The Use of Tricaine Methanesulfonate (MS-222) in Asian Seabass (Lates calcarifer) at Different Temperatures: Study of Optimal Doses, Minimum Effective Concentration, Blood Biochemistry, Immersion Pharmacokinetics, and Tissue Distributions
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Preparation of MS-222 Solutions
2.2. Experimental Fish
2.3. Blood Collection
2.4. Determination of the Optimal Doses after Single MS-222 Immersion
2.5. Determination of the Minimum Effective Concentrations (MECs) by HPLC
2.6. Establishment of the Calibration Curves
2.7. Assessment of Blood Biochemical Parameters
2.8. Pharmacokinetic Study
2.9. Tissue Distribution Study
2.10. Statistical Analysis
3. Results
3.1. Determination of Optimal Doses after Single MS-222 Immersion
3.2. Validation of the HPLC Method for Quantification of MS-222
3.3. Determination of the Minimum Effective Concentration (MEC)
3.4. Assessment of Blood Biochemical Parameters
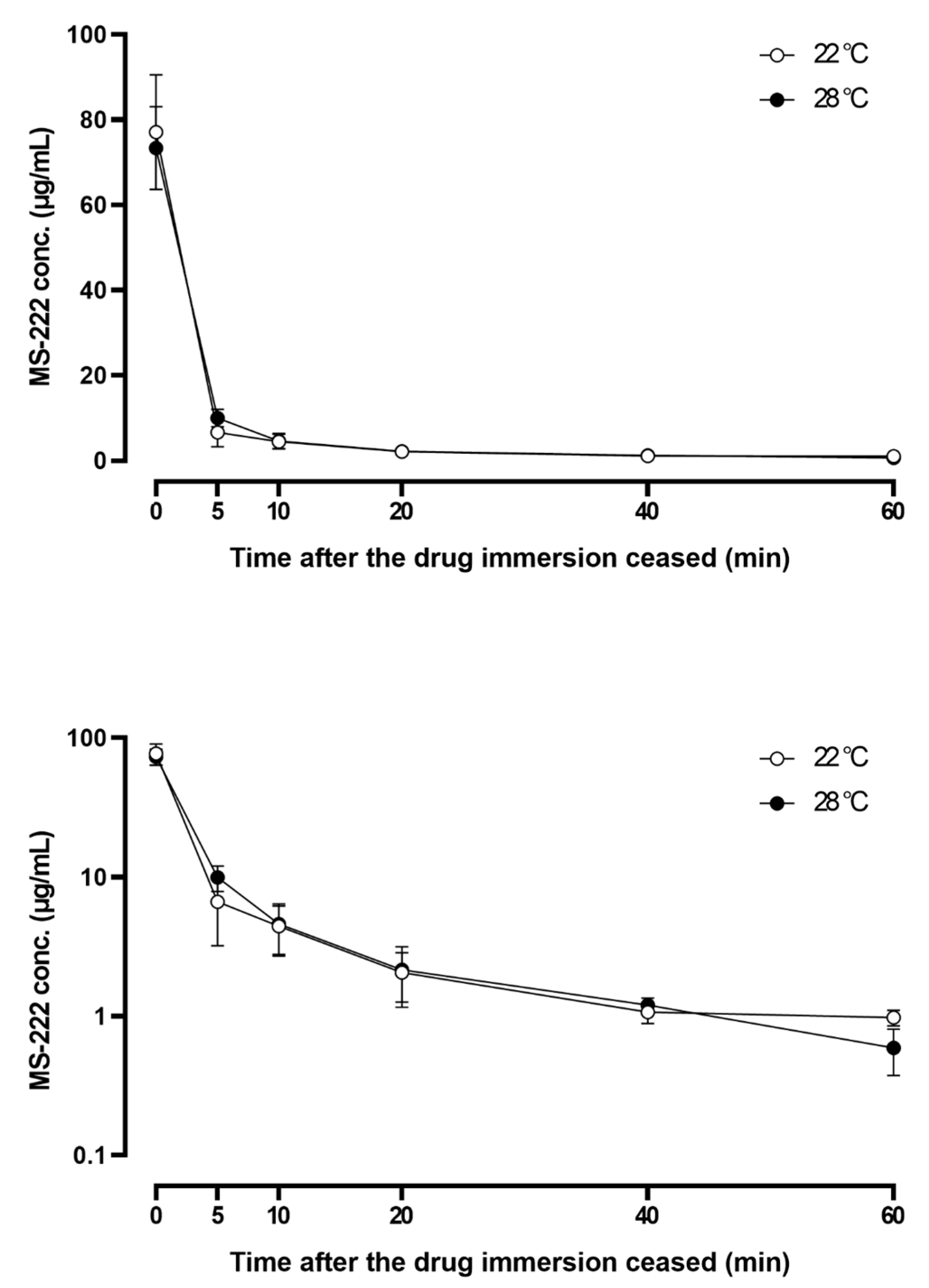
3.5. Pharmacokinetic Study
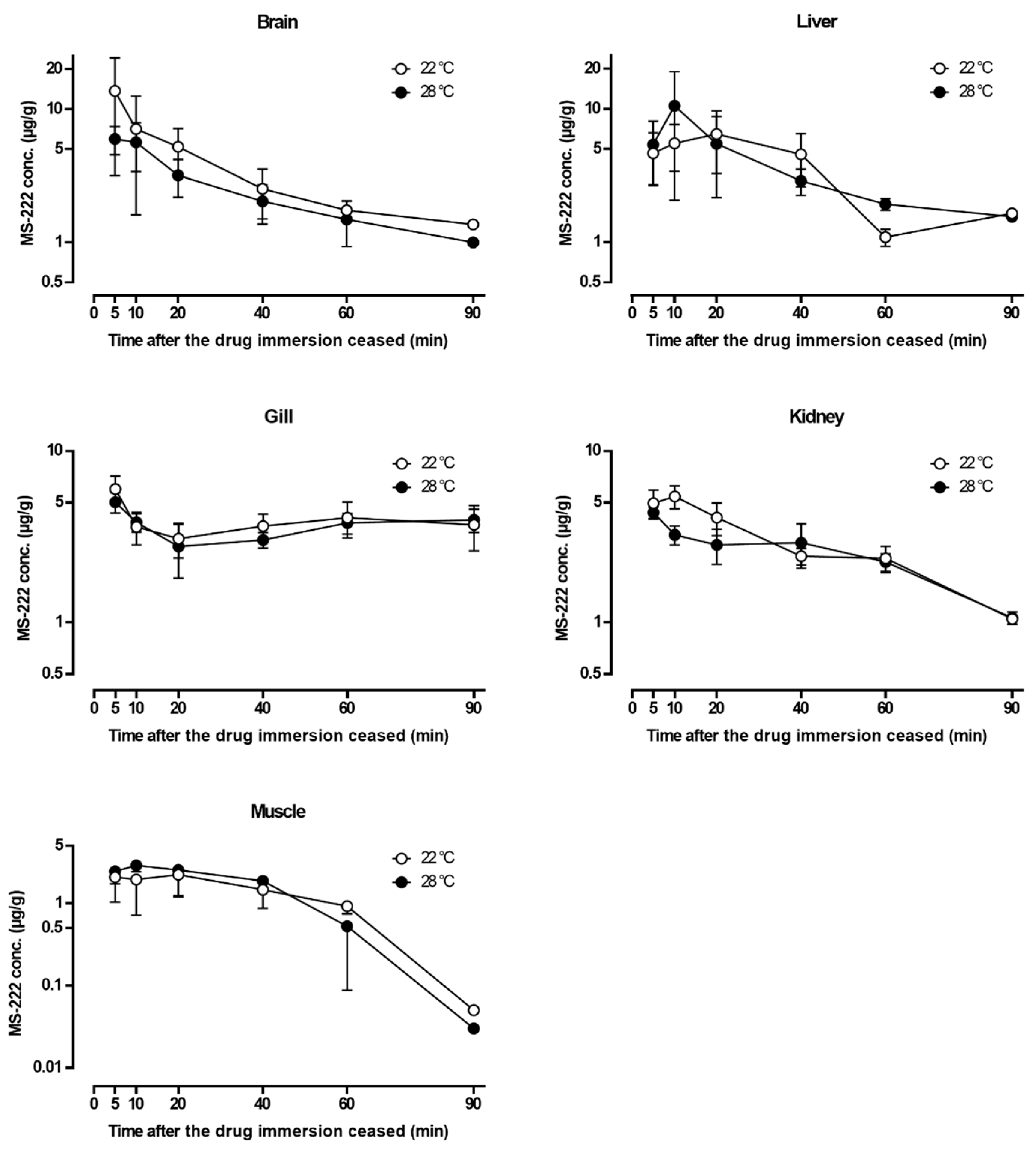
3.6. Tissue Distribution Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghanawi, J.; Monzer, S.; Saoud, I.P. Anaesthetic efficacy of clove oil, benzocaine, 2-phenoxyethanol and tricaine methanesulfonate in juvenile marbled spinefoot (Siganus rivulatus). Aquac. Res. 2013, 44, 359–366. [Google Scholar] [CrossRef]
- Chambel, J.; Pinho, R.; Sousa, R.; Ferreira, T.; Baptista, T.; Severiano, V.; Mendes, S.; Pedrosa, R. The efficacy of MS-222 as anaesthetic agent in four freshwater aquarium fish species. Aquac. Res. 2015, 46, 582–1589. [Google Scholar] [CrossRef]
- Topić Popović, N.; Strunjak-Perović, I.; Čož-Rakovac, R.; Barišić, J.; Jadan, M.; Beraković, A.P.; Klobučar, R.S. Tricaine methane-sulfonate (MS-222) application in fish anaesthesia. J. Appl. Ichthyol. 2012, 28, 553–564. [Google Scholar] [CrossRef]
