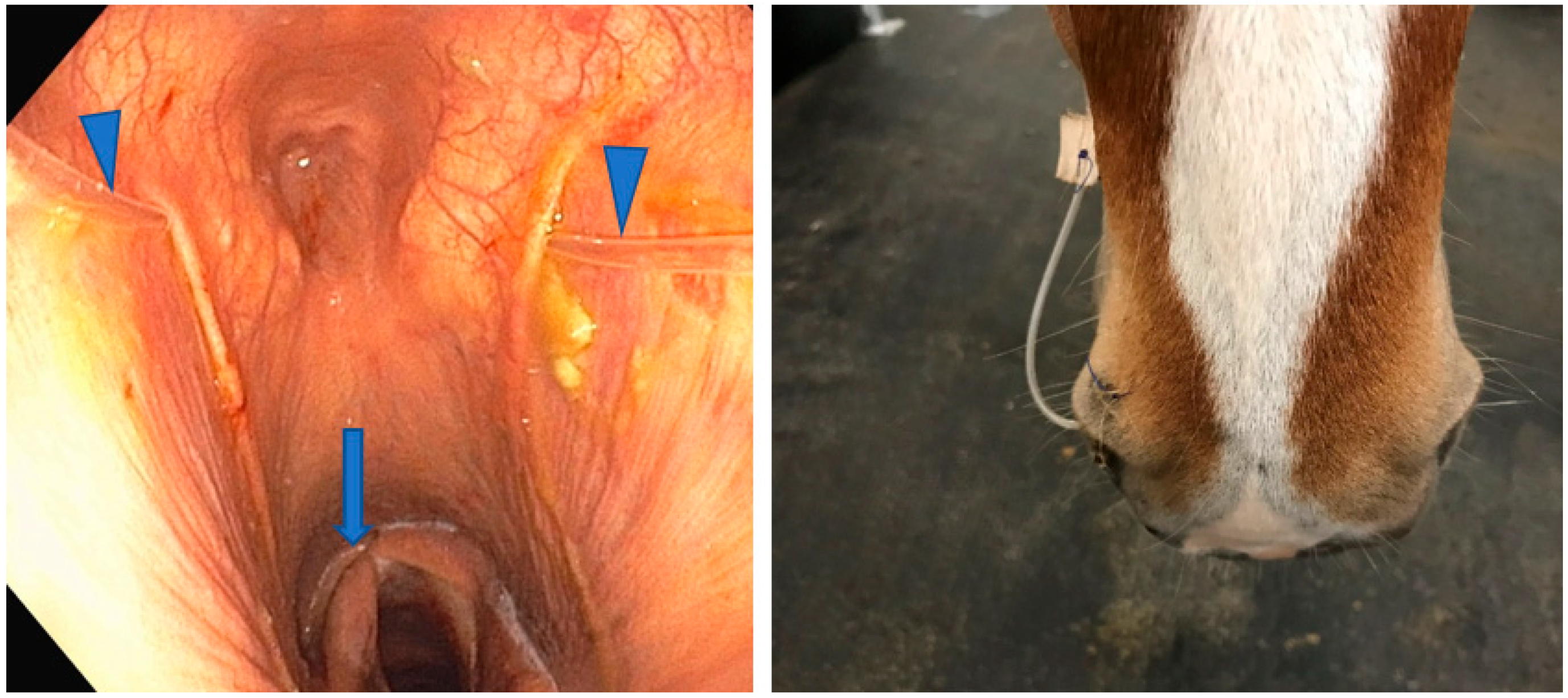
2.2. Step 1: Common Carotid Artery Temporary Ligature
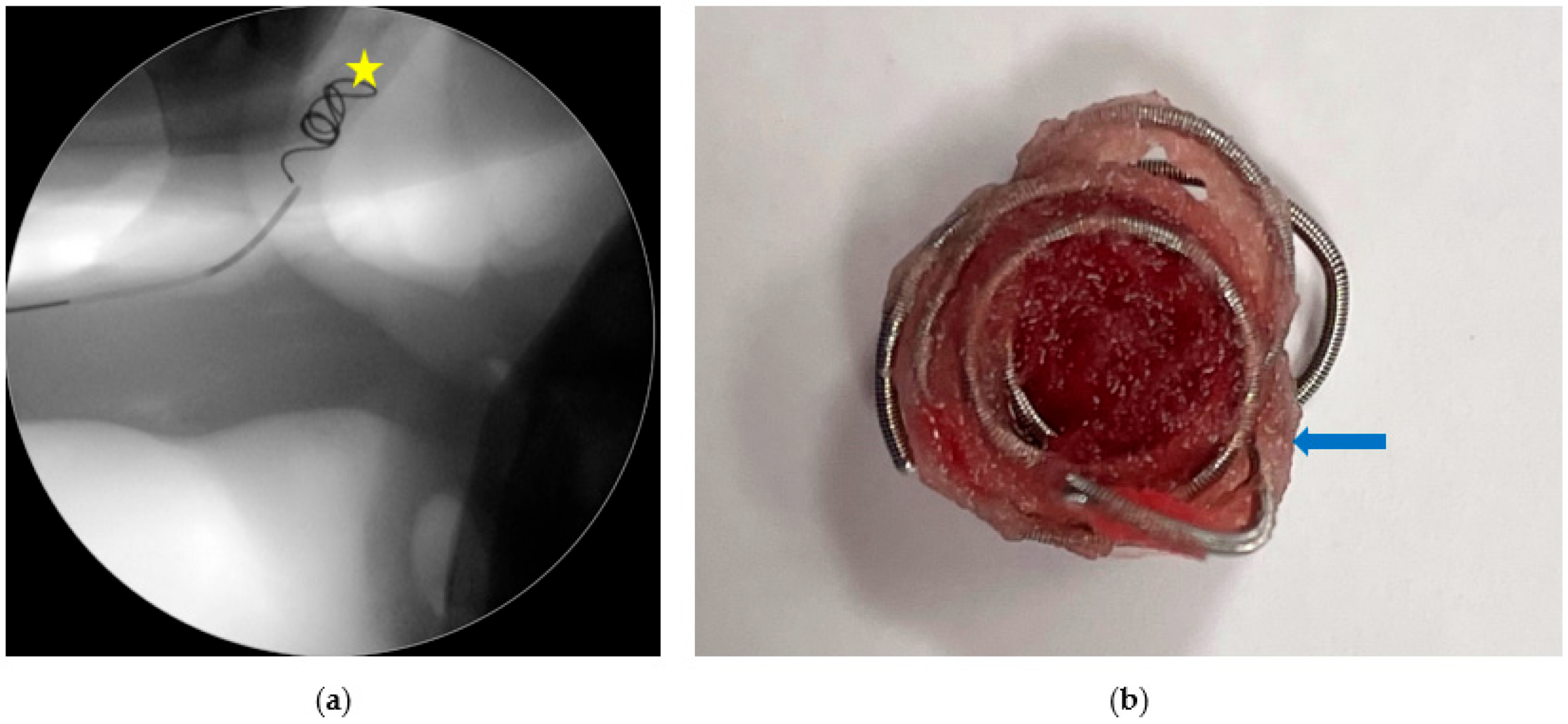
2.3. Step 2: Trans-Arterial Coil Embolization (TACE)
2.4. Step 3: Topical Oxygen Therapy (TOT)
3. Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lepage, O.; Perron, M.-F.; Cadoré, J.-L. The mystery of fungal infection in the guttural pouches. Vet. J. 2004, 168, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Cook, W.R.; Campbell, R.S.; Dawson, C. The pathology and aetiology of guttural pouch mycosis in the horse. Vet. Rec. 1968, 83, 422–428. [Google Scholar] [CrossRef]
- Ludwig, A.; Gatineau, S.; Reynnaud, M.C.; Cadoré, J.L.; Bourdoiseau, G. Fungal isolation and identification in 21 cases of guttural pouch mycosis in horses (1998–2002). Vet. J. 2004, 169, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Piat, P.; Cadoré, J.-L. Endoscopic Anatomy of the Equine Guttural Pouch: An Anatomic Observational Study. Vet. Sci. 2023, 10, 542. [Google Scholar] [CrossRef]
- Lepage, O. Guttural pouch disease. In Comparative Veterinary Anatomy—A Clinical Approach, 1st ed.; Orsini, A., Grenager, N.S., de Lahunta, A., Eds.; Academic Press: Cambridge, MA, USA, 2022; Chapter 10; pp. 611–623. [Google Scholar]
- Baptiste, K.E.; Naylor, J.M.; Bailey, J.; Barber, E.M.; Post, K.; Thornhill, J. A function for guttural pouches in the horse. Nature 2000, 403, 382–383. [Google Scholar] [CrossRef]
- Deluzurieux, M.; Desjardins, I.; Nolf, M.; Guidi, E.; Depecker, M.; Cadoré, J.-L. Endoscopic analysis of guttural pouch opening in horses. J. Exp. Appl. Anim. Sci. 2013, 1, 10. [Google Scholar] [CrossRef]
- Lepage, O.M.; Piccot-Crezollet, C. Transarterial coil embolization in 31 horses (1999–2002) with guttural pouch mycosis: A two years’ follow-up. Equine Vet. J. 2005, 37, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Léveillé, R.; Hardy, J.; Robertson, J.T.; Willis, M.A.; Beard, W.L.; Weisbrode, S.E.; Lepage, O.M. Transarterial occlusion of the internal and external carotid and maxillary arteries for prevention of hemorrhage from guttural pouch mycosis in horses. Vet. Surg. 2000, 29, 389–397. [Google Scholar] [CrossRef]
- Benredouane, K.; Lepage, O.M. Trans-arterial coil embolization of the internal carotid artery in standing horses. Vet. Surg. 2012, 41, 404–409. [Google Scholar] [CrossRef]
