Development and Degeneration of the Intervertebral Disc—Insights from Across Species
Abstract
Simple Summary
Abstract
1. Introduction
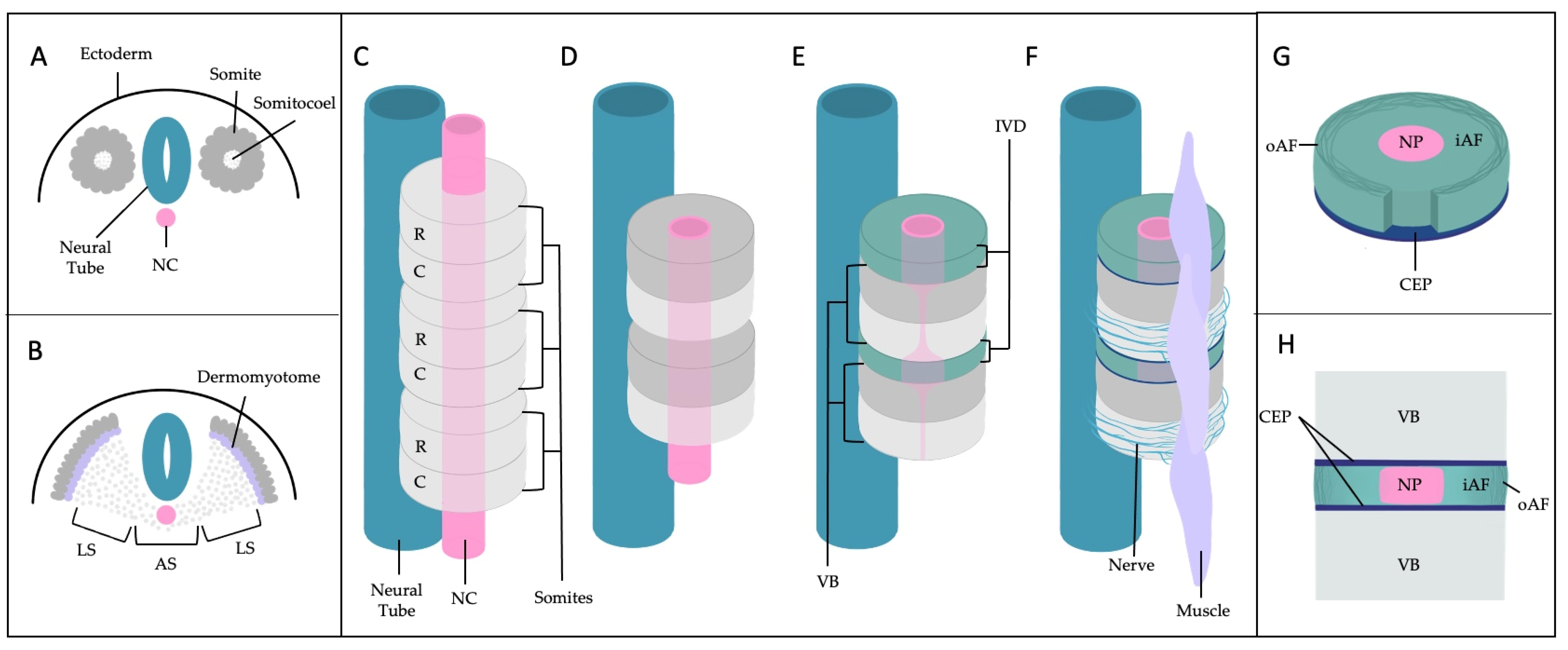
2. A General Understanding of Anatomic and Molecular Features of the IVD
2.1. Importance of the Notochord
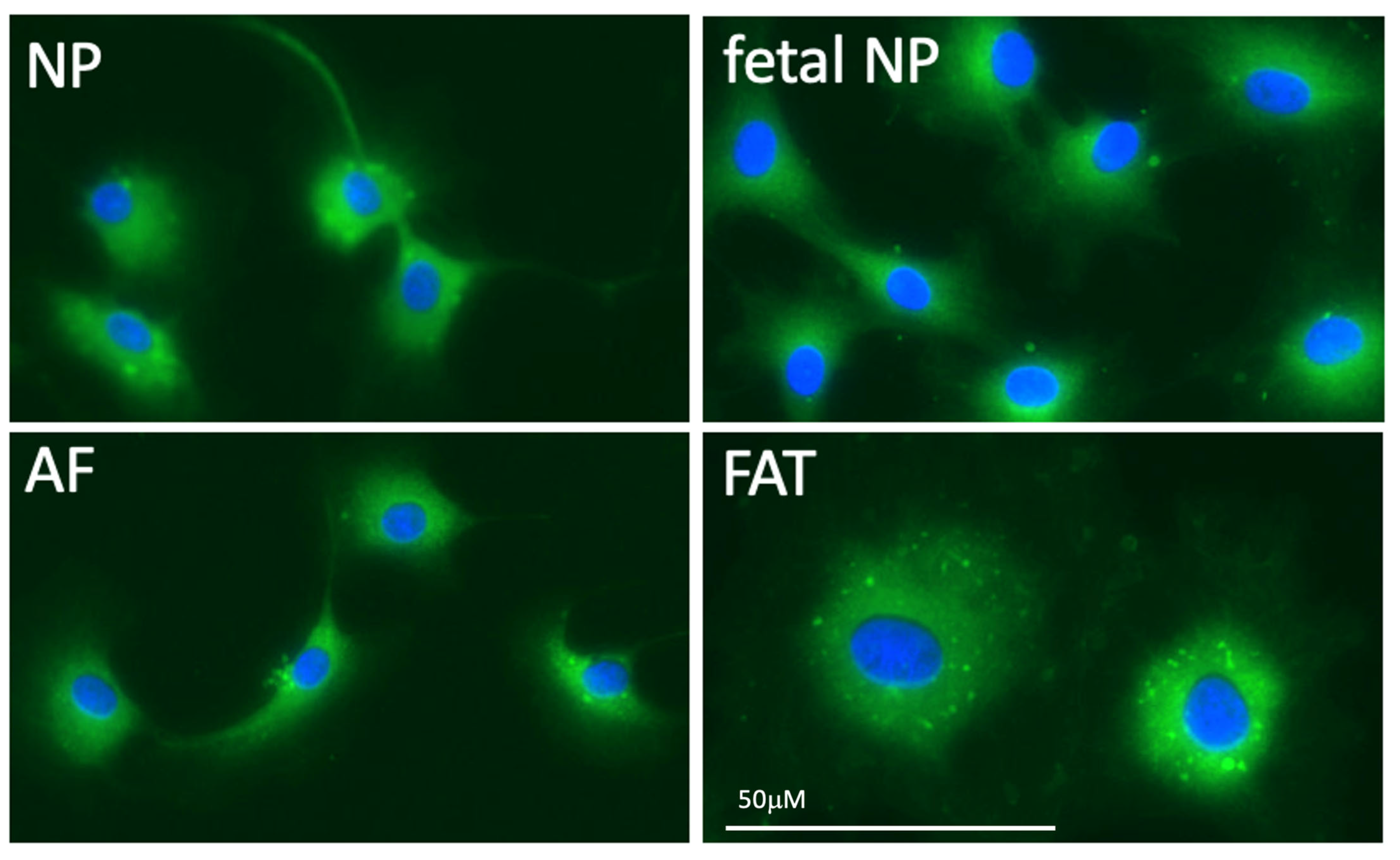
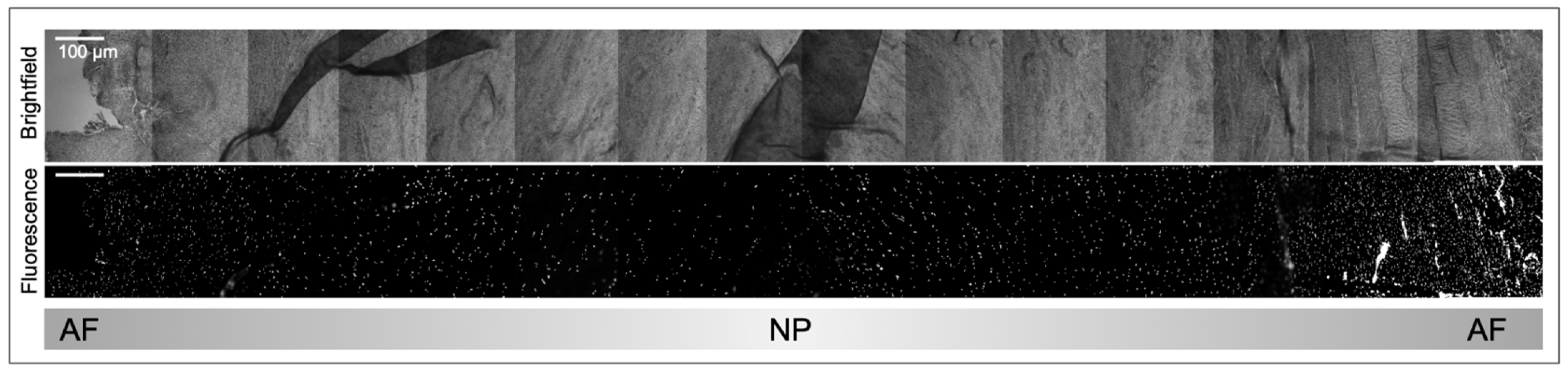
2.2. IVD Structure: Nucleus Pulposus

2.3. IVD Structure: Annulus Fibrosus
2.4. IVD Structure: Cartilage Endplates

2.5. Multi Species scRNA seq Supports Cell Heterogeneity and Clarifies Cell Identities in the IVD
| Species | NC | NP | Inner AF | Outer AF | Method | Source |
|---|---|---|---|---|---|---|
| Human | - | C2ORF40, MGP, MSMP, CHI3L1, LGALS1, ID1, ID3 and TMED | MT1F, PLA2GA, EPYC, PRELP, C10ORF10, FGFBP2 and CHI3L1 | - | Human single-cell transcriptome analysis | [64] |
| - | - | - | COL5A1 | spatiotemporal and single cell transcriptomic analysis | [29] | |
| SOX10, CTSK | - | - | - | Mouse spatiotemporal transcriptional analysis, human embryonic single cell transcriptomic atlas, immunohistochemistry of mouse IVD | [50] | |
| CD24, STMN2, RTN1, PRPH, CXCL-12, IGF-1, MAP1B, ISL1, CLDN1, and THBS2. | - | - | - | Microarray analysis and qPCR validation of human embryonic, fetal, and adult spine | [36] | |
| Rat | - | Krrt7, Prrg4, Akap12, Cxcl3, Rab38 | Mmp3, Bpifb1, Bpifa1a, Mmp13, Il11, Inhba | Fibin, Igfbp5, Tnmd, Myoc, Cilp, Lum | Single-cell transcriptome analysis | [58] |
| - | CD24, Basp1, Ncdn | CD90 | cDNA microarray, RT-PCR, IHC | [65] | ||
| Mouse | - | Anxa3, Cdh2, Cd44, Ca3, Slc2a1, Krt8, Krt19, CD109, and CD81, of which Cdh2, CD44, Slc2a1, and CD81 are exclusive in the NP | Tnmd, Col5a1, Col5a2, Col12a1, and Lect1 of which Tnmd, Col5a2, and Col12a1 are exclusive in the AF | Proteomic analysis of mouse lumbar and tail IVD and comparison to human IVD | [66] | |
| - | Gli1, Gli3, Noto, Scx, Ptpr, Sox2, Zscan10, Loc101904175 | - | Lam1, Thy1 | RISH | [67] | |
| Krt8, Atp6v1g3, C1qtnf3, Cd55, Spp1 | Cp, S100b, H2ac18, Snorc, Creld2, Pdia4, Dnajc3, Chcd7, Rcn2 | Mgp, Comp, Spp1, Gsn, Sod2, Dcn, Fn1, Timp3, Wdr73 | Igfbp6, Ctsk, Lgals1, Ccn3 | Sc RNA seq | [68] | |
| Bovine | - | T, Cd24 and Krt19 | - | Adamts17, Col5a1, Col12a1 and Sfrp2 | qPCR | [69] |
| T | Cdh2, Krt8, Krt18, SNAP25, Sostdc1, Ibsp | - | - | qRT-PCR and Microarray Analysis | [70] | |
| - | Gli1, Gli3, Noto, Ptprc, Scx, Sox2 and Zscan10 | - | Lam1, Thy1 | Fluorescent RISH, confocal microscopy, gaussian mixture modeling | [28] | |
3. Stress and Inflammation in the IVD
3.1. Environmental Stress Factors
3.1.1. Mechanical Stress and Trauma
3.1.2. Metabolic Stress
3.2. Inflammation and Degeneration
3.2.1. Regulated Cell Death and Tissue Homeostasis
3.2.2. Protagonists and Antagonists in the Proinflammatory IVD
| Species | Degenerating IVD | Method | Source |
|---|---|---|---|
| Humans | IL6 serum level is significantly higher In IVDD than disc herniation or control groups | Electrochemiluminescence immunoassays | [114] |
| Elevated CCL5 and CXCL6 plasma levels in moderate to severe IVDD compared to healthy control | ELISA and MRI | [115] | |
| Significant increase in IL18 for degenerated Grade IV/V IVD | IL18 ELISA kit | [116] | |
| Degeneration: MT1G, SPP1, HMGA1, FN1, FBXO2, SPARC, VIM, CTGF, MGST1, TAF1D, CAPS, SPTSSB, S100A1, CHI3L2, PLA2G2A, TNRSF11B, FGFBP2, MGP, SLPI, DCN, MT-ND2, MTCYB, ADIRF, FRZB, CLEC3A, UPP1, S100A2, PRG4, COL2A1, SOD2 and MT2A Verified by protein and mRNA expression: MGST1, vimentin, SOD2 and SYF2 | scRNA seq, quantitative immunofluorescence and Western blotting | [64] | |
| Shear stiffness in both NP and AF correlated with increased Pfirrmann Grade of degeneration | MR elastography shear stiffness measurement | [117] | |
| Cox2+ cell number from Grade 2 IVDD onwards | IHC | [104] | |
| Reduced GRB10 in lumbar IVDD compared to healthy controls. Not detected in piriformis syndrome, sacroiliac joint pain, entrapment neuropathy and lumbar disc herniation, suggesting a biomarker for lumbar IVDD. | miRNA qRT-PCR from plasma sample | [118] | |
| CASP1, IL1β, NLRP3 | mRNA expression, IHC | [119] | |
| SPP1 secreted by NC | Mouse spatiotemporal transcriptional analysis, human embryonic single cell transcriptomic atlas, IHC of mouse IVD | [50] | |
| Increased sensitization of the disc: IL1β, IL6, IL8, IL10, TNFα, IFNy Upregulated in Disc herniation group compared to IVDD: IL4, IL6, IL12, IFNy | IHC | [112,113] | |
| Increased levels in IVDD compared to herniated NP (HNP) groups: TNF-α and IL8 Increased expression levels in IVDD compared to herniated NP (HNP) groups: TGFb, VEGF, and NGF | Western Blot | [120] | |
| CCR7+ and CD163+ cells significantly increase with degeneration in NP, AF, and CEP, while CD206+ cells were present but did not significantly increase with further degeneration. CCR7+, CD163+, and CD206+ cells were not found in healthy IVD. | IHC | [99] | |
| Upregulated in degeneration: SLC7A2, LIF, NAMPT, IL1β, NOD2, CCL20, CCL7, TNFRSF1B, LYN, and GCH1 Inflammatory genes proposed as biomarker of IVDD with positive correlation to infiltration of immune cells: IL1β, LYN, NAMPT | Statistical analysis of available gene expression profiles | [121] | |
| Decreased expression in degenerate IVD: CDH2, KRT8, KRT Increased expression in degenerated AF: VCAN, TNMD, and BASP1 | qRT-PCR and Microarray analysis | [70] | |
| Humans and Rats | Upregulated in dNP: COMP, MGP, FBLN1, BASP1, NCDN and CD155 Downregulated in dNP: SNAP25, KRT8, KRT18, CDH2, KRT19, NRP-1 and CD221 | Cumulative data from rat RT-PCR, human RT-PCR, and rat microarray | [122] |
| Rat | Core genes in IVDD in TSZ-induced T1DM rats: Bmp7, Ripk4, Wnt4, Timp1, Col11a1, Acp5, Vdr, Col8a1, Aldh1a1, and Thbs4 | Microarray analysis and transcriptome sequencing of NP cells followed by interaction analysis | [123] |
| Dog | Clusterin as a cerebrospinal fluid marker for chronic IVDD | Liquid chromatography, mass spectrometry, SDS-page, Western blot, and IHC | [124] |
| Significantly lower GAG content in Grade IV and V degenerated discs | GAG assay | [125] | |
| Cox2+ cell percentage in the NP and dorsal AF is significantly higher in Grade IV and V discs compared to Grades I and II | IHC | [125] | |
| Significantly higher protein levels of IL8 and Tnfα in IVDD versus healthy discs. Significantly higher mRNA levels of IL6 and positive correlation between IL6 and pain severity | qPCR, ELISA, IHC | [103] | |