- Medler, S. Anesthetic MS-222 eliminates nerve and muscle activity in frogs used for physiology teaching laboratories. Adv. Physiol. Educ. 2019, 43, 69–75. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration Approved Aquaculture Drugs. Available online: https://www.fda.gov/animal-veterinary/aquaculture/approved-aquaculture-drugs (accessed on 14 May 2023).
- Council of Agriculture, Executive Yuan, R.O.C. (Taiwan). Guidelines for the Use of Animal Drugs Article 3 Annex I: Code of Practice for Aquatic Animal Drugs. Available online: https://law.coa.gov.tw/glrsnewsout/Download.ashx?FileID=13569 (accessed on 14 May 2023).
- Volkoff, H.; Rønnestad, I. Effects of temperature on feeding and digestive processes in fish. Temperature 2020, 7, 307–320. [Google Scholar] [CrossRef]
- Santos, S.; Ghanawi, J.; Saoud, I.P. Effects of water temperature and body weight on anaesthetic efficiency in marbled rabbitfish (Siganus rivulatus). Aquacult. Res. 2015, 46, 928–936. [Google Scholar] [CrossRef]
- Rożyński, M.; Hopko, M.; Stawecki, K.; Zakęś, Z. Impact of fish size, water temperature, and MS-222 concentration on inducing general anesthesia in pikeperch (Sander lucioperca). Aquac. Res. 2018, 49, 2774–2781. [Google Scholar] [CrossRef]
- Küçük, S. Efficacy of tricaine on Peocilia latipinna at different temperatures and concentrations. Afr. J. Biotechnol. 2012, 9, 755–759. [Google Scholar] [CrossRef]
- Hunn, J.B. Dynamics of MS-222 in the blood and brain of freshwater fishes during anesthesia. Invest. Fish. Control. 1970, 42, 3–8. [Google Scholar]
- Fontana, B.D.; Alnassar, N.; Parker, M.O. Tricaine methanesulfonate (MS222) has short-term effects on young adult zebrafish (Danio rerio) working memory and cognitive flexibility, but not on aging fish. Front. Behav. Neurosci. 2021, 15, 686102. [Google Scholar] [CrossRef] [PubMed]