- Ghoshal, N.G.; Nanda, B.S. Heart and arteries. In Sisson and Grossman’s the Anatomy of the Domestic Animals, 5th ed.; W.B. Saunders: Philadelphia, PA, USA, 1975; Volume 1, pp. 554–618. [Google Scholar]
- Lepage, O.M. Hémorragie dans les poches gutturales. 1. Anatomie, diagnostic et étiologie. Prat. Vet. Eq. 1994, 26, 255–261. [Google Scholar]
- Freeman, D.E. Guttural pouch mycosis. In Current Practice of Equine Surgery; White, N., Moore, J., Eds.; Lippincott: Philadelphia, PA, USA, 1990; pp. 243–249. [Google Scholar]
- Lepage, O.M. Hémorragie dans les poches gutturales. 2. Traitements, pronostic et complications. Prat. Vet. Eq. 1995, 27, 81–89. [Google Scholar]
- Profizi, C.; Lepage, O.M. Réaliser une ligature de l’artère carotide commune en urgence. Prat. Vét. Eq. 2014, 46, 64–65. [Google Scholar]
- Wyn-Jones, G.; Jones, R.S.; Church, S. Temporary bilateral carotid artery occlusion as an aid to nasal surgery in the horse. Equine Vet. J. 1986, 18, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Lepage, O.M. Challenges associated with the diagnosis and management of guttural pouch epistaxis in equids. Equine Vet. Educ. 2015, 28, 372–378. [Google Scholar] [CrossRef]
- Maninchedda, U.; Lepage, O.M.; Gangl, M.; Benredouane, K. Percutaneous ultrasound-guided arterial angiography for trans-arterial coil placement in anesthetized and standing horses. Vet. Surg. 2015, 44, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, A.G.; Scansen, B.A.; Hurcombe, S.D.; Mudge, M.C. Potential for iatrogenic coil embolization of the caudal cerebellar artery during treatment of internal carotid artery bifurcatipon in two horses with gutturalpouch mycosis. J. Am. Vet. Med. Assoc. 2015, 246, 1427–1432. [Google Scholar] [CrossRef] [PubMed]
- Lepage, O.M.; Di Francesco, P.; Moulin, N.; Gangl, M.; Texier, G.; Marchi, J.; Cadoré, J.-L. The effect of topical oxygen therapy in horses affected with mycosis of the guttural pouch: An experimental pilot study and a case series. Animals 2021, 11, 3329. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.M.; Barber, S.M. Guttural pouch hemorrhage associated with lesions of the maxillary artery in two horses. Can. Vet. J. 1984, 25, 239–242. [Google Scholar]
- Sweeney, C.R.; Freeman, D.E.; Sweeney, R.W.; Rubin, J.L.; Maxson, A.D. Hemorrhage into the guttural pouch (auditory tube diverticulum) associated with rupture of the longus capitis muscle in three horses. J. Am. Vet. Med. Assoc. 1993, 202, 1129–1131. [Google Scholar] [CrossRef]