| Increased levels of Tnfα in discs adjacent to IVDD or herniation | qPCR, ELISA, IHC | [103] | |
| Cow | Lam1, Thy1 | RISH | [67] |
| Significantly lower number of CD29+, CD44+, CD45+ and Tie2+ aged NP cells compared to younger NP cells | Flow cytometry, IHC | [126] |
4. Insights from Different Species
4.1. Primates
4.1.1. Humans
4.1.2. Non-Human Primates
4.2. Dogs
4.3. Rodents
4.3.1. Mice
4.3.2. Rats
4.3.3. Rabbits
4.4. Ungulates
4.4.1. Cows
4.4.2. Horses
| Common Region of Degeneration | Clinical Presentation | Radiographic Findings | Biochemical Markers | Serum Analysis | Histological |
|---|---|---|---|---|---|
| Cervical sections (161 cervical degenerated discs (DD): 56 thoracic DD: 48 lumbosacral DD) [162] | Spinal ataxia, more severe in pelvic limbs [170] | Collapse of disc space [171] | Increase in pentosidine in AF and NP [11] | Elevated creatine phosphokinase [171] | Fiber degeneration of white matter [171] |
| Caudo-cervical region showed significantly more severe degeneration compared to other regions. [162] | Limited range of motion in neck [11] | Endplate sclerosis [171] | Advanced glycation end product (AGE) crosslinking [11] | Serum glutamic-oxalocetictransaminase slightly elevated [171] | Poor myelin staining [171] |
| Severe neck pain [11] | Disc protrusion [170,171] | Decrease in hydroxylysine moderate in AF, severe in NP [11] | Elevated blood urea nitrogen [171] | Necrosis of individual neurons in region of disc protrusion [171] | |
| Lameness [170] | Increased Collagen type I in NP [11] | Elevated venous pH values [171] | Scattering of microglial cells [171] | ||
| Spasticity [11] | No change in glycosaminoglycans in NP [11] | CSF showed elevated protein content of xanthochromia [172] | Swollen axons [171] | ||
| Dysmetria [11] | Increased GFAP serum levels [169] | Degeneration of spinal cord in area of disc protrusion [172] | |||
| Normal cutaneous sensation and cranial nerve function [11] | Increased pNF-H serum levels [171] | ||||
| Positive sway response [11] | |||||
| Proprioceptive deficits [11] |
4.5. Cats
4.5.1. Large Cats
4.5.2. Small Cats
4.6. Others
American Black Bear
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cassidy, J.J.; Hiltner, A.; Baer, E. Hierarchical Structure of the Intervertebral Disc. Connect. Tissue Res. 1989, 23, 75–88. [Google Scholar] [CrossRef]
- Dou, Y.; Sun, X.; Ma, X.; Zhao, X.; Yang, Q. Intervertebral Disk Degeneration: The Microenvironment and Tissue Engineering Strategies. Front. Bioeng. Biotechnol. 2021, 9, 592118. [Google Scholar] [CrossRef]
- Harfe, B.D. Intervertebral Disc Repair and Regeneration: Insights from the Notochord. Semin. Cell Dev. Biol. 2022, 127, 3–9. [Google Scholar] [CrossRef]
- Schmorl-Dresden, H. Über Die an Den Wirbelbandscheiben Vorkommenden Ausdehnungs-Und Zerreißungsvorgänge Und Die Dadurch an Ihnen Und Der Wirbelspongiosa Hervorgerufenen Veränderungen’. Verhandlungen Der Dtsch. Pathol. Ges. 1927, 22, 250. [Google Scholar]
- Bergknut, N.; Egenvall, A.; Hagman, R.; Gustås, P.; Hazewinkel, H.A.W.; Meij, B.P.; Lagerstedt, A.S. Incidence of Intervertebral Disk Degeneration–Related Diseases and Associated Mortality Rates in Dogs. J. Am. Vet. Med. Assoc. 2012, 240, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Muhle, C.; Metzner, J.; Weinert, D.; Falliner, A.; Brinkmann, G.; Mehdorn, M.H.; Heller, M.; Resnick, D. Classification System Based on Kinematic MR Imaging in Cervical Spondylitic Myelopathy. Am. J. Neuroradiol. 1998, 19, 1763–1771. [Google Scholar] [PubMed]
- Bertheloot, D.; Latz, E.; Franklin, B.S. Necroptosis, Pyroptosis and Apoptosis: An Intricate Game of Cell Death. Cell Mol. Immunol. 2021, 18, 1106–1121. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kang, R.; Berghe, T.V.; Vandenabeele, P.; Kroemer, G. The Molecular Machinery of Regulated Cell Death. Cell Res. 2019, 29, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Sivakamasundari, V.; Lufkin, T. Bridging the Gap: Understanding Embryonic Intervertebral Disc Development. Cell Dev. Biol. 2012, 1, 103. [Google Scholar] [CrossRef] [PubMed]
- Fenn, J.; Olby, N.J. Classification of Intervertebral Disc Disease. Front. Vet. Sci. 2020, 7, 579025. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, W.; van de Lest, C.; Plomp, S.; Vernooij, J.C.M.; Wijnberg, I.D.; Back, W.; Gröne, A.; Delany, M.W.; Caliskan, N.; Tryfonidou, M.A.; et al. Intervertebral Disc Degeneration in Warmblood Horses: Histological and Biochemical Characterization. Vet. Pathol. 2022, 59, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Knafo, S.E.; Divers, S.J.; Rech, R.; Platt, S.R. Magnetic Resonance Imaging Diagnosis of Intervertebral Disc Disease and Myelomalacia in an American Black Bear (Ursus Americanus). J. Zoo Wildl. Med. 2012, 43, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Kolmstetter, C.; Munson, L.; Ramsay, E.C. Degenerative Spinal Disease in Large Felids. J. Zoo Wildl. Med. 2000, 13, 15–19. [Google Scholar]
- Kraus, P.; Yerden, R.; Kocsis, V.; Lufkin, T. RNA in Situ Hybridization Characterization of Non-Enzymatic Derived Bovine Intervertebral Disc Cell Lineages Suggests Progenitor Cell Potential. Acta Histochem. 2017, 119, 150–160. [Google Scholar] [CrossRef]
- Yurube, T.; Hirata, H.; Kakutani, K.; Maeno, K.; Takada, T.; Zhang, Z.; Takayama, K.; Matsushita, T.; Kuroda, R.; Kurosaka, M.; et al. Notochordal Cell Disappearance and Modes of Apoptotic Cell Death in a Rat Tail Static Compression-Induced Disc Degeneration Model. Arthritis Res. Ther. 2014, 16, R31. [Google Scholar] [CrossRef]
- Bergknut, N.; Smolders, L.A.; Grinwis, G.C.M.; Hagman, R.; Lagerstedt, A.S.; Hazewinkel, H.A.W.; Tryfonidou, M.A.; Meij, B.P. Intervertebral Disc Degeneration in the Dog. Part 1: Anatomy and Physiology of the Intervertebral Disc and Characteristics of Intervertebral Disc Degeneration. Vet. J. 2013, 195, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Fournier, D.E.; Kiser, P.K.; Shoemaker, J.K.; Battié, M.C.; Séguin, C.A. Vascularization of the Human Intervertebral Disc: A Scoping Review. JOR Spine 2020, 3, e1123. [Google Scholar] [CrossRef]
- Williams, S.; Alkhatib, B.; Serra, R. Development of the Axial Skeleton and Intervertebral Disc. In Current Topics in Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 133, pp. 49–90. ISBN 978-0-12-810487-3. [Google Scholar]
