The Effect of Dietary Protein Concentration on the Fecal Microbiome and Serum Concentrations of Gut-Derived Uremic Toxins in Healthy Adult Cats
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. The Study Design and Cats
2.2. Foods
2.3. Serum Uremic Toxin Assay
2.4. Shallow Shotgun Metagenomic Sequencing
2.5. Statistical Analysis
3. Results
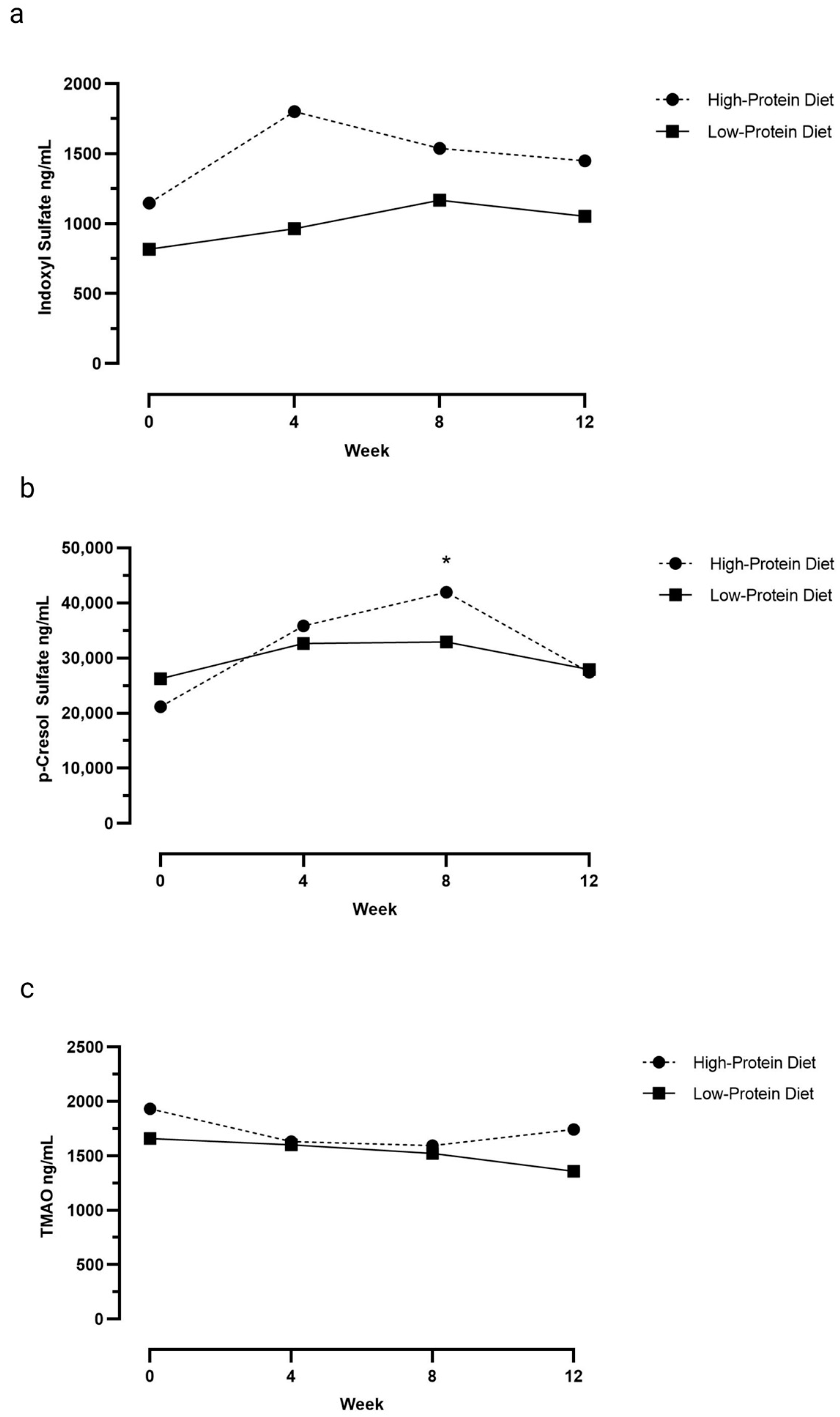
3.1. Serum Uremic Toxin Concentrations
3.2. Fecal Microbiome
3.2.1. Alpha Diversity Metrics
3.2.2. Beta Diversity Metric
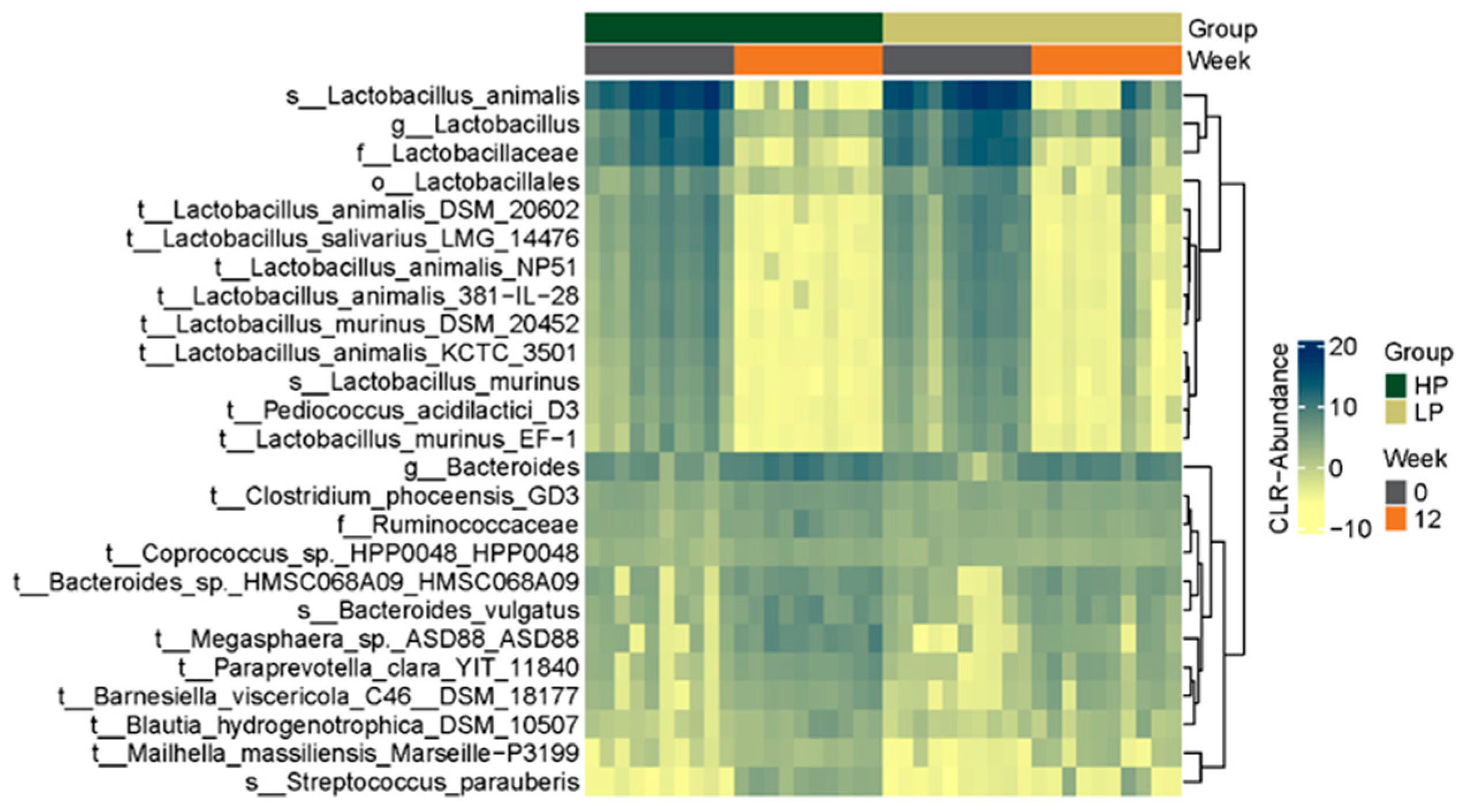
3.2.3. Microbial Composition
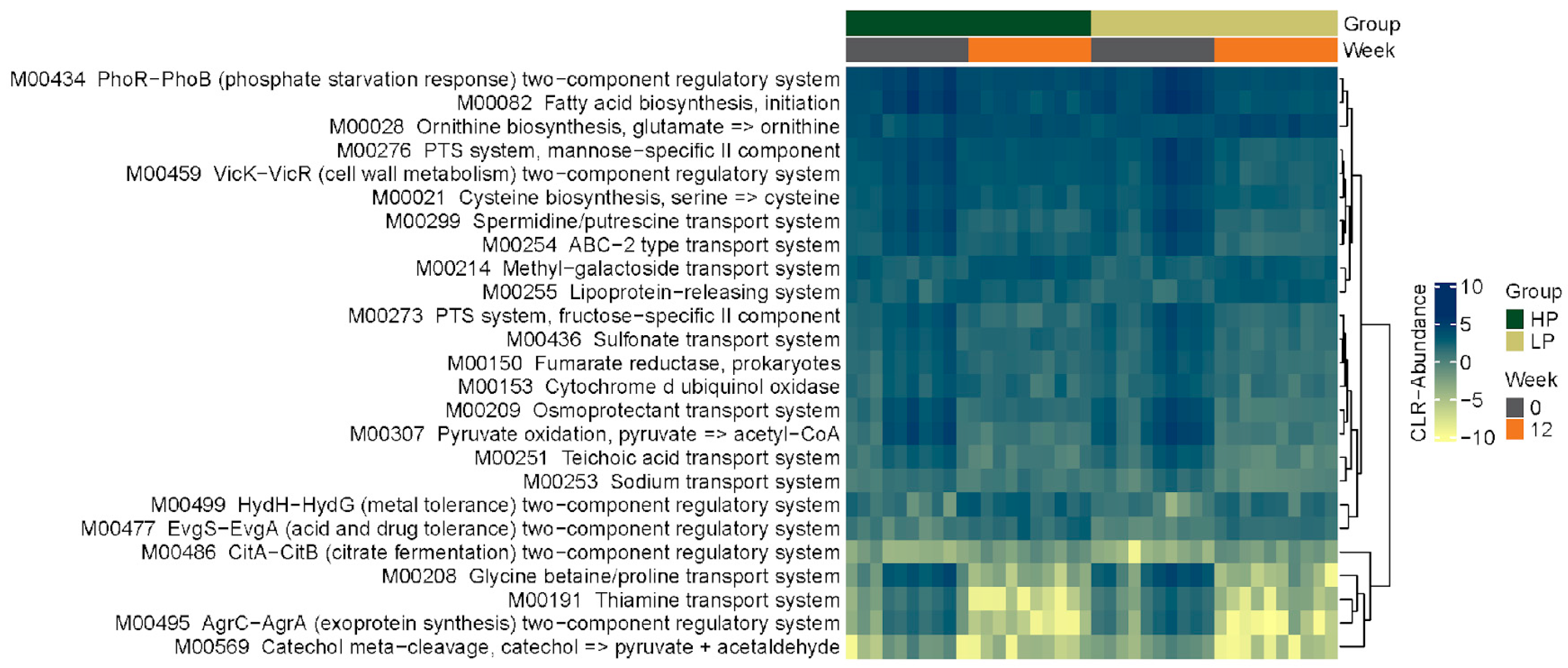
3.2.4. KEGG Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wernimont, S.M.; Radosevich, J.; Jackson, M.I.; Ephraim, E.; Badri, D.V.; MacLeay, J.M.; Jewell, D.E.; Suchodolski, J.S. The effects of nutrition on the gastrointestinal microbiome of cats and dogs: Impact on health and disease. Front. Microbiol. 2020, 11, 1266. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, X.; Liu, H.; Brown, M.A.; Qiao, S. Dietary protein and gut microbiota composition and function. Curr. Protein Pept. Sci. 2019, 20, 145–154. [Google Scholar] [CrossRef]
- Ephraim, E.; Cochrane, C.Y.; Jewell, D.E. Varying protein levels influence metabolomics and the gut microbiome in healthy adult dogs. Toxins 2020, 12, 517. [Google Scholar] [CrossRef]
- Badri, D.V.; Jackson, M.I.; Jewell, D.E. Dietary protein and carbohydrate levels affect the gut microbiota and clinical assessment in healthy adult cats. J. Nutr. 2021, 151, 3637–3650. [Google Scholar] [CrossRef] [PubMed]