- Knight, A.P. Dysphagia resulting from unilateral rupture of the rectus capitis ventralis muscles in a horse. J. Am. Vet. Med. Assoc. 1977, 170, 735–738. [Google Scholar]
- Nation, P.N. Epistaxis of guttural pouch origin in horses: Pathology of three cases. Can. Vet. J. 1978, 19, 194–197. [Google Scholar]
- McIlwraith, C.W. (Ed.) Extra diverticular ligation of the internal carotid artery for guttural pouch mycosis. In Equine Surgery Advanced Techniques; Lea & Febiger: Philadelphia, PA, USA, 1987; pp. 228–234. [Google Scholar]
- Greet, T.R.C. Outcome of treatment in 35 cases of guttural pouch mycosis. Equine Vet. J. 1987, 19, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Freeman, D.E.; Donawick, W.J. Occlusion of internal carotid artery in the horse by means of a balloon tipped catheter: Clinical use of a method to prevent epistaxis caused by guttural pouch mycosis. J. Am. Vet. Med. Assoc. 1980, 176, 236–240. [Google Scholar]
- Freeman, D.E.; Ross, M.W.; Donawick, W.J.; Hamir, A.N. Occlusion of the external carotid and maxillary arteries in the horse to prevent hemorrhage from guttural pouch mycosis. Vet. Surg. 1989, 18, 39–47. [Google Scholar] [CrossRef]
- Genton, M.; Farfan, M.; Tesson, C.; Laclaire, A.-L.; Rossignol, F.; Mespoulhes-Rivière, C. Balloon catheter occlusion of the maxillary, internal, and external carotid arteries in standing horses. Vet. Surg. 2021, 50, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Jennings, A.; Lepage, O.M.; Mair, T.; Sherlock, C. Surgical site infection after occlusion of the internal carotid artery with a thrombectomy catheter: Five cases. Equine vet. Educ. 2019, 31, 37–44. [Google Scholar] [CrossRef]
- Hardy, J.; Robertson, J.T.; Wilkie, D.A. Ischemic optic neuropathy and blindness after arterial occlusion for treatment of guttural pouch mycosis in two horses. J. Am. Vet. Med. Assoc. 1990, 196, 1631–1634. [Google Scholar] [CrossRef] [PubMed]
- Munoz, J.; Iglesias, M.; Lloret Chao, E.; Bussy, C. Ultrasound guided transarterial coil placement in the internal and external carotid artery in horses. Vet. Surg. 2015, 44, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Arantza, V.; Laborda, A.; Serrano-Casorran, C.; Fuente, S.; Romero, A.; Vazquez, F.J. Percutaneous ultrasound guided carotid access and puncture closure with angio-seal in horses. Animals 2022, 12, 1481. [Google Scholar] [CrossRef]