- Ramlochansingh, C.; Branoner, F.; Chagnaud, B.P.; Straka, H. Efficacy of tricaine methanesulfonate (MS-222) as an anesthetic agent for blocking sensory-motor responses in Xenopus laevis tadpoles. PLoS ONE 2014, 9, e101606. [Google Scholar] [CrossRef] [PubMed]
- Treves-Brown, K.M. Applied Fish Pharmacology; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 209–210. [Google Scholar]
- Stoskopf, M.K. Laboratory Animal Medicine; Fox, J.G., Anderson, L.C., Otto, G.M., Pritchett-Corning, K.R., Whary, M.T., Eds.; Elsevier Inc.: London, UK, 2015; Volume 3, Chapter 21; pp. 1063–1086. [Google Scholar]
- Xue, Y.J.; Chang, C.C.; Lai, J.M.; Wang, J.H. Determining the tranquilization dose and residue of tricaine methanesulfonate (MS-222) in sea bass Lates calcarifer tissue. Fish. Sci. 2017, 83, 625–633. [Google Scholar] [CrossRef]
- Palić, D.; Herolt, D.M.; Andreasen, C.B.; Menzel, B.W.; Roth, J.A. Anesthetic efficacy of tricaine methanesulfonate, metomidate and eugenol: Effects on plasma cortisol concentration and neutrophil function in fathead minnows (Pimephales promelas Rafinesque, 1820). Aquaculture 2006, 254, 675–685. [Google Scholar] [CrossRef]
- Weber, R.A.; Peleteiro, J.B.; García Martín, L.O.; Aldegunde, M. The efficacy of 2-phenoxyethanol, metomidate, clove oil and MS-222 as anaesthetic agents in the Senegalese sole (Solea senegalensis Kaup 1858). Aquaculture 2009, 288, 147–150. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, X.W.; Ding, H.T.; Dong, X.J.; Zhang, J.J.; Zheng, Y.C.; Chen, X.N.; Cheng, H.L.; Ding, Z.J.; Xu, J.H. Effects of tricaine methanesulfonate (MS-222) on sedation and responses of yellow catfish (Pelteobagrus fulvidraco) subjected to simulated transportation stress. Aquaculture 2022, 549, 737789. [Google Scholar] [CrossRef]
