Thermographic Image of the Hoof Print in Leisure and Cross-Country Warmblood Horses: A Pilot Study
Abstract
Simple Summary
Abstract
1. Introduction
- to investigate the hoof print of non-lame horses;
- to calculate and propose a temperature reference value for six areas from the hoofprint surface;
- to check for any differences in temperature between the horses used for leisure and those trained for cross-country;
- to compare the results obtained in different locations.
2. Materials and Methods
2.1. Animal Selection
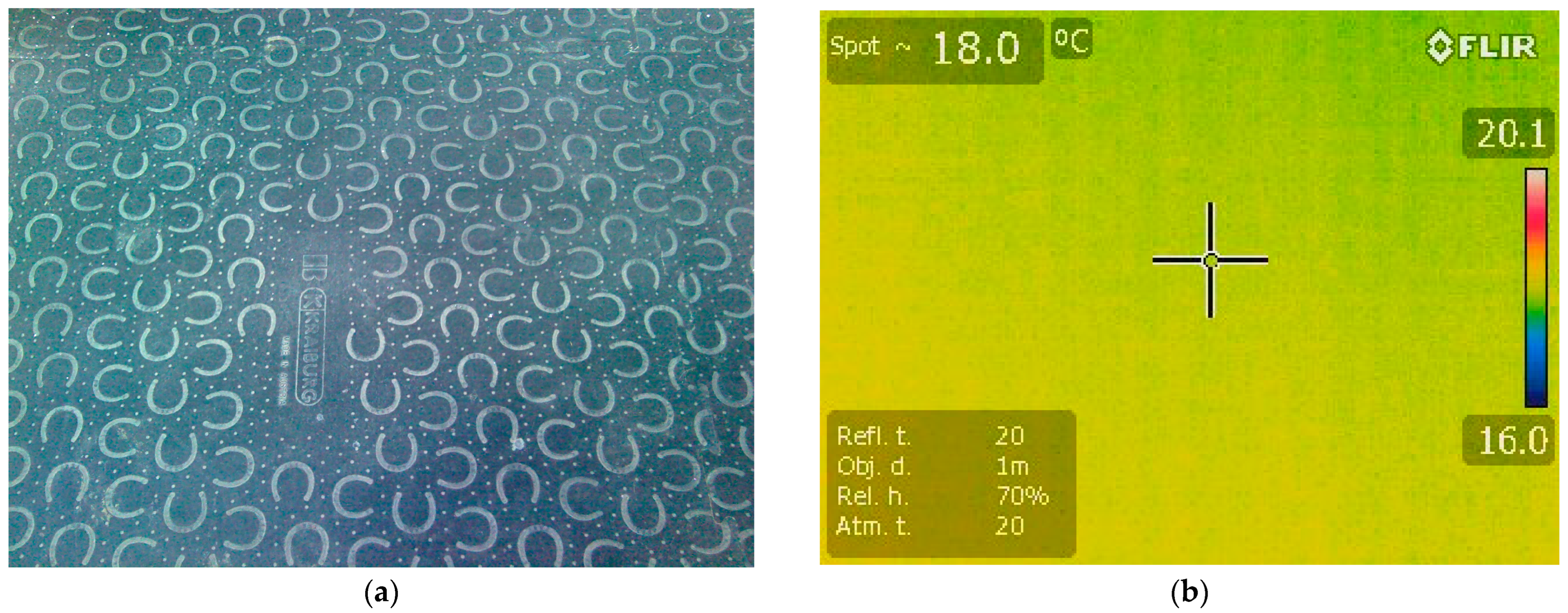