- Christ, B.; Huang, R.; Scaal, M. Amniote Somite Derivatives. Dev. Dyn. 2007, 236, 2382–2396. [Google Scholar] [CrossRef]
- Ward, L.; Evans, S.E.; Stern, C.D. A Resegmentation-Shift Model for Vertebral Patterning. J. Anat. 2017, 230, 290–296. [Google Scholar] [CrossRef]
- Pang, D.; Thompson, D.N.P. Embryology and Bony Malformations of the Craniovertebral Junction. Child’s Nerv. Syst. 2011, 27, 523–564. [Google Scholar] [CrossRef]
- Remak, R. Untersuchungen über die Entwicklung der Wirbeltiere; G. Reimer: Berlin, Germany, 1855. [Google Scholar]
- Hayes, A.; Isaacs, M.; Hughes, C.; Caterson, B.; Ralphs, J. Collagen Fibrillogenesis in the Development of the Annulus Fibrosus of the Intervertebral Disc. Eur. Cell Mater. 2011, 22, 226–241. [Google Scholar] [CrossRef] [PubMed]
- Christ, B.; Wilting, J. From Somites to Vertebral Column. Ann. Anat.-Anat. Anz. 1992, 174, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Lawson, L.; Harfe, B.D. Notochord to Nucleus Pulposus Transition. Curr. Osteoporos Rep. 2015, 13, 336–341. [Google Scholar] [CrossRef] [PubMed]
- McCann, M.R.; Séguin, C.A. Notochord Cells in Intervertebral Disc Development and Degeneration. J. Dev. Biol. 2016, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Kraus, P.; Li, K.; Sipes, D.; Varden, L.; Yerden, R.; Henderson, A.; Sur, S.; Lufkin, T. Single-Cell Phenotyping of Complex Heterogeneous Tissue. Handbook of Single-Cell Technologies; Springer: Singapore, 2022; pp. 633–649. [Google Scholar] [CrossRef]
- Li, K.; Kapper, D.; Mondal, S.; Lufkin, T.; Kraus, P. Quantitative Single-Cell Transcript Assessment of Biomarkers Supports Cellular Heterogeneity in the Bovine IVD. Vet. Sci. 2019, 6, 42. [Google Scholar] [CrossRef]
- Torre, O.M.; Mroz, V.; Bartelstein, M.K.; Huang, A.H.; Iatridis, J.C. Annulus Fibrosus Cell Phenotypes in Homeostasis and Injury: Implications for Regenerative Strategies. Ann. N. Y. Acad. Sci. 2019, 1442, 61. [Google Scholar] [CrossRef]
- Trout, J.J.; Buckwalter, J.A.; Moore, K.C.; Landas, S.K. Ultrastructureofthe Human Intervertebral Disc. I. Changes in Notochordal Cells with Age. Tissue Cell 1982, 14, 359–369. [Google Scholar] [CrossRef]
- Lufkin, L.; Samanta, A.; Baker, D.V.; Lufkin, S.; Schulze, J.H.; Ellis, B.; Rose, J.; Lufkin, T.; Kraus, P. Glis1 and Oxaloacetate in Nucleus Pulposus Stromal Cell Somatic Reprogramming and Survival. Front. Mol. Biosci. 2022, 9, 1009402. [Google Scholar] [CrossRef]
- Séguin, C.A.; Chan, D.; Dahia, C.L.; Gazit, Z. Latest Advances in Intervertebral Disc Development and Progenitor Cells. JOR Spine 2018, 1, e1030. [Google Scholar] [CrossRef]
- Walmsley, R. The Development and Growth of the Intervertebral Disc. Edinb. Med. J. 1953, 60, 341. [Google Scholar]
- Choi, K.S.; Harfe, B.D. Hedgehog Signaling Is Required for Formation of the Notochord Sheath and Patterning of Nuclei Pulposi within the Intervertebral Discs. Proc. Natl. Acad. Sci. USA 2011, 108, 9484–9489. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Yan, P.; Zhu, J.; Huang, S.; Wang, Z.; Hu, O.; Jin, H.; Li, Y.; Zhang, L.; Zhao, J.; et al. Spatially Multicellular Variability of Intervertebral Disc Degeneration by Comparative Single-cell Analysis. In Cell Proliferation; Wiley Online Library: Hoboken, NJ, USA, 2023; p. e13464. [Google Scholar] [CrossRef]
- Rodrigues-Pinto, R.; Ward, L.; Humphreys, M.; Zeef, L.A.H.; Berry, A.; Hanley, K.P.; Hanley, N.; Richardson, S.M.; Hoyland, J.A. Human Notochordal Cell Transcriptome Unveils Potential Regulators of Cell Function in the Developing Intervertebral Disc. Sci. Rep. 2018, 8, 12866. [Google Scholar] [CrossRef] [PubMed]
- Tessier, S.; Risbud, M.V. Understanding Embryonic Development for Cell-Based Therapies of Intervertebral Disc Degeneration: Toward an Effort to Treat Disc Degeneration Subphenotypes. Dev. Dyn. 2021, 250, 302–317. [Google Scholar] [CrossRef]
- Gan, Y.; He, J.; Zhu, J.; Xu, Z.; Wang, Z.; Yan, J.; Hu, O.; Bai, Z.; Chen, L.; Xie, Y.; et al. Spatially Defined Single-Cell Transcriptional Profiling Characterizes Diverse Chondrocyte Subtypes and Nucleus Pulposus Progenitors in Human Intervertebral Discs. Bone Res. 2021, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Jiang, B.; Xing, W.; Xie, Z.; Luo, Z.; Zou, W. Discovery and Application of Postnatal Nucleus Pulposus Progenitors Essential for Intervertebral Disc Homeostasis and Degeneration. Adv. Sci. 2022, 9, 2104888. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.A. Early Human Development. Clin. Obstet. Gynecol. 2007, 50, 2–9. [Google Scholar] [CrossRef]
- Evans, H.E.; Sack, W.O. Prenatal Development of Domestic and Laboratory Mammals: Growth Curves, External Features and Selected References. Anat. Histol. Embryol. 1973, 2, 11–45. [Google Scholar] [CrossRef]
- Urban, J.P.G.; Roberts, S.; Ralphs, J.R. The Nucleus of the Intervertebral Disc from Development to Degeneration. Am. Zool. 2000, 40, 53–061. [Google Scholar] [CrossRef]
- Hunter, C.J.; Matyas, J.R.; Duncan, N.A. Cytomorphology of Notochordal and Chondrocytic Cells from the Nucleus Pulposus: A Species Comparison. J. Anat. 2004, 205, 357–362. [Google Scholar] [CrossRef]
- Bedore, J.; Leask, A.; Séguin, C.A. Targeting the Extracellular Matrix: Matricellular Proteins Regulate Cell–Extracellular Matrix Communication within Distinct Niches of the Intervertebral Disc. Matrix Biol. 2014, 37, 124–130. [Google Scholar] [CrossRef]
- Pattappa, G.; Li, Z.; Peroglio, M.; Wismer, N.; Alini, M.; Grad, S. Diversity of Intervertebral Disc Cells: Phenotype and Function: Diversity of Intervertebral Disc Cells. J. Anat. 2012, 221, 480–496. [Google Scholar] [CrossRef]
- Shnayder, N.A.; Ashhotov, A.V.; Trefilova, V.V.; Nurgaliev, Z.A.; Novitsky, M.A.; Vaiman, E.E.; Petrova, M.M.; Nasyrova, R.F. Cytokine Imbalance as a Biomarker of Intervertebral Disk Degeneration. Int. J. Mol. Sci. 2023, 24, 2360. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Zhang, Y.; Zhang, X.; Zhang, H.; Meng, S.; Kong, M.; Liu, X.; Ma, X. Single-Cell RNA Sequencing of the Nucleus Pulposus Reveals Chondrocyte Differentiation and Regulation in Intervertebral Disc Degeneration. Front. Cell Dev. Biol. 2022, 10, 824771. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.P.G. The Role of the Physicochemical Environment in Determining Disc Cell Behaviour. Biochem. Soc. Trans. 2002, 30, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Binch, A.L.A.; Cole, A.A.; Breakwell, L.M.; Michael, A.L.R.; Chiverton, N.; Creemers, L.B.; Cross, A.K.; Le Maitre, C.L. Nerves Are More Abundant than Blood Vessels in the Degenerate Human Intervertebral Disc. Arthritis Res. Ther. 2015, 17, 370. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Chen, Y.; Liao, Z.; Zhang, L.; Su, D.; Li, Z.; Yang, X.; Ke, X.; Liu, H.; Chen, Y.; et al. Spatiotemporal Characterization of Human Early Intervertebral Disc Formation at Single-Cell Resolution. Adv. Sci. 2023, 10, 2206296. [Google Scholar] [CrossRef]