- Lubbs, D.C.; Vester, B.M.; Fastinger, N.D.; Swanson, K.S. Dietary protein concentration affects intestinal microbiota of adult cats: A study using DGGE and qPCR to evaluate differences in microbial populations in the feline gastrointestinal tract. J. Anim. Physiol. Anim. Nutr. 2009, 93, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Summers, S.C.; Quimby, J.M.; Isaiah, A.; Suchodolski, J.S.; Lunghofer, P.J.; Gustafson, D.L. The fecal microbiome and serum concentrations of indoxyl sulfate and p-cresol sulfate in cats with chronic kidney disease. J. Vet. Intern. Med. 2018, 33, 662–669. [Google Scholar] [CrossRef]
- Cheng, F.P.; Hsieh, M.J.; Chou, C.C.; Hsu, W.L.; Lee, Y.J. Detection of indoxyl sulfate levels in dogs and cats suffering from naturally occurring kidney diseases. Vet. J. 2015, 205, 399–403. [Google Scholar] [CrossRef]
- Wong, J.; Piceno, Y.M.; Desantis, T.Z.; Pahl, M.; Andersen, G.L.; Vaziri, N.D. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am. J. Nephrol. 2014, 39, 230–237. [Google Scholar] [CrossRef]
- Gryp, T.; De Paepe, K.; Vanholder, R.; Kerckhof, F.M.; Van Biesen, W.; Van de Wiele, T.; Verbeke, F.; Speeckaert, M.; Joossens, M.; Coutenye, M.M.; et al. Gut microbiota generation of protein-bound uremic toxins and related metabolites is not altered at different stages of chronic kidney disease. Kidney Int. 2020, 97, 1230–1242. [Google Scholar] [CrossRef] [PubMed]
- Gryp, T.; Huys, G.R.B.; Joossens, M.; Van Biesen, W.; Glorieux, G.; Vaneechoutte, M. Isolation and quantification of uremic toxin precursor-generating gut bacteria in chronic kidney disease patients. Int. J. Mol. Sci. 2020, 21, 1986. [Google Scholar] [CrossRef]
- Patel, K.P.; Luo, F.J.G.; Plummer, N.S.; Hostetter, T.H.; Meyer, T.W. The production of p-cresol sulfate and indoxyl sulfate in vegetarians versus omnivores. Clin. J. Am. Soc. Nephrol. 2012, 7, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Marzocco, S.; Dal Piaz, F.; Di Micco, L.; Torraca, S.; Sirico, M.L.; Tartaglia, D.; Autore, G.; Di Iorio, B. Very low-protein diet reduces indoxyl sulfate levels in chronic kidney disease. Blood Purif. 