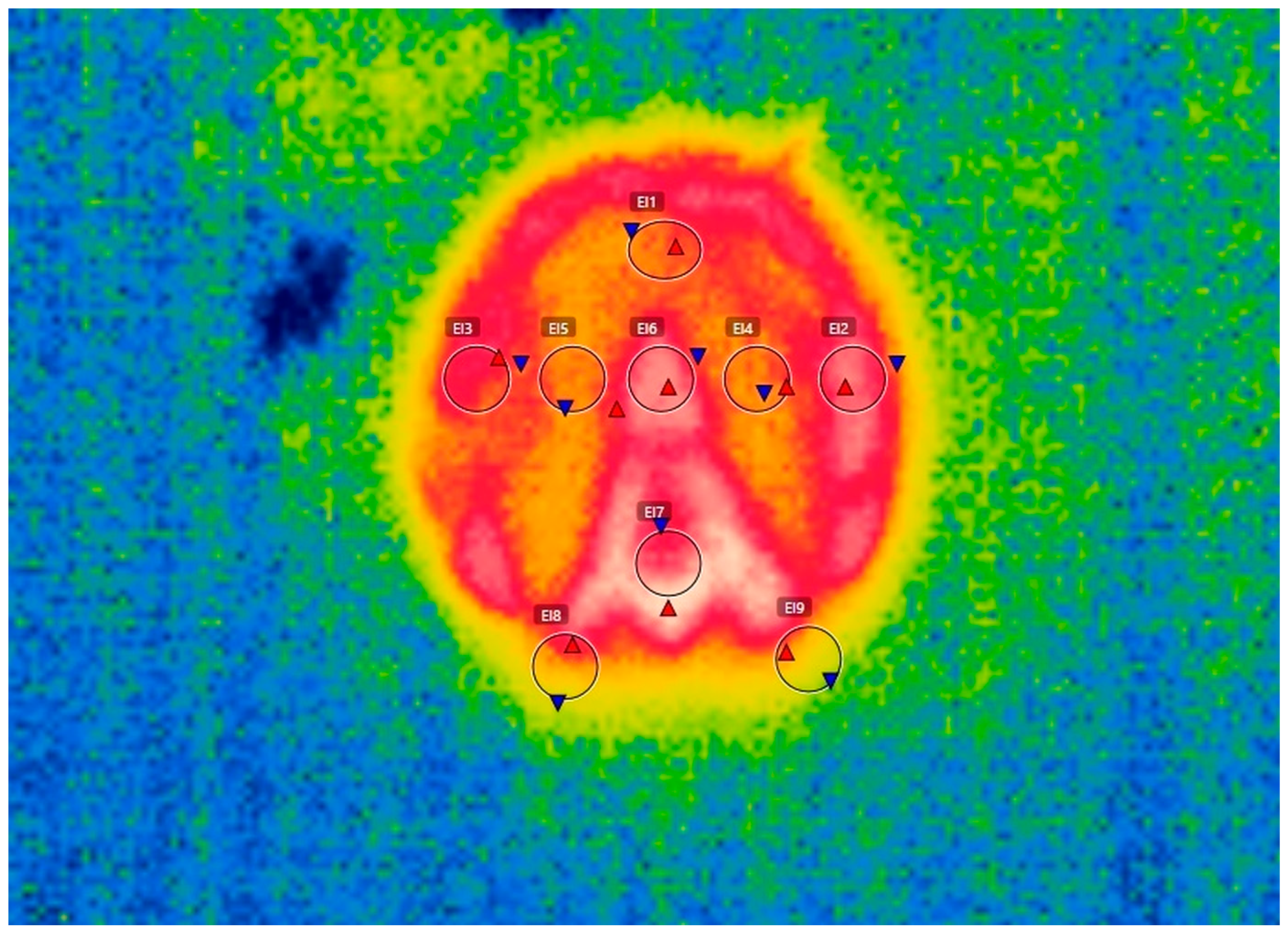
2.2. Thermal Imaging and Data Recording
2.3. Statistical Analysis
3. Results
3.1. Thermographic Scan of the Hoofprints
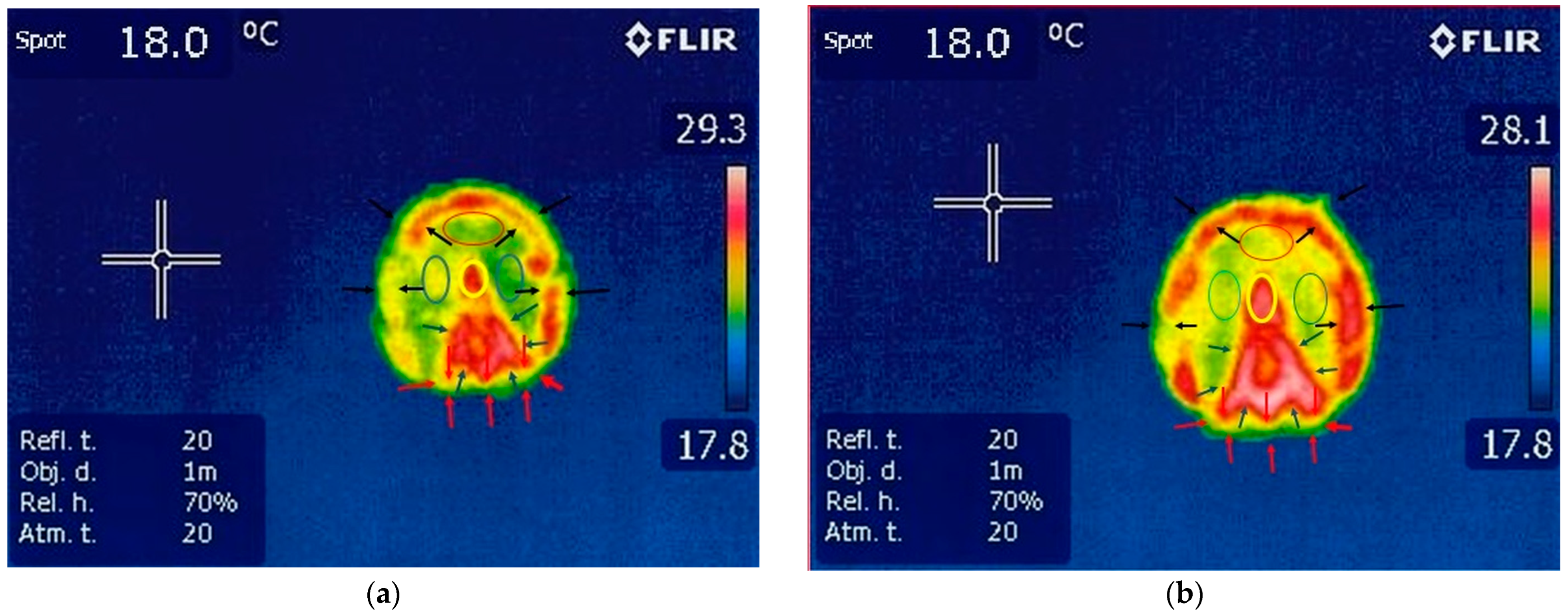
3.1.1. Thermography of the Forelimbs
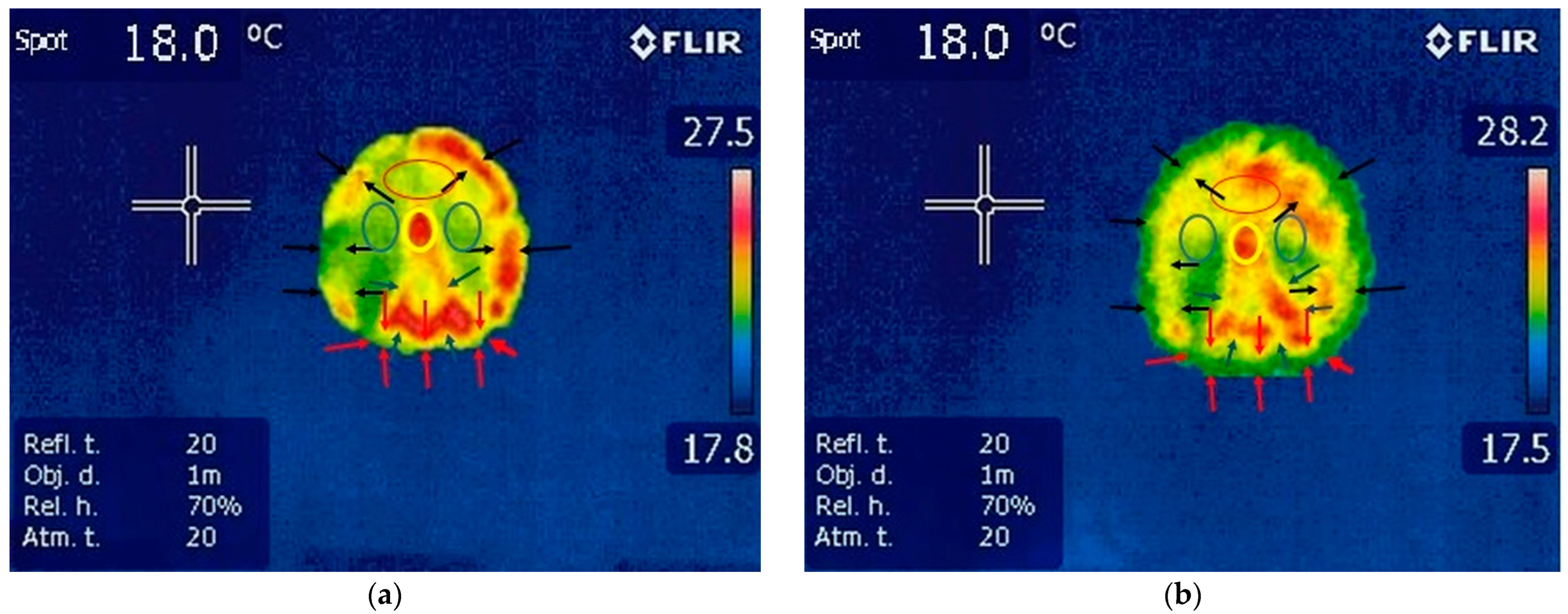
3.1.2. Thermography of the Hindlimbs
3.2. Statistical Analysis—Group Comparisons
3.2.1. Group Comparison Warmblood Horses
3.2.2. Group Comparisons
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gilbert, M.; Gillett, J. Equine athletes and interspecies sport. Int. Rev. Sociol. Sport 2012, 47, 632–643. [Google Scholar] [CrossRef]