- Ryan, S. The dynamics of MS-222 anaesthesia in a marine teleost (Pagrus auratus: Sparidae). Comp. Biochem. Physiol. C Comp. Pharmacol. Toxicol. 1992, 101, 593–600. [Google Scholar] [CrossRef]
- Skår, M.W.; Haugland, G.T.; Powell, M.D.; Wergeland, H.I.; Samuelsen, O.B. Development of anaesthetic protocols for lumpfish (Cyclopterus lumpus L.): Effect of anaesthetic concentrations, sea water temperature and body weight. PLoS ONE 2017, 12, e0179344. [Google Scholar] [CrossRef]
- Rairat, T.; Chi, Y.; Hsieh, C.Y.; Liu, Y.K.; Chuchird, N.; Chou, C.C. Determination of optimal doses and minimum effective concentrations of tricaine methanesulfonate, 2-phenoxyethanol and eugenol for laboratory managements in Nile tilapia (Oreochromis niloticus). Animals 2021, 11, 1521. [Google Scholar] [CrossRef]
- Kucuk, S.; Coban, D. Effects of tricaine as an anaesthetics on goldfish, Carassius auratus (Linnaeus 1758) at different salinities and concentrations. Turkish. J. Fish. Aquat. Sci. 2016, 16, 611–616. [Google Scholar] [CrossRef]
- Kiessling, A.; Johansson, D.; Zahl, I.H.; Samuelsen, O.B. Pharmacokinetics, plasma cortisol and effectiveness of benzocaine, MS-222 and isoeugenol measured in individual dorsal aorta-cannulated Atlantic salmon (Salmo salar) following bath administration. Aquaculture 2009, 286, 301–308. [Google Scholar] [CrossRef]
- Vaclavik, L.; Cajka, T.; Zhou, W.; Wang, P.G. High-Throughput Analysis for Food Safety; Wang, P.G., Vitha, M.F., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; Volume 1, Chapter 2; pp. 15–72. [Google Scholar]
- American Veterinary Medical Association AVMA Guidelines for the Euthanasia of Animals: 2020 Edition. Available online: https://www.avma.org/sites/default/files/2020-02/Guidelines-on-Euthanasia-2020.pdf (accessed on 14 May 2023).
