Intranasal and Serum Gentamicin Concentration: Comparison of Three Topical Administration Protocols in Dogs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Dog Population
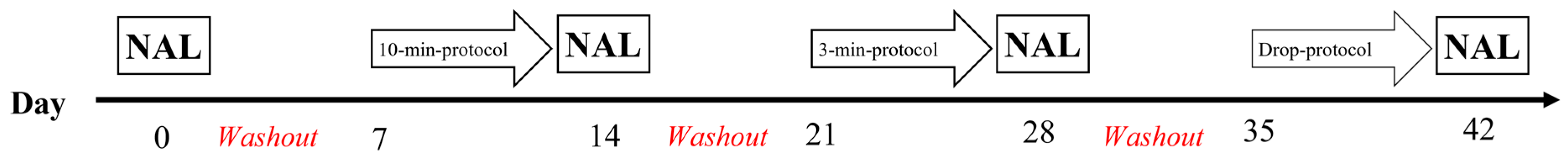
2.2. Protocol
2.3. Sample Collection
2.4. Sample Processing
2.5. Gentamicin Concentration
2.6. Statistical Analysis
3. Results
3.1. Nasal Lavage and Serum Sampling
3.2. Total Cell Counts and Differential Cell Counts in Nasal Lavage Fluid
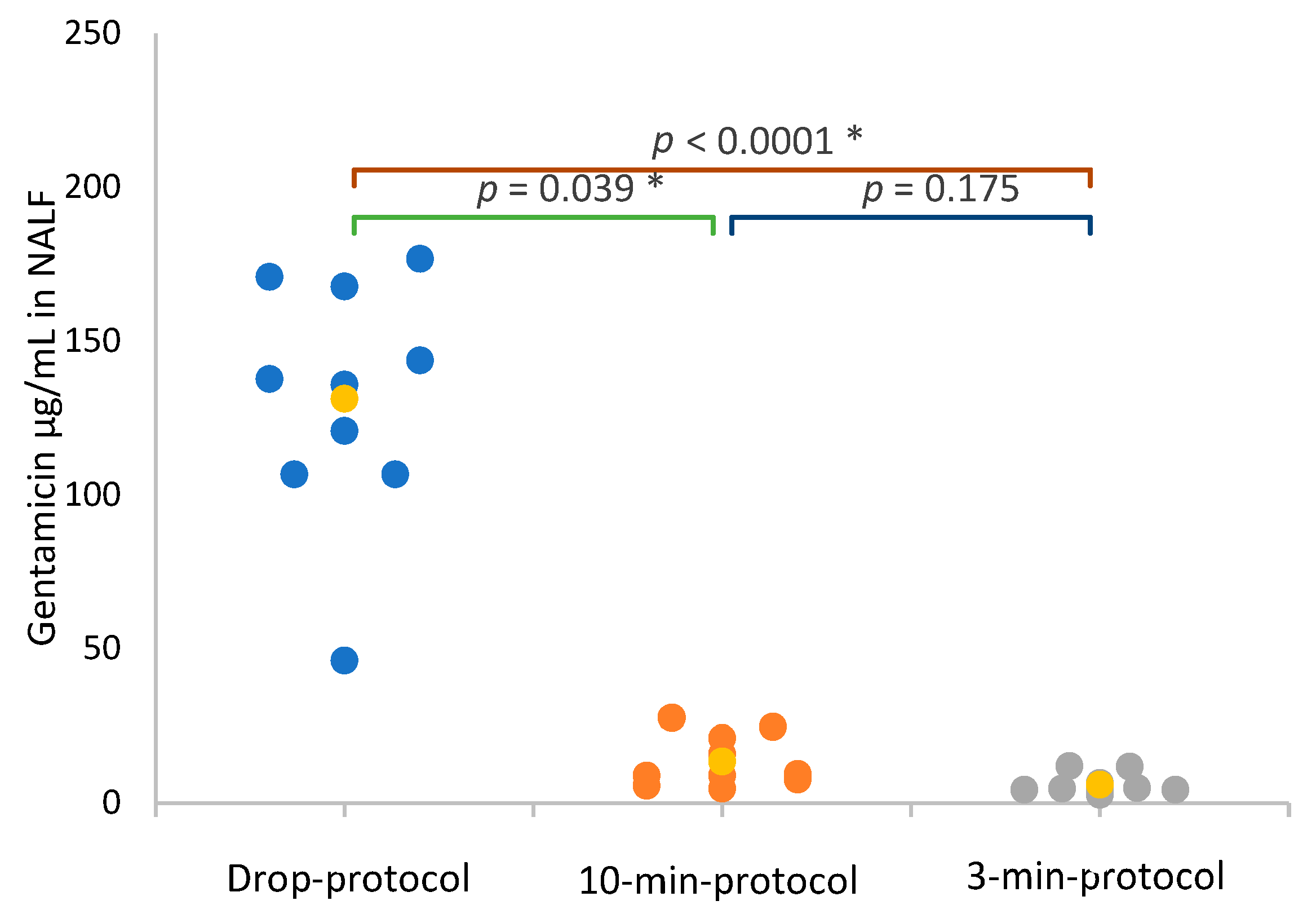
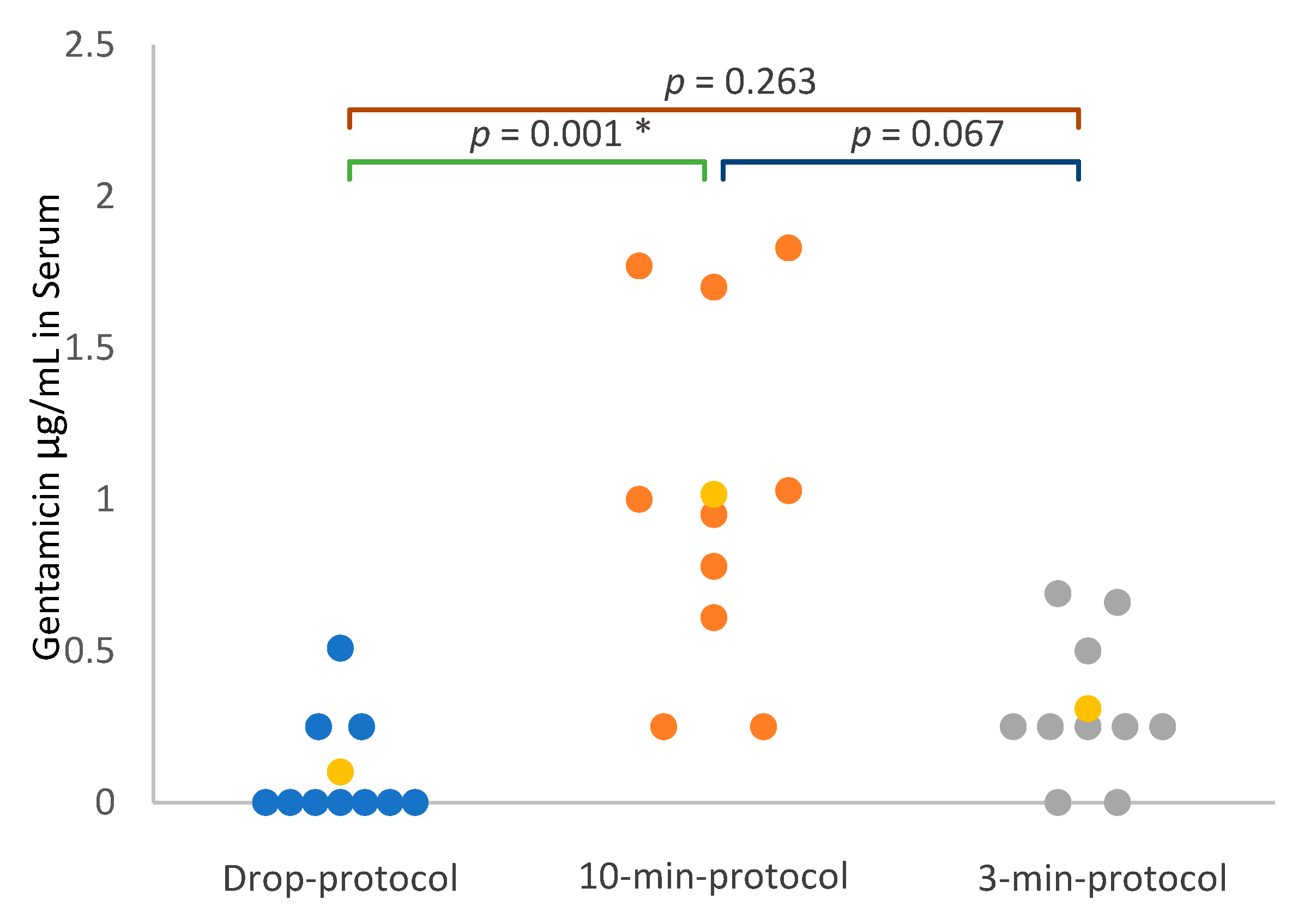
3.3. Gentamicin Concentration in Nasal Lavage Fluid and Serum
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CMAX | Maximum concentration |
| Drop protocol | Administration of 0.25 mL of gentamicin 5% in each nostril, twice daily, for 1 week |
| ELF | Epithelial lining fluid |
| LOQ | Lower limit of quantification |
| MIC | Minimum inhibitory concentration |
| NAL | Nasal lavage |
| NALF | Nasal lavage fluid |
| 3-min protocol | Inhalation of gentamicin 5% for 3 min, twice daily, for 1 week |
| 10-min protocol | Inhalation of gentamicin 5% for 10 min, twice daily, for 1 week |
References
- Tasker, S.; Knottenbelt, C.M.; Munro, E.A.C.; Stonehewer, J.; Simpson, J.W.; Mackin, A.J. Aetiology and diagnosis of persistent nasal disease in the dog: A retrospective study of 42 cases. J. Small Anim. Pract. 1999, 40, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Meler, E.; Dunn, M.; Lecuyer, M. A retrospective study of canine persistent nasal disease: 80 cases (1998–2003). Can. Vet. J. 2008, 49, 71–76. [Google Scholar]
- Lobetti, R.G. A retrospective study of chronic nasal disease in 75 dogs. J. S. Afr. Vet. Assoc. 2009, 80, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Peeters, D.; Clercx, C. Update on canine sinonasal aspergillosis. Vet. Clin. N. Am. Small Anim. Pract. 2007, 37, 901–916. [Google Scholar] [CrossRef]
- Eloy, J.A.; Govindaraj, S. Microbiology and Immunology of Rhinosinusitis. In Rhinosinusitis; Thaler, E., Kennedy, D., Eds.; Springer: New York, NY, USA, 2008; pp. 1–12. [Google Scholar]