- Speirs, V.C.; Harrison, I.W.; Veenendaal, J.C.; Baumgartner, T.; Josseck, H.H.; Reutter, H. Is specific antifungal therapy necessary for the treatment of guttural pouch mycosis in horses? Equine Vet. J. 1995, 27, 151–152. [Google Scholar] [CrossRef]
- Cousty, M.; Tricaud, C.; De Beauregard, T.; Picandet, V.; Bizon-Mercier, C.; Tessier, C. Ligation of the ipsilateral common carotid artery and topical treatment for the prevention of epistaxis from guttural pouch mycosis in horses. Vet. Rec. 2016, 178, 44. [Google Scholar] [CrossRef]
- Greppi, M.C.; Guillot, J.; Melloul, E.; Bourdoiseau, G.; Lepage, O.; Cadoré, J.-L. Experimental induction of mycotic plaques in the guttural pouches of horses. Med. Mycol. 2016, 55, 308–313. [Google Scholar] [CrossRef]
- Watkins, A.R.; Parente, E.J. Salpingopharyngeal fistula as a treatment for guttural pouch mycosis in seven horses. Equine Vet. J. 2018, 50, 781–786. [Google Scholar] [CrossRef]
- García-Covarrubias, L.; Barratt, D.M.; Bartlett, R.; Metzinger, S.; Van Meter, K. Invasive aspergillosis treated with adjunctive hyperbaric oxygenation: A retrospective clinical series at a single institution. South. Med. J. 2002, 95, 450–456. [Google Scholar] [CrossRef]
- Dobesova, O.; Schwarz, B.; Velde, K.; Jahn, P.; Zert, Z.; Bezdekova, B. Guttural pouch mycosis in horses: A retrospective study of 28 cases. Vet. Rec. 2012, 171, 561. [Google Scholar] [CrossRef]
- Di Francesco, P.; Lepage, O.M.; Moulin, N.; Cadoré, J.-L. Gli effetti e l’utilizzo dell’ossigeno iperbarico nell’Aspergillosi delle tasche gutturali del cavallo: Studio preliminare. In Proceedings of the 22nd SIVE International Congress, Milan, Italy, 14–15 October 2016; pp. 308–309. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lepage, O.M. Guttural Pouch Mycosis: A Three-Step Therapeutic Approach. Vet. Sci. 2024, 11, 41. https://doi.org/10.3390/vetsci11010041
Lepage OM. Guttural Pouch Mycosis: A Three-Step Therapeutic Approach. Veterinary Sciences. 2024; 11(1):41. https://doi.org/10.3390/vetsci11010041
Chicago/Turabian StyleLepage, Olivier M. 2024. "Guttural Pouch Mycosis: A Three-Step Therapeutic Approach" Veterinary Sciences 11, no. 1: 41. https://doi.org/10.3390/vetsci11010041
APA StyleLepage, O. M. (2024). Guttural Pouch Mycosis: A Three-Step Therapeutic Approach. Veterinary Sciences, 11(1), 41. https://doi.org/10.3390/vetsci11010041