- Hoskonen, P.; Prihonen, J. Temperature effects on anaesthesia with clove oil in six temperate-zone fishes. J. Fish. Biol. 2004, 64, 1136–1137. [Google Scholar] [CrossRef]
- Park, I.S.; Gil, H.W.; Lee, T.H.; Nam, Y.K.; Lim, S.G.; Kim, D.S. Effects of clove oil and lidocaine-HCl anesthesia on water parameter during simulated transportation in the marine medaka, Oryzias dancena. Dev. Reprod. 2017, 21, 19–33. [Google Scholar] [CrossRef]
- Jacobsen, J.V.; Steen, K.; Nilssen, K.J. Anaesthetic efficacy of Aqui-S, Benzoak, and MS-222 on lumpfish (Cyclopterus lumpus) fries. Impact from temperature, salinity, and fasting. PLOS One 2019, 14, e0211080. [Google Scholar] [CrossRef] [PubMed]
- Killen, S.S.; Atkinson, D.; Glazier, D.S. The intraspecific scaling of metabolic rate with body mass in fishes depends on lifestyle and temperature. Ecol. Lett. 2010, 13, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Zahl, I.H.; Kiessling, A.; Samuelsen, O.B.; Hansen, M.K. Anaesthesia of Atlantic halibut (Hippoglossus hippoglossus) effect of pre-anaesthetic sedation, and importance of body weight and water temperature. Aquacult. Res. 2010, 42, 1235–1245. [Google Scholar] [CrossRef]
- Rairat, T.; Hsieh, M.K.; Ho, W.C.; Lu, Y.P.; Fu, Z.Y.; Chuchird, N.; Chou, C.C. Effects of temperature on the pharmacokinetics, optimal dosage, tissue residue, and withdrawal time of florfenicol in asian seabass (lates calcarifer). Food. Addit. Contam. Part. A Chem. Anal. Control. Expo. Risk. Assess. 2003, 40, 235–246. [Google Scholar] [CrossRef]
- Cao, C.; Liu, Y.; Zhang, G.; Dong, J.; Xu, N.; Zhou, S.; Yang, Y.; Yang, Q.; Ai, X. Temperature-dependent residue depletion regularities of tiamulin in Nile tilapia (Oreochromis niloticus) following multiple oral administrations. Front. Vet. Sci. 2021, 8, 679657. [Google Scholar] [CrossRef]
- Xu, N.; Li, M.; Lin, Z.; Ai, X. Comparative pharmacokinetics of sulfadiazine and its metabolite N4-acetyl sulfadiazine in grass carp (Ctenopharyngodon idella) at different temperatures after oral administration. Pharmaceutics 2002, 14, 712. [Google Scholar] [CrossRef]
- Charoendat, U.; Areechon, N.; Srisapoome, P.; Chantasart, D. Efficacy of synthetic eugenol as an anesthetic for Nile tilapia (Oreochromis niloticus Linn.). Kasetsart. J. 2009, 43, 132–140. Available online: https://li01.tci-thaijo.org/index.php/anres/article/view/244804 (accessed on 1 March 2023).