- Götz, W.; Barnert, S.; Bertagnoli, R.; Miosge, N.; Kresse, H.; Herken, R. Immunohistochemical Localization of the Small Proteoglycans Decorin and Biglycan in Human Intervertebral Discs. Cell Tissue Res. 1997, 289, 185–190. [Google Scholar] [CrossRef]
- Raj, P.P. Intervertebral Disc: Anatomy-Physiology-Pathophysiology-Treatment. Pain Pract. 2008, 8, 18–44. [Google Scholar] [CrossRef]
- Mohd Isa, I.L.; Mokhtar, S.A.; Abbah, S.A.; Fauzi, M.B.; Devitt, A.; Pandit, A. Intervertebral Disc Degeneration: Biomaterials and Tissue Engineering Strategies toward Precision Medicine. Adv. Healthc. Mater. 2022, 11, 2102530. [Google Scholar] [CrossRef]
- Sheehan, D.; Hrapchak, B. Theory and Practice of Histotechnology, 2nd ed.; Battelle Press: Detroit, MI, USA, 1980. [Google Scholar]
- Sakai, D.; Nakamura, Y.; Nakai, T.; Mishima, T.; Kato, S.; Grad, S.; Alini, M.; Risbud, M.V.; Chan, D.; Cheah, K.S.E.; et al. Exhaustion of Nucleus Pulposus Progenitor Cells with Ageing and Degeneration of the Intervertebral Disc. Nat. Commun. 2012, 3, 1264. [Google Scholar] [CrossRef]
- Hagizawa, H.; Koyamatsu, S.; Okada, S.; Kaito, T.; Tsumaki, N. Chondrocyte-like Cells in Nucleus Pulposus and Articular Chondrocytes Have Similar Transcriptomic Profiles and Are Paracrine-Regulated by Hedgehog from Notochordal Cells and Subchondral Bone. Front. Cell Dev. Biol. 2023, 11, 1151947. [Google Scholar] [CrossRef] [PubMed]
- Calió, M.; Gantenbein, B.; Egli, M.; Poveda, L.; Ille, F. The Cellular Composition of Bovine Coccygeal Intervertebral Discs: A Comprehensive Single-Cell Rnaseq Analysis. Int. J. Mol. Sci. 2021, 22, 4917. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, Y.; Huang, L.; Shi, K.; Zhu, C.; Li, L.; Zhang, L.; Feng, G.; Liu, L.; Song, Y. Novel Biomarkers of Intervertebral Disc Cells and Evidence of Stem Cells in the Intervertebral Disc. Osteoarthr. Cartil. 2021, 29, 389–401. [Google Scholar] [CrossRef]
- Hickman, T.T.; Rathan-Kumar, S.; Peck, S.H. Development, Pathogenesis, and Regeneration of the Intervertebral Disc: Current and Future Insights Spanning Traditional to Omics Methods. Front. Cell Dev. Biol. 2022, 10, 841831. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ye, D.; Dai, L.; Xu, Y.; Wu, H.; Luo, W.; Liu, Y.; Yao, X.; Wang, P.; Miao, H.; et al. Single-Cell RNA Sequencing Reveals the Difference in Human Normal and Degenerative Nucleus Pulposus Tissue Profiles and Cellular Interactions. Front. Cell Dev. Biol. 2022, 10, 910626. [Google Scholar] [CrossRef]
- Fang, X.; Zheng, P.; Tang, J.; Liu, Y. CD24: From A to Z. Cell Mol. Immunol. 2010, 7, 100–103. [Google Scholar] [CrossRef]
- Tekari, A.; Chan, S.C.W.; Sakai, D.; Grad, S.; Gantenbein, B. Angiopoietin-1 Receptor Tie2 Distinguishes Multipotent Differentiation Capability in Bovine Coccygeal Nucleus Pulposus Cells. Stem. Cell Res. Ther. 2016, 7, 75. [Google Scholar] [CrossRef]
- Tang, Y.; Harrington, A.; Yang, X.; Friesel, R.E.; Liaw, L. The Contribution of the Tie2+ Lineage to Primitive and Definitive Hematopoietic Cells. Genesis 2010, 48, 563. [Google Scholar] [CrossRef]
- Cherif, H.; Mannarino, M.; Pacis, A.S.; Ragoussis, J.; Rabau, O.; Ouellet, J.A.; Haglund, L. Single-Cell RNA-Seq Analysis of Cells from Degenerating and Non-Degenerating Intervertebral Discs from the Same Individual Reveals New Biomarkers for Intervertebral Disc Degeneration. Int. J. Mol. Sci. 2022, 23, 3993. [Google Scholar] [CrossRef]
- Tang, X.; Jing, L.; Chen, J. Changes in the Molecular Phenotype of Nucleus Pulposus Cells with Intervertebral Disc Aging. PLoS ONE 2012, 7, e52020. [Google Scholar] [CrossRef]
- Kudelko, M.; Chen, P.; Tam, V.; Zhang, Y.; Kong, O.Y.; Sharma, R.; Au, T.Y.K.; To, M.K.T.; Cheah, K.S.E.; Chan, W.C.W.; et al. PRIMUS: Comprehensive Proteomics of Mouse Intervertebral Discs That Inform Novel Biology and Relevance to Human Disease Modelling. Matrix Biol. Plus 2021, 12, 100082. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Kapper, D.; Youngs, B.; Kocsis, V.; Mondal, S.; Kraus, P.; Lufkin, T. Potential Biomarkers of the Mature Intervertebral Disc Identified at the Single Cell Level. J. Anat. 2019, 234, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Panebianco, C.J.; Dave, A.; Charytonowicz, D.; Sebra, R.; Iatridis, J.C. Single-Cell RNA-Sequencing Atlas of Bovine Caudal Intervertebral Discs: Discovery of Heterogeneous Cell Populations with Distinct Roles in Homeostasis. FASEB J. 2021, 35, e21919. [Google Scholar] [CrossRef]
- Van den Akker, G.G.H.; Koenders, M.I.; van de Loo, F.A.J.; van Lent, P.L.E.M.; Blaney Davidson, E.; van der Kraan, P.M. Transcriptional Profiling Distinguishes Inner and Outer Annulus Fibrosus from Nucleus Pulposus in the Bovine Intervertebral Disc. Eur. Spine J. 2017, 26, 2053–2062. [Google Scholar] [CrossRef]
- Minogue, B.M.; Richardson, S.M.; Zeef, L.A.H.; Freemont, A.J.; Hoyland, J.A. Transcriptional Profiling of Bovine Intervertebral Disc Cells: Implications for Identification of Normal and Degenerate Human Intervertebral Disc Cell Phenotypes. Arthritis Res. Ther. 2010, 12, R22. [Google Scholar] [CrossRef] [PubMed]
- Teraguchi, M.; Yoshimura, N.; Hashizume, H.; Muraki, S.; Yamada, H.; Minamide, A.; Oka, H.; Ishimoto, Y.; Nagata, K.; Kagotani, R.; et al. Prevalence and Distribution of Intervertebral Disc Degeneration over the Entire Spine in a Population-Based Cohort: The Wakayama Spine Study. Osteoarthr. Cartil. 2014, 22, 104–110. [Google Scholar] [CrossRef]
- Stott, B.; Driscoll, M. Biomechanical Evaluation of the Thoracolumbar Spine Comparing Healthy and Irregular Thoracic and Lumbar Curvatures. Comput. Biol. Med. 2023, 160, 106982. [Google Scholar] [CrossRef]
- Jiong Guo, J.; Du, J.; Xu, Y.; Liu, A.; Yang, H. Catching the Circadian Rhythm of Intervertebral Disc and Association with Clinical Outcomes by Twice-a-Day Magnetic Resonance Imaging. Eur. J. Radiol. 2022, 147, 110130. [Google Scholar] [CrossRef]