2013, 35, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Black, A.P.; Anjos, J.S.; Cardozo, L.; Carmo, F.L.; Dolenga, C.J.; Nakao, L.S.; de Carvalho Ferreira, D.; Rosado, A.; Carraro Eduardo, J.C.; Mafra, D. Does Low-protein diet influence the uremic toxin serum levels from the gut microbiota in nondialysis chronic kidney disease patients? J. Ren. Nutr. 2018, 28, 208–214. [Google Scholar] [CrossRef]
- Rysz, J.; Franczyk, B.; Ciałkowska-Rysz, A.; Gluba-Brzózka, A. The effect of diet on the survival of patients with chronic kidney disease. Nutrients 2017, 9, 495. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.; Rawlings, J.M.; Markwell, P.J.; Barber, P.J. Survival of cats with naturally occurring chronic renal failure: Effect of dietary management. J. Small Anim. Pract 2000, 41, 235–242. [Google Scholar] [CrossRef]
- Ross, S.J.; Osborne, C.A.; Kirk, C.A.; Lowry, S.R.; Koehler, L.A.; Polzin, D.J. Clinical evaluation of dietary modification for treatment of spontaneous chronic kidney disease in cats. J. Am. Vet. Med. Assoc. 2006, 229, 949–957. [Google Scholar] [CrossRef]
- Conroy, M.; Brodbelt, D.C.; O’Neill, D.; Chang, Y.M.; Elliott, J. Chronic kidney disease in cats attending primary care practice in the UK: A VetCompass study. Vet. Rec. 2019, 184, 526. [Google Scholar] [CrossRef]
- Association of American Feed Control Officials. Model regulations for pet food and specialty pet food under the model bill. In 2019 Official Publication: Association of American Feed Control Officials; Association of American Feed Control Officials: Champaign, IL, USA, 2019. [Google Scholar]
- Ephraim, E.; Jewell, D.E. High protein consumption with controlled phosphorus level increases plasma concentrations of uremic toxins in cats with early chronic kidney disease. J. Food Sci. Nutr. 2021, 7, 1–8. [Google Scholar] [CrossRef]