- Tsantilas, H.; Galatos, A.D.; Athanassopoulou, F.; Prassinos, N.N.; Kousoulaki, K. Efficacy of 2-phenoxyethanol as an anaesthetic for two size classes of white sea bream, Diplodus sargus L. and sharp snout sea bream, Diplodus puntazzo C. Aquaculture 2006, 253, 64–70. [Google Scholar] [CrossRef]
- Jia, Y.; Xie, T.; Gao, Y.; Qin, H.; Guan, C. Anesthetics efficacy and physiological response of MS222 and clove oil in spotted knifejaw Oplegnathus punctatus. Aquac. Rep. 2022, 25, 101201. [Google Scholar] [CrossRef]
- Iwama, G.K.; McGeer, J.C.; Pawluk, M.P. The effects of five fish anaesthetics on acid-base balance, hematocrit, blood gases, cortisol, and adrenaline in rainbow trout. Can. J. Zool. 1989, 67, 2065–2073. [Google Scholar] [CrossRef]
- Hanley, C.S.; Clyde, V.L.; Wallace, R.S.; Paul-Murphy, J.; Patterson, T.A.; Keuler, N.S.; Sladky, K.K. Effects of anesthesia and surgery on serial blood gas values and lactate concentrations in yellow perch (Perca flavescens), walleye pike (Sander vitreus), and koi (Cyprinus carpio). J. Am. Vet. Med. Assoc. 2010, 236, 1104–1108. [Google Scholar] [CrossRef]
- Garcia, A.J.; Ramirez, J.M. Keeping carbon dioxide in check. eLife 2017, 6, e27563. [Google Scholar] [CrossRef] [PubMed]
- Suski, C.D.; Cooke, S.J.; Danylchuk, A.J.; O’Connor, C.M.; Gravel, M.A.; Redpath, T.; Hanson, K.C.; Gingerich, A.J.; Murchie, K.J.; Danylchuk, S.E.; et al. Physiological disturbance and recovery dynamics of bonefish (Albula vulpes), a tropical marine fish, in response to variable exercise and exposure to air. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 148, 664–673. [Google Scholar] [CrossRef]
- Monteiro, M.; Sousa, C.; Coutinho, F.; Castro, C.; Fontinha, F.; Guerreiro, I.; Pousão, P.; Matos, E.; Díaz-Rosales, P.; Oliva-Teles, A.; et al. Functional feeds to tackle meagre (Argyrosomus regius) stress: Physiological responses under acute stressful handling conditions. Mar. Drugs. 2021, 19, 598. [Google Scholar] [CrossRef]
- Maccormack, T.J.; Driedzic, W.R. Cardiorespiratory and tissue adenosine responses to hypoxia and reoxygenation in the short-horned sculpin Myoxocephalus scorpius. J. Exp. Biol. 2004, 207, 4157–4164. [Google Scholar] [CrossRef][Green Version]
- Fraser, T.W.K.; Mayer, I.; Skjæraasen, J.E.; Hansen, T.; Fjelldal, P.G. The effect of triploidy on the efficacy and physiological response to anesthesia with MS 222 and isoeugenol in Atlantic salmon post-smolts. Aquac. Int. 2014, 22, 1347–1359. [Google Scholar] [CrossRef]
- Welker, T.; Lim, C.; Yildirim-Aksoy, M. Effect of buffered and unbuffered tricaine methanesulfonate (MS-222) at different concentrations on the stress responses of channel catfish, Ictalurus punctatus Rafinesque. J. Appl. Aquac. 2007, 19, 1–18. [Google Scholar] [CrossRef]
- Witeska, M.; Teodorczuk, B.; Lugowska, K. Hematological effects of etomidate and tricaine in common carp. Turkish J. Vet. Anim. Sci. 2017, 41, 93–98. [Google Scholar] [CrossRef]