- Ren, P.; Chen, P.; Reeves, R.A.; Buchweitz, N.; Niu, H.; Gong, H.; Mercuri, J.; Reitman, C.A.; Yao, H.; Wu, Y. Diffusivity of Human Cartilage Endplates in Healthy and Degenerated Intervertebral Disks. J. Biomech. Eng. 2023, 145, 71006. [Google Scholar] [CrossRef]
- Walter, B.A.; Korecki, C.L.; Purmessur, D.; Roughley, P.J.; Michalek, A.J.; Iatridis, J.C. Complex Loading Affects Intervertebral Disc Mechanics and Biology. Osteoarthr. Cartil. OARS Osteoarthr. Res. Soc. 2011, 19, 1011. [Google Scholar] [CrossRef]
- Walter, B.A.; Likhitpanichkul, M.; Illien-Junger, S.; Roughley, P.J.; Hecht, A.C.; Iatridis, J.C. TNFα Transport Induced by Dynamic Loading Alters Biomechanics of Intact Intervertebral Discs. PLoS ONE 2015, 10, e0118358. [Google Scholar] [CrossRef] [PubMed]
- Hutton, W.C.; Ganey, T.M.; Elmer, W.A.; Kozlowska, E.; Ugbo, J.L.; Doh, E.S.; Whitesides, T.E. Does Long-Term Compressive Loading on the Intervertebral Disc Cause Degeneration? Spine 2000, 25, 2993–3004. [Google Scholar] [CrossRef] [PubMed]
- Rohanifar, M.; Clayton, S.W.; Easson, G.W.D.; Patil, D.S.; Lee, F.; Jing, L.; Barcellona, M.N.; Speer, J.E.; Stivers, J.J.; Tang, S.Y.; et al. Single Cell RNA-Sequence Analyses Reveal Uniquely Expressed Genes and Heterogeneous Immune Cell Involvement in the Rat Model of Intervertebral Disc Degeneration. Appl. Sci. 2022, 12, 8244. [Google Scholar] [CrossRef] [PubMed]
- Mosley, G.E.; Wang, M.; Nasser, P.; Lai, A.; Charen, D.A.; Zhang, B.; Iatridis, J.C. Males and Females Exhibit Distinct Relationships between Intervertebral Disc Degeneration and Pain in a Rat Model. Sci. Rep. 2020, 10, 15120. [Google Scholar] [CrossRef] [PubMed]
- Glaeser, J.D.; Tawackoli, W.; Ju, D.G.; Yang, J.H.; Kanim, L.E.; Salehi, K.; Yu, V.; Saidara, E.; Vit, J.-P.; Khnkoyan, Z.; et al. Optimization of a Rat Lumbar IVD Degeneration Model for Low Back Pain. JOR Spine 2020, 3, e1092. [Google Scholar] [CrossRef]
- Ohshima, H.; Urban, J.P.G. The Effect of Lactate and PH on Proteoglycan and Protein Synthesis Rates in the Intervertebral Disc. Spine 1992, 17, 1079–1082. [Google Scholar] [CrossRef]
- Bibby, S.R.S.; Jones, D.A.; Ripley, R.M.; Urban, J.P.G. Metabolism of the Intervertebral Disc: Effects of Low Levels of Oxygen, Glucose, and PH on Rates of Energy Metabolism of Bovine Nucleus Pulposus Cells. Spine 2005, 30, 487–496. [Google Scholar] [CrossRef]
- Gilbert, H.T.J.; Hodson, N.; Baird, P.; Richardson, S.M.; Hoyland, J.A. Acidic PH Promotes Intervertebral Disc Degeneration: Acid-Sensing Ion Channel-3 as a Potential Therapeutic Target. Sci. Rep. 2016, 6, 37360. [Google Scholar] [CrossRef]
- Gonzales, S.; Wang, C.; Levene, H.; Cheung, H.S.; Huang, C.Y.C. ATP Promotes Extracellular Matrix Biosynthesis of Intervertebral Disc Cells. Cell Tissue Res. 2015, 359, 635–642. [Google Scholar] [CrossRef]
- Ge, Y.; Chen, Y.; Guo, C.; Luo, H.; Fu, F.; Ji, W.; Wu, C.; Ruan, H. Pyroptosis and Intervertebral Disc Degeneration: Mechanistic Insights and Therapeutic Implications. J. Inflamm. Res. 2022, 15, 5857. [Google Scholar] [CrossRef]
- Ma, H.; Xie, C.; Chen, Z.; He, G.; Dai, Z.; Cai, H.; Zhang, H.; Lu, H.; Wu, H.; Hu, X.; et al. MFG-E8 Alleviates Intervertebral Disc Degeneration by Suppressing Pyroptosis and Extracellular Matrix Degradation in Nucleus Pulposus Cells via Nrf2/TXNIP/NLRP3 Axis. Cell Death Discov. 2022, 8, 209. [Google Scholar] [CrossRef]
- Zhao, C.Q.; Zhang, Y.H.; Jiang, S.D.; Jiang, L.S.; Dai, L.Y. Both Endoplasmic Reticulum and Mitochondria Are Involved in Disc Cell Apoptosis and Intervertebral Disc Degeneration in Rats. Age 2010, 32, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Dolma, S.; Lessnick, S.L.; Hahn, W.C.; Stockwell, B.R. Identification of Genotype-Selective Antitumor Agents Using Synthetic Lethal Chemical Screening in Engineered Human Tumor Cells. Cancer Cell 2003, 3, 285–296. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Xu, W.; Zheng, H.; Zheng, X.; Li, B.; Jiang, L.; Jiang, S. Involvement of Oxidative Stress-induced Annulus Fibrosus Cell and Nucleus Pulposus Cell Ferroptosis in Intervertebral Disc Degeneration Pathogenesis. J. Cell Physiol. 2021, 236, 2725–2739. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jing, X.; Du, T.; Ren, J.; Liu, X.; Chen, F.; Shao, Y.; Sun, S.; Yang, G.; Cui, X. Iron Overload Promotes Intervertebral Disc Degeneration via Inducing Oxidative Stress and Ferroptosis in Endplate Chondrocytes. Free Radic. Biol. Med. 2022, 190, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Chen, Z.; Tang, H.-B.; Shan, L.-Q.; Chen, Z.-Y.; Liu, S.-C.; Zhang, Y.-Y.; Guo, X.-Y.; Yang, H.; Hao, D.-J. Necroptosis of Nucleus Pulposus Cells Involved in Intervertebral Disc Degeneration through MyD88 Signaling. Front. Endocrinol. 2022, 13, 994307. [Google Scholar] [CrossRef]
- McIlwain, D.R.; Berger, T.; Mak, T.W. Caspase Functions in Cell Death and Disease. Cold Spring Harb. Perspect. Biol. 2013, 5, a008656. [Google Scholar] [CrossRef]
- Avenoso, A.; D’Ascola, A.; Scuruchi, M.; Mandraffino, G.; Calatroni, A.; Saitta, A.; Campo, S.; Campo, G.M. Hyaluronan in Experimental Injured/Inflamed Cartilage: In Vivo Studies. Life Sci. 2018, 193, 132–140. [Google Scholar] [CrossRef]
- Hwang, H.S.; Park, S.J.; Cheon, E.J.; Lee, M.H.; Kim, H.A. Fibronectin Fragment-Induced Expression of Matrix Metalloproteinases Is Mediated by MyD88-Dependent TLR-2 Signaling Pathway in Human Chondrocytes. Arthritis Res. Ther. 2015, 17, 320. [Google Scholar] [CrossRef]
- Feldman, N.; Rotter-Maskowitz, A.; Okun, E. DAMPs as Mediators of Sterile Inflammation in Aging-Related Pathologies. Ageing Res. Rev. 2015, 24, 29–39. [Google Scholar] [CrossRef]
- Lambert, C.; Zappia, J.; Sanchez, C.; Florin, A.; Dubuc, J.-E.; Henrotin, Y. The Damage-Associated Molecular Patterns (DAMPs) as Potential Targets to Treat Osteoarthritis: Perspectives From a Review of the Literature. Front. Med. 2021, 7, 607186. [Google Scholar] [CrossRef]
- Schmidli, M.R.; Sadowska, A.; Cvitas, I.; Gantenbein, B.; Lischer, H.E.L.; Forterre, S.; Hitzl, W.; Forterre, F.; Wuertz-Kozak, K. Fibronectin Fragments and Inflammation During Canine Intervertebral Disc Disease. Front. Vet. Sci. 2020, 7, 547644. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, K.R.; Walter, B.A.; Laudier, D.M.; Krishnamoorthy, D.; Mosley, G.E.; Spiller, K.L.; Iatridis, J.C. Accumulation and Localization of Macrophage Phenotypes with Human Intervertebral Disc Degeneration. Spine J. 2018, 18, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.J.; Ferreira, J.R.; Cunha, C.; Corte-Real, J.V.; Bessa-Gonçalves, M.; Barbosa, M.A.; Santos, S.G.; Gonçalves, R.M. Macrophages Down-Regulate Gene Expression of Intervertebral Disc Degenerative Markers under a Proinflammatory Microenvironment. Front. Immunol. 2019, 10, 1508. [Google Scholar] [CrossRef]