- Summers, S.; Quimby, J.; Yao, L.; Hess, A.; Broeckling, C.; Lappin, M. Biological variation of major gut-derived uremic toxins in the serum of healthy adult cats. J. Vet. Intern. Med. 2021, 35, 902–911. [Google Scholar] [CrossRef]
- O’Leary, N.A.; Wright, M.W.; Brister, J.R.; Ciufo, S.; Haddad, D.; McVeigh, R.; Rajput, B.; Robbertse, B.; Smith-White, B.; Ako-Adjei, D.; et al. Reference sequence (RefSeq) database at NCBI: Current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 2016, 44, D733–D745. [Google Scholar] [CrossRef]
- Al-Ghalith, G.; Knights, D. BURST enables mathematically optimal short-read alignment for big data. BioRxiv 2020. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Schroeder, B.O.; Birchenough, G.M.H.; Ståhlman, M.; Arike, L.; Johansson, M.E.V.; Hansson, G.C.; Bäckhed, F. Bifidobacteria or fiber protects against diet-induced microbiota-mediated colonic mucus deterioration. Cell Host Microbe 2018, 23, 27–40.e7. [Google Scholar] [CrossRef]
- Zentek, J.; Marquart, B.; Pietrzak, T.; Ballèvre, O.; Rochat, F. Dietary effects on bifidobacteria and Clostridium perfringens in the canine intestinal tract. J. Anim. Physiol. Anim. Nutr. 2003, 87, 397–407. [Google Scholar] [CrossRef]
- Xu, J.; Verbrugghe, A.; Lourenço, M.; Cools, A.; Liu, D.J.X.; Van de Wiele, T.; Marzorati, M.; Eeckhaut, V.; Van Immerseel, F.; Vanhaecke, L.; et al. The response of canine faecal microbiota to increased dietary protein is influenced by body condition. BMC Vet. Res. 2017, 13, 374. [Google Scholar] [CrossRef]
- Pinna, C.; Vecchiato, C.G.; Bolduan, C.; Grandi, M.; Stefanelli, C.; Windisch, W.; Zaghini, G.; Biagi, G. Influence of dietary protein and fructooligosaccharides on fecal fermentative end-products, fecal bacterial populations and apparent total tract digestibility in dogs. BMC Vet. Res. 2018, 14, 106. [Google Scholar] [CrossRef]