- Fabbri, E.; Capuzzo, A.; Moon, T.W. The role of circulating catecholamines in the regulation of fish metabolism: An overview. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1998, 120, 177–192. [Google Scholar] [CrossRef]
- Pankhurst, N.W. The endocrinology of stress in fish: An environmental perspective. Gen. Comp. Endocrinol. 2010, 170, 265–275. [Google Scholar] [CrossRef]
- Roubach, R.; Gomes, L.C.; Val, A.L. Safest level of tricaine methanesulfonate (MS-222) to induce anesthesia in juveniles of Matrinxã, Brycon cephalus. Acta. Amaz. 2001, 31, 159–163. [Google Scholar] [CrossRef]
- Houston, A.H.; Madden, J.A.; Woods, R.J.; Miles, H.M. Some physiological effects of handling and tricaine methane-sulphonate anesthetization upon the brook trout, Salvelinus fontinalis. J. Fish. Res. Board. Can. 1971, 28, 625–633. [Google Scholar] [CrossRef]
- Parker-Graham, C.A.; Lima, K.M.; Soto, E. The effect of anesthetic time and concentration on blood gases, acid-base status, and electrolytes in koi (cyprinus carpio) anesthetized with buffered tricaine methanesulfonate (MS-222). J. Zoo. Wildl. Med. 2020, 51, 102–109. [Google Scholar] [CrossRef]
- Clarke, A.; Johnston, N.M. Scaling of metabolic rate with body mass and temperature in teleost fish. J. Anim. Ecol. 1999, 68, 893–905. [Google Scholar] [CrossRef]
- Rairat, T.; Hsieh, C.Y.; Thongpiam, W.; Sung, C.H.; Chou, C.C. Temperature-dependent pharmacokinetics of florfenicol in Nile tilapia (Oreochromis niloticus) following single oral and intravenous administration. Aquaculture 2019, 503, 483–488. [Google Scholar] [CrossRef]
- Hedaya, M.A. Basic Pharmacokinetics; Taylor & Francis Group: Boca Raton, FL, USA, 2012; pp. 13–22. [Google Scholar]
- Fan, J.; de Lannoy, I.A. Pharmacokinetics. Biochem. Pharmacol. 2014, 87, 93–120. [Google Scholar] [CrossRef]
- Hunn, J.B.; Allen, J.L. Movement of drugs across the gills of fishes. Annu. Rev. Pharmacol. 1974, 14, 47–54. [Google Scholar] [CrossRef]
- Hunn, J.B.; Schoettger, R.A.; Willford, W.A. Turnover and urinary excretion of free and acetylated MS 222 by rainbow trout, Salmo gairdneri. J. Fish. Res. Board. Can. 1968, 25, 25–31. [Google Scholar] [CrossRef]
- Stenger, V.G.; Maren, T.H. The pharmacology of MS 222 (ethyl-m-aminobenzoate) in Squalus acanthias. Comp. Gen. Pharmacol. 1974, 5, 23–35. [Google Scholar] [CrossRef] [PubMed]

| Temperature | Dose | Induction Time | Induction Time < 5 min (Fish Number) | Recovery Time | Recovery Time < 5 min (Fish Number) | Minimum Effective Concentration (MEC) | Grand Mean of MEC |
|---|---|---|---|---|---|---|---|
| (°C) | (µg/mL) | (min) | (min) | (µg/mL) | (µg/mL) | ||
| 22 | 100 | 16.41 ± 1.80 a | 0/7 | 2.68 ± 1.30 a | 7/7 | 58.20 ± 12.22 a | |
| 140 | 4.17 ± 0.45 b | 7/7 | 2.95 ± 1.04 a | 7/7 | 74.44 ± 24.61 a | 70.48 ± 21.56 | |
| 175 | 2.64 ± 0.60 c | 7/7 | 5.02 ± 1.02 b | 4/7 | 78.80 ± 22.80 a | ||
| 28 | 100 | 29.37 ± 3.81 a | 0/7 | 2.77 ± 1.43 a | 7/7 | 76.84 ± 8.57 a | |
| 150 | 3.25 ± 0.83 b | 7/7 | 2.80 ± 0.30 a | 7/7 | 76.03 ± 15.33 a | 78.27 ± 16.22 | |
| 200 | 1.32 ± 0.35 c | 7/7 | 3.92 ± 0.98 a | 6/7 | 81.96 ± 23.34 a |