- Li, X.C.; Luo, S.J.; Fan, W.; Zhou, T.L.; Huang, C.M.; Wang, M.S. M2 Macrophage-Conditioned Medium Inhibits Intervertebral Disc Degeneration in a Tumor Necrosis Factor-α-Rich Environment. J. Orthop. Res. 2022, 40, 2488–2501. [Google Scholar] [CrossRef]
- Du, J.; Pfannkuche, J.J.; Lang, G.; Häckel, S.; Creemers, L.B.; Alini, M.; Grad, S.; Li, Z. Proinflammatory Intervertebral Disc Cell and Organ Culture Models Induced by Tumor Necrosis Factor Alpha. JOR Spine 2020, 3, e1104. [Google Scholar] [CrossRef] [PubMed]
- Monchaux, M.; Forterre, S.; Spreng, D.; Karol, A.; Forterre, F.; Wuertz-Kozak, K. Inflammatory Processes Associated with Canine Intervertebral Disc Herniation. Front. Immunol. 2017, 8, 1681. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liang, G.; Deng, Z.; Tan, J.; Zheng, Q.; Lyu, F.-J. The Upregulation of COX2 in Human Degenerated Nucleus Pulposus: The Association of Inflammation with Intervertebral Disc Degeneration. Mediat. Inflamm. 2021, 2021, 2933199. [Google Scholar] [CrossRef]
- Maidhof, R.; Jacobsen, T.; Papatheodorou, A.; Chahine, N.O. Inflammation Induces Irreversible Biophysical Changes in Isolated Nucleus Pulposus Cells. PLoS ONE 2014, 9, e99621. [Google Scholar] [CrossRef]
- Ruiz-Fernández, C.; Ait, D.; González-Rodríguez, M.; Cordero Barreal, A.; Farrag, Y.; García-Caballero, L.; Lago, F.; Mobasheri, A.; Sakai, D.; Pino, J.; et al. Monomeric CRP Regulates Inflammatory Responses in Human Intervertebral Disc Cells. Bone Jt. Res. 2023, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Xu, K.; Liu, W.; Liu, X.; Yuan, P.; Xu, P.; Li, H. Monomeric C-Reactive Protein Level Is Associated with Osteoarthritis. Exp. Ther. Med. 2022, 23, 277. [Google Scholar] [CrossRef] [PubMed]
- Zeller, J.; Shing, K.S.C.T.; Nero, T.L.; McFadyen, J.D.; Krippner, G.; Bogner, B.; Kreuzaler, S.; Kiefer, J.; Horner, V.K.; Braig, D.; et al. A Novel Phosphocholine-Mimetic Inhibits a pro-Inflammatory Conformational Change in C-Reactive Protein. EMBO Mol. Med. 2023, 15, e16236. [Google Scholar] [CrossRef] [PubMed]
- Durdag, E.; Albayrak, S.; Atci, I.B.; Civi, S.; Kardes, O.; Koca, S.S. The Importance of C-Reactive Protein in Discogenic Low Back Pain: The Analysis of 444 Patients. J. Turk. Spinal Surg. 2018, 29, 115–118. [Google Scholar]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Ge, J.; Yan, Q.; Wang, Y.; Cheng, X.; Song, D.; Wu, C.; Yu, H.; Yang, H.; Zou, J. IL-10 Delays the Degeneration of Intervertebral Discs by Suppressing the P38 MAPK Signaling Pathway. Free Radic. Biol. Med. 2020, 147, 262–270. [Google Scholar] [CrossRef]
- Shamji, M.F.; Setton, L.A.; Jarvis, W.; So, S.; Chen, J.; Jing, L.; Bullock, R.; Isaacs, R.E.; Brown, C.; Richardson, W.J. Pro-Inflammatory Cytokine Expression Profile in Degenerative and Herniated Human Intervertebral Disc Tissues. Arthritis Rheum. 2010, 62, 1974–1982. [Google Scholar] [CrossRef]
- Sadowska, A.; Hausmann, O.N.; Wuertz-Kozak, K. Inflammaging in the Intervertebral Disc. Clin. Transl. Neurosci. 2018, 2, 2514183X1876114. [Google Scholar] [CrossRef]
- Weber, K.T.; Alipui, D.O.; Sison, C.P.; Bloom, O.; Quraishi, S.; Overby, M.C.; Levine, M.; Chahine, N.O. Serum Levels of the Proinflammatory Cytokine Interleukin-6 Vary Based on Diagnoses in Individuals with Lumbar Intervertebral Disc Diseases. Arthritis Res. Ther. 2016, 18, 3. [Google Scholar] [CrossRef]
- Grad, S.; Bow, C.; Karppinen, J.; Luk, K.; Cheung, K.; Alini, M.; Samartzis, D. Systemic Blood Plasma CCL5 and CXCL6: Potential Biomarkers for Human Lumbar Disc Degeneration. Eur. Cell Mater 2016, 31, 1–10. [Google Scholar] [CrossRef]
- Ye, S.; Ju, B.; Wang, H.; Lee, K.B. Bone Morphogenetic Protein-2 Provokes Interleukin-18-Induced Human Intervertebral Disc Degeneration. Bone Jt. Res. 2016, 5, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Walter, B.A.; Mageswaran, P.; Mo, X.; Boulter, D.J.; Mashaly, H.; Nguyen, X.V.; Prevedello, L.M.; Thoman, W.; Raterman, B.D.; Kalra, P.; et al. MR Elastography–Derived Stiffness: A Biomarker for Intervertebral Disc Degeneration. Radiology 2017, 285, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Guo, J.; Zhai, W.; Xie, Y.; Jia, Y. CircRNA GRB10 Is a Novel Biomarker for the Accurate Diagnosis of Lumbar Degenerative Disc Disease. Mol. Biotechnol. 2023, 65, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Jin, S.; Wang, M.; Jin, X.; Lv, C.; Deng, Y.; Wang, J. Enhanced NLRP3, Caspase-1, and IL- 1β Levels in Degenerate Human Intervertebral Disc and Their Association with the Grades of Disc Degeneration. Anat. Rec. 2015, 298, 720–726. [Google Scholar] [CrossRef]
- Lee, S.; Moon, C.S.; Sul, D.; Lee, J.; Bae, M.; Hong, Y.; Lee, M.; Choi, S.; Derby, R.; Kim, B.J.; et al. Comparison of Growth Factor and Cytokine Expression in Patients with Degenerated Disc Disease and Herniated Nucleus Pulposus. Clin. Biochem. 2009, 42, 1504–1511. [Google Scholar] [CrossRef]
- Lan, T.; Hu, Z.; Guo, W.; Yan, B.; Zhang, Y. Development of a Novel Inflammatory-Associated Gene Signature and Immune Infiltration Patterns in Intervertebral Disc Degeneration. Oxid. Med. Cell. Longev. 2022, 2022, 2481071. [Google Scholar] [CrossRef]
- Lv, F.; Leung, V.Y.L.; Huang, S.; Huang, Y.; Sun, Y.; Cheung, K.M.C. In Search of Nucleus Pulposus-Specific Molecular Markers. Rheumatology 2014, 53, 600–610. [Google Scholar] [CrossRef]
- Yu, X.-J.; Wang, Y.-G.; Lu, R.; Guo, X.-Z.; Qu, Y.-K.; Wang, S.-X.; Xu, H.-R.; Kang, H.; You, H.-B.; Xu, Y. BMP7 Ameliorates Intervertebral Disc Degeneration in Type 1 Diabetic Rats by Inhibiting Pyroptosis of Nucleus Pulposus Cells and NLRP3 Inflammasome Activity. Mol. Med. 2023, 29, 30. [Google Scholar] [CrossRef]
- Shafie, I.N.F.; McLaughlin, M.; Burchmore, R.; Lim, M.A.A.; Montague, P.; Johnston, P.E.J.; Penderis, J.; Anderson, T.J. The Chaperone Protein Clusterin May Serve as a Cerebrospinal Fluid Biomarker for Chronic Spinal Cord Disorders in the Dog. Cell Stress Chaperones 2014, 19, 311. [Google Scholar] [CrossRef]