- Wu, S.; Bhat, Z.F.; Gounder, R.S.; Mohamed Ahmed, I.A.; Al-Juhaimi, F.Y.; Ding, Y.; Bekhit, A.E.A. Effect of dietary protein and processing on gut microbiota—A systematic review. Nutrients 2022, 14, 453. [Google Scholar] [CrossRef]
- Lin, X.; Liang, W.; Li, L.; Xiong, Q.; He, S.; Zhao, J.; Guo, X.; Xiang, S.; Zhang, P.; Wang, H.; et al. The accumulation of gut microbiome-derived indoxyl sulfate and p-cresyl sulfate in patients with end-stage renal disease. J. Ren. Nutr. 2021, 32, 578–586. [Google Scholar] [CrossRef]
- Fernandes, A.L.F.; Borges, N.A.; Black, A.P.; Anjos, J.D.; Silva, G.S.D.; Nakao, L.S.; Mafra, D. Dietary intake of tyrosine and phenylalanine, and p-cresyl sulfate plasma levels in non-dialyzed patients with chronic kidney disease. Braz. J. Nephrol. 2020, 42, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Wang, Z.; Tang, W.H.W.; Hazen, S.L. Gut microbe-generated trimethylamine n-oxide from dietary choline is prothrombotic in subjects. Circulation 2017, 135, 1671–1673. [Google Scholar] [CrossRef] [PubMed]
- Thalacker-Mercer, A.E.; Campbell, W.W. Dietary protein intake affects albumin fractional synthesis rate in younger and older adults equally. Nutr. Rev. 2008, 66, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Coltherd, J.C.; Staunton, R.; Colyer, A.; Thomas, G.; Gilham, M.; Logan, D.W.; Butterwick, R.; Watson, P. Not all forms of dietary phosphorus are equal: An evaluation of postprandial phosphorus concentrations in the plasma of the cat. Br. J. Nutr. 2019, 121, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Coltherd, J.C.; Staunton, R.; Colyer, A.; Gilham, M.; Rawlings, J.; Alexander, J.E.; Logan, D.W.; Butterwick, R.; Watson, P.; Bakke, A.M. Dietary calcium to phosphorus ratio affects postprandial phosphorus concentrations in feline plasma. Br. J. Nutr. 2022, 128, 1689–1699. [Google Scholar] [CrossRef]