| Parameters | Unit | Temperature | |||
|---|---|---|---|---|---|
| 22 °C | 28 °C | ||||
| Before | After * | Before | After * | ||
| Lactate | mmol/L | 0.50 ± 0.00 a | 1.36 ± 0.61 b | 0.52 ± 0.06 a | 2.29 ± 0.91 b |
| Glucose | mg/dL | 30.71 ± 3.25 a | 104.43 ± 35.43 b | 29.14 ± 1.86 a | 106.57 ± 55.78 b |
| BUN | mg/dL | 2.43 ± 1.13 a | 2.43 ± 1.13 a | 1.86 ± 0.90 a | 2.14 ± 1.07 a |
| Calcium | mg/dL | 9.64 ± 0.21 a | 10.24 ± 0.52 b | 9.77 ± 0.08 a | 10.64 ± 0.40 b |
| Magnesium | mg/dL | 2.76 ± 0.15 a | 3.16 ± 0.32 b | 2.63 ± 0.16 a | 3.10 ± 0.16 b |
| Sodium | mEq/L | 166.14 ± 2.61 a | 170.14 ± 3.18 b | 165.43 ± 3.26 a | 171.86 ± 3.44 b |
| Potassium | mEq/L | 3.57 ± 0.43 a | 2.93 ± 0.39 b | 4.31 ± 0.83 a | 4.06 ± 1.33 a |
| Chloride | mEq/L | 145.71 ± 1.80 a | 147.29 ± 2.50 a | 146.57 ± 1.72 a | 149.14 ± 2.48 b |
| pH | 7.69 ± 0.02 a | 7.62 ± 0.05 b | 7.57 ± 0.04 a | 7.48 ± 0.10 b | |
| HCO3− | mmol/L | 9.16 ± 0.79 a | 9.86 ± 0.85 a | 8.89 ± 1.04 a | 8.84 ± 1.90 a |
| pCO2 | mmHg | 7.14 ± 0.69 a | 9.14 ± 0.69 b | 9.57 ± 1.40 a | 11.29 ± 1.80 a |
| tCO2 | mmol/L | 9.60 ± 0.82 a | 10.40 ± 0.85 a | 9.30 ± 1.12 a | 9.37 ± 1.92 a |
| Anion gap | mEq/L | 22.95 ± 1.33 a | 23.38 ± 1.19 a | 22.86 ± 1.41 a | 24.91 ± 1.55 b |
| Parameters | Unit | Temperature | |
|---|---|---|---|
| 22 °C | 28 °C | ||
| λ | 1/min | 0.019 | 0.038 |
| t1/2 λ | min | 37.01 | 18.43 |
| AUC | min·µg/mL | 304.48 | 306.68 |
| MRT | min | 28.43 | 14.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, J.C.-N.; Rairat, T.; Lu, Y.-P.; Chou, C.-C. The Use of Tricaine Methanesulfonate (MS-222) in Asian Seabass (Lates calcarifer) at Different Temperatures: Study of Optimal Doses, Minimum Effective Concentration, Blood Biochemistry, Immersion Pharmacokinetics, and Tissue Distributions. Vet. Sci. 2023, 10, 539. https://doi.org/10.3390/vetsci10090539
Hsu JC-N, Rairat T, Lu Y-P, Chou C-C. The Use of Tricaine Methanesulfonate (MS-222) in Asian Seabass (Lates calcarifer) at Different Temperatures: Study of Optimal Doses, Minimum Effective Concentration, Blood Biochemistry, Immersion Pharmacokinetics, and Tissue Distributions. Veterinary Sciences. 2023; 10(9):539. https://doi.org/10.3390/vetsci10090539
Chicago/Turabian StyleHsu, Julia Chu-Ning, Tirawat Rairat, Yi-Ping Lu, and Chi-Chung Chou. 2023. "The Use of Tricaine Methanesulfonate (MS-222) in Asian Seabass (Lates calcarifer) at Different Temperatures: Study of Optimal Doses, Minimum Effective Concentration, Blood Biochemistry, Immersion Pharmacokinetics, and Tissue Distributions" Veterinary Sciences 10, no. 9: 539. https://doi.org/10.3390/vetsci10090539
APA StyleHsu, J. C.-N., Rairat, T., Lu, Y.-P., & Chou, C.-C. (2023). The Use of Tricaine Methanesulfonate (MS-222) in Asian Seabass (Lates calcarifer) at Different Temperatures: Study of Optimal Doses, Minimum Effective Concentration, Blood Biochemistry, Immersion Pharmacokinetics, and Tissue Distributions. Veterinary Sciences, 10(9), 539. https://doi.org/10.3390/vetsci10090539