- Chiu, A.G. Osteitis in chronic rhinosinusitis. Otolaryngol. Clin. N. Am. 2005, 38, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.T.; Kennedy, D.W.; Palmer, J.N.; Feldman, M.; Chiu, A.G. The incidence of concurrent osteitis in patients with chronic rhinosinusitis: A clinicopathological study. Am. J. Rhinol. 2006, 20, 278–282. [Google Scholar] [CrossRef]
- Kisiel, M.; Sjölander, I.; Klar, A.; Asplund Stenkvist, M.; Laurell, G. Development of bacterial resistance during treatment with topical gentamicin for chronic rhinosinusitis in patients with cystic fibrosis and primary ciliary dyskinesis. Retrospective case series. Otolaryngol. Pol. 2020, 74, 33–40. [Google Scholar] [CrossRef]
- Nicolau, D.P.; Freeman, C.D.; Belliveau, P.P.; Nightingale, C.H.; Ross, J.W.; Quintiliani, R. Experience with a once daily aminoglycoside program administered to 2184 adult patients. Antimicrob. Agents Chemother. 1995, 39, 650–655. [Google Scholar] [CrossRef]
- Whatley, W.S.; Chandra, R.K.; MacDonald, C.B. Systemic absorption of gentamicin nasal irrigations. Am. J. Rhinol. 2006, 20, 251–254. [Google Scholar] [CrossRef]
- Belfield, K.; Bayston, R.; Birchall, J.P.; Daniel, M. Do orally administered antibiotics reach concentrations in the middle ear sufficient to eradicate planktonic and biofilm bacteria? A review. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 296–300. [Google Scholar] [CrossRef]
- Ebert, S.C.; Craig, W.A. Pharmacodynamic properties of antibiotics: Application to drug monitoring and dosage regimen design. Infect. Control Hosp. Epidemiol. 1990, 11, 319–326. [Google Scholar] [CrossRef]
- Moore, R.D.; Lietman, P.S.; Smith, C.R. Clinical response to aminoglycoside therapy: Importance of the ratio of peak concentration to minimal inhibitory concentration. J. Infect. Dis. 1987, 155, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Boisson, M.; Mimoz, O.; Hadzic, M.; Marchand, S.; Adier, C.; Couet, W.; Grégoire, N. Pharmacokinetics of intravenous and nebulized gentamicin in critically ill patients. J. Antimicrob. Chemother. 2018, 73, 2830–2837. [Google Scholar] [CrossRef] [PubMed]