- Akimbekov, N.S.; Digel, I.; Sherelkhan, D.K.; Razzaque, M.S. Vitamin D and phosphate interactions in health and disease. Adv. Exp. Med. Biol. 2022, 1362, 37–46. [Google Scholar] [CrossRef]

| Nutrient | Pre-Trial Diet 1 | Low-Protein Diet 2 | High-Protein Diet 3 | |
|---|---|---|---|---|
| Caloric Density, Macronutrient, and Mineral Content | ||||
| Caloric density DM | kcal/100 gr | 389 | 415 | 413 |
| Protein | g/100 kcal | 8.7 | 7.4 | 11.0 |
| Fat | g/100 kcal | 3.6 | 4.6 | 4.4 |
| Carbohydrate | g/100 kcal | 11.1 | 10.1 | 6.8 |
| Soluble fiber | g/100 kcal | 0.4 | 0.4 | 0.3 |
| Insoluble fiber | g/100 kcal | 2.4 | 2.2 | 2.4 |
| Total fiber | g/100 kcal | 2.8 | 2.7 | 2.7 |
| Calcium | mg/100 kcal | 348.5 | 185.2 | 179.5 |
| Phosphorus | mg/100 kcal | 313.2 | 106.8 | 117.0 |
| Potassium | mg/100 kcal | 209.5 | 340.8 | 378.6 |
| Sodium | mg/100 kcal | 117.6 | 74.2 | 72.2 |
| Magnesium | mg/100 kcal | 36.6 | 23.8 | 23.4 |
| Amino Acid Concentration | ||||
| Alanine | g/100 kcal | 0.56 | 0.43 | 0.72 |
| Arginine | g/100 kcal | 0.49 | 0.37 | 0.49 |
| Aspartic acid | g/100 kcal | 0.70 | 0.63 | 0.86 |
| Cysteine | g/100 kcal | 0.13 | 0.11 | 0.17 |
| Glutamic acid | g/100 kcal | 1.59 | 1.21 | 2.04 |
| Glycine | g/100 kcal | 0.50 | 0.30 | 0.40 |
| Histidine | g/100 kcal | 0.20 | 0.18 | 0.26 |
| Isoleucine | g/100 kcal | 0.33 | 0.30 | 0.45 |
| Leucine | g/100 kcal | 0.81 | 0.68 | 1.23 |
| Lysine | g/100 kcal | 0.43 | 0.44 | 0.59 |
| Methionine | g/100 kcal | 0.17 | 0.17 | 0.26 |
| Phenylalanine | g/100 kcal | 0.42 | 0.37 | 0.60 |
| Proline | g/100 kcal | 0.57 | 0.41 | 0.73 |
| Serine | g/100 kcal | 0.39 | 0.34 | 0.52 |
| Threonine | g/100 kcal | 0.31 | 0.29 | 0.41 |
| Tyrosine | g/100 kcal | 0.30 | 0.27 | 0.47 |
| Tryptophan | g/100 kcal | 0.06 | 0.07 | 0.08 |