- Willems, N.; Tellegen, A.R.; Bergknut, N.; Creemers, L.B.; Wolfswinkel, J.; Freudigmann, C.; Benz, K.; Grinwis, G.C.M.; Tryfonidou, M.A.; Meij, B.P. Inflammatory Profiles in Canine Intervertebral Disc Degeneration. BMC Vet. Res. 2016, 12, 10. [Google Scholar] [CrossRef]
- Molinos, M.; Fiordalisi, M.F.; Caldeira, J.; Almeida, C.R.; Barbosa, M.A.; Gonçalves, R.M. Alterations of Bovine Nucleus Pulposus Cells with Aging. Aging Cell 2023, 2023, e13873. [Google Scholar] [CrossRef] [PubMed]
- De Bree, K.; De Bakker, B.S.; Oostra, R.J. The Development of the Human Notochord. PLoS ONE 2018, 13, e0205752. [Google Scholar] [CrossRef] [PubMed]
- Scaal, M. Early Development of the Vertebral Column. Semin. Cell Dev. Biol. 2016, 49, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Gomerčić, H.; Vukovic, S.; Gomerčić, V.; Škrtić, D. Histological and Histochemical Characteristics of the Bovine Notochord. Int. J. Dev. Biol. 1991, 35, 353–358. [Google Scholar] [PubMed]
- Stoeckelhuber, M.; Brueckner, S.; Spohr, G.; Welsch, U. Proteoglycans and Collagen in the Intervertebral Disc of the Rhesus Monkey (Macaca mulatta). Ann. Anat.-Anat. Anz. 2005, 187, 35–42. [Google Scholar] [CrossRef]
- Guo, C.; Hu, Y.; Wu, X.; An, F. The development of degenerative disc animal model in rhesus monkey. Zhonghua Wai Ke Za Zhi 2000, 38, 548–551. [Google Scholar]
- Platenberg, R.C.; Hubbard, G.B.; Ehler, W.J.; Hixson, C.J. Spontaneous Disc Degeneration in the Baboon Model: Magnetic Resonance Imaging and Histopathologic Correlation. J. Med. Primatol. 2001, 30, 268–272. [Google Scholar] [CrossRef]
- Longo, G.; Ripalda, P.; Denaro, V.; Forriol, F. Morphologic Comparison of Cervical, Thoracic, Lumbar Intervertebral Discs of Cynomolgus Monkey (Macaca fascicularis). Eur. Spine J. 2006, 15, 1845–1851. [Google Scholar] [CrossRef]
- Longo, U.; Ripalda, P.; Salvatore, G.; Berton, A.; Khan, W.; Maffulli, N.; Forriol, F.; Denaro, V. Fibronectin Expression in the Intervertebral Disc of Monkey. Curr. Stem. Cell Res. Ther. 2014, 10, 64–68. [Google Scholar] [CrossRef]
- Bailey, J.F.; Fields, A.J.; Liebenberg, E.; Mattison, J.A.; Lotz, J.C.; Kramer, P.A. Comparison of Vertebral and Intervertebral Disc Lesions in Aging Humans and Rhesus Monkeys. Osteoarthr. Cartil. 2014, 22, 980–985. [Google Scholar] [CrossRef]
- Duncan, A.E.; Colman, R.J.; Kramer, P.A. Sex Differences in Spinal Osteoarthritis in Humans and Rhesus Monkeys (Macaca Mulatta). Spine 2012, 37, 915–922. [Google Scholar] [CrossRef]
- Hansen, H.-J. A Pathologic-Anatomical Study on Disc Degeneration in Dog: With Special Reference to the So-Called Enchondrosis Intervertebralis. Acta Orthop. Scand. 1952, 23, 1–130. [Google Scholar] [CrossRef]
- Lee, N.N.; Kramer, J.S.; Stoker, A.M.; Bozynski, C.C.; Cook, C.R.; Stannard, J.T.; Choma, T.J.; Cook, J.L. Canine Models of Spine Disorders. JOR Spine 2020, 3, e1109. [Google Scholar] [CrossRef] [PubMed]
- Smolders, L.A.; Bergknut, N.; Grinwis, G.C.M.; Hagman, R.; Lagerstedt, A.S.; Hazewinkel, H.A.W.; Tryfonidou, M.A.; Meij, B.P. Intervertebral Disc Degeneration in the Dog. Part 2: Chondrodystrophic and Non-Chondrodystrophic Breeds. Vet. J. 2013, 195, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Rusbridge, C. Canine Chondrodystrophic Intervertebral Disc Disease (Hansen Type I Disc Disease). BMC Musculoskelet Disord. 2015, 16, S11. [Google Scholar] [CrossRef]
- Stigen, Ø.; Ciasca, T.; Kolbjørnsen, Ø. Calcification of Extruded Intervertebral Discs in Dachshunds: A Radiographic, Computed Tomographic and Histopathological Study of 25 Cases. Acta Vet. Scand. 2019, 61, 13. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, M.S.; Scheibye-Alsing, K.; Karlskov-Mortensen, P.; Proschowsky, H.F.; Jensen, V.F.; Bak, M.; Tommerup, N.; Kadarmideen, H.N.; Fredholm, M. Validation of Genome-Wide Intervertebral Disk Calcification Associations in Dachshund and Further Investigation of the Chromosome 12 Susceptibility Locus. Front. Genet. 2012, 3, 225. [Google Scholar] [CrossRef]
- Brisson, B.A. Intervertebral Disc Disease in Dogs. Vet. Clin. NA Small Anim. Pract. 2010, 40, 829–858. [Google Scholar] [CrossRef] [PubMed]
- Henke, D.; Vandevelde, M.; Doherr, M.G.; Stöckli, M.; Forterre, F. Correlations between Severity of Clinical Signs and Histopathological Changes in 60 Dogs with Spinal Cord Injury Associated with Acute Thoracolumbar Intervertebral Disc Disease. Vet. J. 2013, 198, 70–75. [Google Scholar] [CrossRef]
- Hansen, T.; Smolders, L.A.; Tryfonidou, M.A.; Meij, B.P.; Vernooij, J.C.M.; Bergknut, N.; Grinwis, G.C.M. The Myth of Fibroid Degeneration in the Canine Intervertebral Disc: A Histopathological Comparison of Intervertebral Disc Degeneration in Chondrodystrophic and Nonchondrodystrophic Dogs. Vet. Pathol. 2017, 54, 945–952. [Google Scholar] [CrossRef]
- Priester, W.A. Canine Intervertebral Disc Disease—Occurrence by Age, Breed, and Sex among 8,117 Cases. Theriogenology 1976, 6, 293–303. [Google Scholar] [CrossRef]
- Packer, R.M.A.; Seath, I.J.; O’Neill, D.G.; De Decker, S.; Volk, H.A. DachsLife 2015: An Investigation of Lifestyle Associations with the Risk of Intervertebral Disc Disease in Dachshunds. Canine Genet. Epidemiol. 2016, 3, 8. [Google Scholar] [CrossRef]
- Harrison, M.; O’Brien, A.; Adams, L.; Cowin, G.; Ruitenberg, M.J.; Sengul, G.; Watson, C. Vertebral Landmarks for the Identification of Spinal Cord Segments in the Mouse. Neuroimage 2013, 68, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.J. The Anatomy of the Laboratory Mouse; Acadmic Press: London, UK; New York, NY, USA, 1965. [Google Scholar]
- Tang, S.N.; Walter, B.A.; Heimann, M.K.; Gantt, C.C.; Khan, S.N.; Kokiko-Cochran, O.N.; Askwith, C.C.; Purmessur, D. In Vivo Mouse Intervertebral Disc Degeneration Models and Their Utility as Translational Models of Clinical Discogenic Back Pain: A Comparative Review. Front. Pain Res. 2022, 3, 894651. [Google Scholar] [CrossRef]
- Brendler, J.; Winter, K.; Lochhead, P.; Schulz, A.; Ricken, A.M. Histological Differences between Lumbar and Tail Intervertebral Discs in Mice. J. Anat. 2022, 240, 84. [Google Scholar] [CrossRef] [PubMed]
- Koolhaas, J.M. The Laboratory Rat. In The UFAW Handbook on the Care and Management of Laboratory and Other Research Animals; Hubrecht, R.C., Kirkwood, J., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 1–848. [Google Scholar]
- Stöckl, S.; Reichart, J.; Zborilova, M.; Johnstone, B.; Grässel, S. Semaphorin 3A-Neuropilin-1 Signaling Modulates MMP13 Expression in Human Osteoarthritic Chondrocytes. Int. J. Mol. Sci. 2022, 23, 14180. [Google Scholar] [CrossRef]