- Begg, E.J.; Peddie, B.A.; Chambers, S.T.; Boswell, D.R. Comparison of gentamicin dosing regimens using an in-vitro model. J. Antimicrob. Chemother. 1992, 29, 427–433. [Google Scholar] [CrossRef]
- Skopnik, H.; Wallraf, R.; Nies, B.; Tröster, K.; Heimann, G. Pharmacokinetics and antibacterial activity of daily gentamicin. Arch. Dis. Child. 1992, 67, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Destache, C.J. Aminoglycoside-induced nephrotoxicity—A focus on monitoring: A review of literature. J. Pharm. Pract. 2014, 27, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.L.; Sykes, K.J.; Johnson, P.; He, J.; Mayo, M.S. Safety and efficacy of once-daily nasal irrigation for the treatment of pediatric chronic rhinosinusitis. Laryngoscope 2011, 121, 1989–2000. [Google Scholar] [CrossRef] [PubMed]
- Bexfield, N.H.; Foale, R.D.; Davison, L.J.; Watson, P.J.; Skelly, B.J.; Herrtage, M.E. Management of 13 cases of canine respiratory disease using inhaled corticosteroids. J. Small Anim. Pract. 2006, 47, 377–382. [Google Scholar] [CrossRef]
- Morgane Canonne, A.; Roels, E.; Menard, M.; Desquilbet, L.; Billen, F.; Clercx, C. Clinical response to 2 protocols of aerosolized gentamicin in 46 dogs with Bordetella bronchiseptica infection (2012–2018). J. Vet. Intern. Med. 2020, 34, 2078–2085. [Google Scholar] [CrossRef]
- Erdő, F.; Bors, L.A.; Farkas, D.; Bajza, Á.; Gizurarson, S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res. Bull. 2018, 143, 155–170. [Google Scholar] [CrossRef]
- López-Ramis, V.; Canfrán, S.; Gómez de Segura, I.A. Comparison of the sedative effects of intranasal or intramuscular dexmedetomidine at low doses in healthy dogs: A randomized clinical trial. Vet. Anaesth. Analg. 2022, 49, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.A.; Gow, S.P.; Lee, L.B.; Lacoste, S.; Ball, E.C. Comparative efficacy of intranasal and injectable vaccines in stimulating Bordetella bronchiseptica-reactive anamnestic antibody responses in household dogs. Can. Vet. J. 2017, 58, 809–815. [Google Scholar]