| Valine | g/100 kcal | 0.43 | 0.39 | 0.57 |
| Low-Protein Diet (n = 10) | High-Protein Diet (n = 10) | ||||
|---|---|---|---|---|---|
| Reference Interval | Week 0 | Week 12 | Week 0 | Week 12 | |
| Body weight (kg) | 5.0 (2.9–6.7) | 4.9 (3.0–6.6) | 4.5 (3.0–6.1) | 4.4 (2.8–6.0) | |
| BCS | 0–9 | 6 (5–8) | 6 (5–8) | 5.5 (5–6) | 6 (5–7) |
| MCS | 0–3 | 0.5 (0–1) | 1 (0–1) | 0 (0–1) | 0 (0–1) |
| Fecal score | 1–7 | 2.5 (2–3) | 2 (2–4) | 3 (2–4) | 2 (2–3) |
| Blood urea nitrogen | 18–35 mg/dL | 25 (21–31) | 26 (20–28) | 25 (21–34) | 24 (18–32) |
| Creatinine | 0.8–2.4 mg/dL | 1.4 (1.0–1.7) | 1.4 (1.1–2.0) | 1.4 (1.0–1.7) | 1.4 (1.0–1.8) |
| Albumin | 3.1–4.4 g/dL | 3.5 (3.2–3.7) a | 3.8 (3.2–4.0) b | 3.3 (3.2–4.2) a | 3.7 (3.2–4.7) b |
| Phosphorus | 3.0–6.0 mg/dL | 4.1 (3.5–5.2) | 5.2 (3.0–6.1) | 3.9 (3.3–4.4) a | 5.1 (4.3–6.3) b |
| Calcium | 9.2–11.1 mg/dL | 9.7 (9.1–10.1) | 9.7 (8.6–10.9) | 9.6 (9.3–10.0) | 9.7 (9.4–11.0) |
| Urine specific gravity | >1.035 | 1.056 (1.032–1.066) | 1.053 (1.044–1.057) | 1.055 (1.050–1.066) | 1.052 (1.044–1.068) |
| Low-Protein Diet (n = 10) | High-Protein Diet (n = 10) | |
|---|---|---|
| p-Cresol Sulfate | ||
| Week 0 | 26,288 (7391–51,217) | 21,184 (13,171–66,635) * |
| Week 4 | 32,647 (16,506–57,342) | 35,851 (9944–86,416) |
| Week 8 | 32,944 (14,727–52,337) | 41,969 (14,438–83,490) * |
| Week 12 | 27,942 (14,419–46,022) | 27,449 (8158–49,244) |
| Indoxyl Sulfate | ||
| Week 0 | 817 (428–2945) | 1147 (736–4755) |
| Week 4 | 964 (500–2216) | 1802 (942–3079) |
| Week 8 | 1167 (688–1710) | 1537 (1201–2982) |
| Week 12 | 1053 (702–2336) | 1449 (796–2429) |
| Trimethylamine-n-oxide | ||
| Week 0 | 1660 (932–2703) | 1932 (1326–3867) |
| Week 4 | 1602 (862–3155) | 1631 (1086–2665) |
| Week 8 | 1523 (1035–2761) | 1593 (1338–2531) |
| Week 12 | 1357 (915–1614) | 1741 (684–2322) |