- Poletto, D.L.; Crowley, J.D.; Tanglay, O.; Walsh, W.R.; Pelletier, M.H. Preclinical in Vivo Animal Models of Intervertebral Disc Degeneration. Part 1: A Systematic Review. JOR Spine 2023, 6, e1234. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, K.; Chamoli, U.; Masuda, K.; Miyazaki, S.; Kato, K.; Diwan, A.D. A Novel Magnetic Resonance Imaging Postprocessing Technique for the Assessment of Intervertebral Disc Degeneration—Correlation with Histological Grading in a Rabbit Disc Degeneration Model. JOR Spine 2019, 2, e1060. [Google Scholar] [CrossRef]
- Alini, M.; Eisenstein, S.M.; Ito, K.; Little, C.; Kettler, A.A.; Masuda, K.; Melrose, J.; Ralphs, J.; Stokes, I.; Wilke, H.J. Are Animal Models Useful for Studying Human Disc Disorders/Degeneration? Eur. Spine J. 2008, 17, 2–19. [Google Scholar] [CrossRef]
- Kim, K.-W.; Lim, T.-H.; Kim, J.G.; Jeong, S.-T.; Masuda, K.; An, H.S. The Origin of Chondrocytes in the Nucleus Pulposus and Histologic Findings Associated With the Transition of a Notochordal Nucleus Pulposus to a Fibrocartilaginous Nucleus Pulposus in Intact Rabbit Intervertebral Discs. Spine 2003, 28, 982–990. [Google Scholar] [CrossRef]
- Ashinsky, B.G.; Bonnevie, E.D.; Mandalapu, S.A.; Pickup, S.; Wang, C.; Han, L.; Mauck, R.L.; Smith, H.E.; Gullbrand, S.E. Intervertebral Disc Degeneration Is Associated With Aberrant Endplate Remodeling and Reduced Small Molecule Transport. J. Bone Miner. Res. 2020, 35, 1572–1581. [Google Scholar] [CrossRef]
- Caldeira, J.; Santa, C.; Osório, H.; Molinos, M.; Manadas, B.; Gonçalves, R.; Barbosa, M. Matrisome Profiling During Intervertebral Disc Development And Ageing. Sci. Rep. 2017, 7, 11629. [Google Scholar] [CrossRef]
- Bonnaire, F.C.; Danalache, M.; Sigwart, V.A.; Breuer, W.; Rolauffs, B.; Hofmann, U.K. The Intervertebral Disc from Embryonic Development to Disc Degeneration: Insights into Spatial Cellular Organization. Spine J. 2021, 21, 1387–1398. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.M.; Levine, G.J.; Hoffman, A.G.; Bratton, G. Comparative Anatomy of the Horse, Ox, and Dog:The Brain and Associated Vessels*. Compend. Equine 2008, 3, 153–164. [Google Scholar]
- Bergmann, W.; Bergknut, N.; Veraa, S.; Gröne, A.; Vernooij, H.; Wijnberg, I.D.; Back, W.; Grinwis, G.C.M. Intervertebral Disc Degeneration in Warmblood Horses: Morphology, Grading, and Distribution of Lesions. Vet. Pathol. 2018, 55, 442–452. [Google Scholar] [CrossRef]
- Townsend, H.G.G.; Leach, D.H.; Doiget, C.E.; Kirkaldy-Willis, W.H. Relationship between Spinal Biomechanics Anid Pathological Changes in the Equine Thoracolumbar Spine. Equine Vet. J. 1986, 18, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Bollwein, A.; Hänichen, T. Age-Related Changes in the Intervertebral Disks of the Cervical Vertebrae of the Horse. Tierarztl. Prax. 1989, 17, 73–76. [Google Scholar]
- Krueger, C.; Gold, J.; Barrett, M.; Aboellail, T. Diagnosis of Lumbosacral Intervertebral Disc Disease and Protrusion in a Horse Using Ultrasonographic Evaluation and Computed Tomography. Equine Vet. Educ. 2016, 28, 685–689. [Google Scholar] [CrossRef]
- Barreto, R.d.S.N.; Rodrigues, M.N.; Carvalho, R.C.; Fernanda, F.M.; Rigoglio, N.N.; Jacob, J.C.F.; Gastal, E.L.; Miglino, M.A. Organogenesis of the Musculoskeletal System in Horse Embryos and Early Fetuses. Anat. Rec. 2016, 299, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Story, M.R.; Haussler, K.K.; Nout-Lomas, Y.S.; Aboellail, T.A.; Kawcak, C.E.; Barrett, M.F.; Frisbie, D.D.; Wayne Mcilwraith, C. Equine Cervical Pain and Dysfunction: Pathology, Diagnosis and Treatment. Animals 2021, 11, 422. [Google Scholar] [CrossRef]
- Dyson, S.; Busoni, V.; Salciccia, A. Intervertebral Disc Disease of the Cervical and Cranial Thoracic Vertebrae in Equidae: Eight Cases. Equine Vet. Educ. 2020, 32, 437–443. [Google Scholar] [CrossRef]
- Mayaki, A.M.; Razak, I.-S.A.; Adzahan, N.M.; Mazlan, M.; Abdullah, R. Investigation of Potential Serum Biomarkers for the Diagnosis of Chronic Back Pain in Horses. Maced. Vet. Rev. 2023, 46, 79–87. [Google Scholar] [CrossRef]
- Veraa, S.; Bergmann, W.; Wijnberg, I.D.; Back, W.; Vernooij, H.; Nielen, M.; den Belt, A.M. Equine Cervical Intervertebral Disc Degeneration Is Associated with Location and MRI Features. Vet. Radiol. Ultrasound 2019, 60, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Foss, R.R.; Genetzky, R.M.; Riedesel, E.A.; Graham, C. Cervical Intervertebral Disc Protrusion in Two Horses. Can. Vet. J. 1983, 24, 188–191. [Google Scholar]
- Nappert, G.; Vrins, A.; Breton, L.; Beauregard, M. A Retrospective Study of Nineteen Ataxic Horses. Can. Vet. J. 1989, 30, 802. [Google Scholar]
- Magi, G.; Cherubini, G.B.; Taeymans, O. Sacrocaudal (Sacrococcygeal) Intervertebral Disc Protrusion in 2 Cats. Can. Vet. J. 2018, 59, 388–392. [Google Scholar]
- Knipe, M.; Vernau, K.; Hornof, W.; LeCouteur, R. Intervertebral Disc Extrusion in Six Cats. J. Feline Med. Surg. 2001, 3, 161–168. [Google Scholar] [CrossRef]
- Debreuque, M.; Valin, I.; Prata, D.; De Fornel, P.; Thibaud, J.-L. Case Report: Intramedullary Intervertebral Disk Extrusion in a Cat: Clinical, Computed Tomographic, High-Field Magnetic Resonance Imaging, and Outcome Findings. Front. Vet. Sci. 2020, 7, 583892. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. Gene; National Library of Medicine (US), National Center for Biotechnology Information: Bethesda, MD, USA, 2004. Available online: https://www.ncbi.nlm.nih.gov/gene/ (accessed on 30 June 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murphy, K.; Lufkin, T.; Kraus, P. Development and Degeneration of the Intervertebral Disc—Insights from Across Species. Vet. Sci. 2023, 10, 540. https://doi.org/10.3390/vetsci10090540
Murphy K, Lufkin T, Kraus P. Development and Degeneration of the Intervertebral Disc—Insights from Across Species. Veterinary Sciences. 2023; 10(9):540. https://doi.org/10.3390/vetsci10090540
Chicago/Turabian StyleMurphy, Kathryn, Thomas Lufkin, and Petra Kraus. 2023. "Development and Degeneration of the Intervertebral Disc—Insights from Across Species" Veterinary Sciences 10, no. 9: 540. https://doi.org/10.3390/vetsci10090540
APA StyleMurphy, K., Lufkin, T., & Kraus, P. (2023). Development and Degeneration of the Intervertebral Disc—Insights from Across Species. Veterinary Sciences, 10(9), 540. https://doi.org/10.3390/vetsci10090540