- Granstrand, P.; Nylander-French, L.; Holmstrom, M. Biomarkers of nasal inflammation in wood-surface coating industry workers. Am. J. Ind. Med. 1998, 33, 392–399. [Google Scholar] [CrossRef]
- Howarth, P.H.; Persson, C.G.; Meltzer, E.O.; Jacobson, M.R.; Durham, S.R.; Silkoff, P.E. Objective monitoring of nasal airway inflammation in rhinitis. J. Allergy Clin. Immunol. 2005, 115, S414–S441. [Google Scholar] [CrossRef]
- Larsson, B.M.; Palmberg, L.; Malmberg, P.O.; Larsson, K. Effect of exposure to swine dust on levels of IL-8 in airway lavage fluid. Thorax 1997, 52, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Castano, R.; Thériault, G.; Maghni, K.; Ghezzo, H.; Malo, J.L.; Gautrin, D. Reproducibility of nasal lavage in the context of the inhalation challenge investigation of occupational rhinitis. Am. J. Rhinol. 2008, 22, 271–275. [Google Scholar] [CrossRef]
- Dubois, N.; Sqalli, G.; Gilson, M.; Charlier, C. Analytical validation of a quantitative method for therapeutic drug monitoring on the Alinity®c Abbott. Ann. Biol. Clin. 2020, 78, 147–155. [Google Scholar] [CrossRef]
- Albarellos, G.; Montoya, L.; Ambros, L. Multiple once-daily dose pharmacokinetics and renal safety of gentamicin in dogs. J. Vet Pharmacol. Ther. 2004, 27, 21–25. [Google Scholar] [CrossRef]
- Rubinstein, A. Intranasal administration of gentamicin in human subjects. Antimicrob. Agents Chemother. 1983, 23, 778–779. [Google Scholar] [CrossRef]
- Koltai, P.J.; Maisel, B.O.; Goldstein, J.C. Pseudomonas aeruginosa in chronic maxillary sinusitis. Laryngoscope 1985, 95, 34–37. [Google Scholar] [CrossRef]
- Kovalhuk, L.C.S.; Telles, E.Q.; Lima, M.N.; Rosario Filho, N.A. Nasal lavage cytology and mucosal histopathological alterations in patients with rhinitis. Braz. J. Otorhinolaryngol. 