| Low-Protein Diet (n = 10) | High-Protein Diet (n = 10) | |||
|---|---|---|---|---|
| Week 0 | Week 12 | Week 0 | Week 12 | |
| Taxonomic Level | ||||
| Observed OTU | 633 (482–735) | 618 (486–723) | 639 (385–720) | 662 (385–710) |
| Chao1 | 702 (524–790) | 664 (562–796) | 719 (461–774) | 695 (621–732) |
| Shannon | 2.9 (2.2–3.8) | 3.3 (2.7–3.5) | 3.2 (2.6–3.7) a | 3.5 (3.3–4.1) b |
| KEGG Functional Level | ||||
| Observed OTUs | 1431 (1217–1644) | 1440 (1310–1569) | 1452 (1116–1548) a | 1532 (1470–1569) b |
| Chao1 | 1544 (1311–1780) | 1526 (1397–1646) | 1537 (1346–1657) | 1605 (1470–1653) |
| Shannon | 6.5 (6.2–6.6) | 6.5 (6.4–6.6) | 6.5 (6.3–6.6) a | 6.6 (6.5–6.7) b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Summers, S.; Quimby, J.; Gagné, J.; Lappin, M. The Effect of Dietary Protein Concentration on the Fecal Microbiome and Serum Concentrations of Gut-Derived Uremic Toxins in Healthy Adult Cats. Vet. Sci. 2023, 10, 497. https://doi.org/10.3390/vetsci10080497
Summers S, Quimby J, Gagné J, Lappin M. The Effect of Dietary Protein Concentration on the Fecal Microbiome and Serum Concentrations of Gut-Derived Uremic Toxins in Healthy Adult Cats. Veterinary Sciences. 2023; 10(8):497. https://doi.org/10.3390/vetsci10080497
Chicago/Turabian StyleSummers, Stacie, Jessica Quimby, Jason Gagné, and Michael Lappin. 2023. "The Effect of Dietary Protein Concentration on the Fecal Microbiome and Serum Concentrations of Gut-Derived Uremic Toxins in Healthy Adult Cats" Veterinary Sciences 10, no. 8: 497. https://doi.org/10.3390/vetsci10080497
APA StyleSummers, S., Quimby, J., Gagné, J., & Lappin, M. (2023). The Effect of Dietary Protein Concentration on the Fecal Microbiome and Serum Concentrations of Gut-Derived Uremic Toxins in Healthy Adult Cats. Veterinary Sciences, 10(8), 497. https://doi.org/10.3390/vetsci10080497