2020, 86, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Scherzad, A.; Hagen, R.; Hackenberg, S. Current Understanding of Nasal Epithelial Cell Mis-Differentiation. J. Inflamm. Res. 2019, 12, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Baughman, R.P. Technical aspects of bronchoalveolar lavage: Recommendations for a standard procedure. Semin. Respir. Crit. Care Med. 2007, 28, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Rennard, S.I.; Basset, G.; Lecossier, D.; O’Donnell, K.M.; Pinkston, P.; Martin, P.G.; Crystal, R.G. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J. Appl. Physiol. 1986, 60, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Melamies, M.A.; Järvinen, A.K.; Seppälä, K.M.; Rita, H.J.; Rajamäki, M.M. Comparison of results for weight-adjusted and fixed-amount bronchoalveolar lavage techniques in healthy Beagles. Am. J. Vet. Res. 2011, 72, 694–698. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biénès, T.; Lyssens, A.; Machiels, H.; Hercot, M.E.; Fastres, A.; Alexandru-Cosmin, T.; Deville, M.; Charlier, C.; Billen, F.; Clercx, C. Intranasal and Serum Gentamicin Concentration: Comparison of Three Topical Administration Protocols in Dogs. Vet. Sci. 2023, 10, 490. https://doi.org/10.3390/vetsci10080490
Biénès T, Lyssens A, Machiels H, Hercot ME, Fastres A, Alexandru-Cosmin T, Deville M, Charlier C, Billen F, Clercx C. Intranasal and Serum Gentamicin Concentration: Comparison of Three Topical Administration Protocols in Dogs. Veterinary Sciences. 2023; 10(8):490. https://doi.org/10.3390/vetsci10080490
Chicago/Turabian StyleBiénès, Tom, Aurélie Lyssens, Hélène Machiels, Marie Eve Hercot, Aline Fastres, Tutunaru Alexandru-Cosmin, Marine Deville, Corinne Charlier, Frédéric Billen, and Cécile Clercx. 2023. "Intranasal and Serum Gentamicin Concentration: Comparison of Three Topical Administration Protocols in Dogs" Veterinary Sciences 10, no. 8: 490. https://doi.org/10.3390/vetsci10080490
APA StyleBiénès, T., Lyssens, A., Machiels, H., Hercot, M. E., Fastres, A., Alexandru-Cosmin, T., Deville, M., Charlier, C., Billen, F., & Clercx, C. (2023). Intranasal and Serum Gentamicin Concentration: Comparison of Three Topical Administration Protocols in Dogs. Veterinary Sciences, 10(8), 490. https://doi.org/10.3390/vetsci10080490