- Stachurska, A.; Pieta, M.; Ussing, A.P.; Kapron, A.; Kwiecinska, N. Difficulty of cross-country obstacles for horses competing in Three Day Events. Appl. Anim. Behav. Sci. 2010, 123, 101–107. [Google Scholar] [CrossRef]
- Dyson, S. Lameness and poor performance in the sport horse: Dressage, show jumping and horse trials. J. Equine Vet. Sci. 2001, 22, 145–150. [Google Scholar] [CrossRef]
- Wallsten, H.; Olsson, K.; Dahlborn, K. Temperature regulation in horses during exercise and recovery in a cool environment. Acta Vet. Scand. 2012, 54, 42. [Google Scholar] [CrossRef] [PubMed]
- Soroko, M.; Howell, K. Infrared thermography: Current applications in Equine Medicine. J. Equine Vet. Sci. 2018, 60, 90–96. [Google Scholar] [CrossRef]
- Loughin, C.A.; Marino, D.J. Evaluation of thermographic imaging of the limbs of healthy dogs. Am. J. Vet. Res. 2007, 68, 1064–1069. [Google Scholar] [CrossRef]
- Igna, C.; Mavromatis, S.; Sicoe, B.; Schuszler, L. Assessment of the thermal paw print symmetry of the hind legs in healthy dogs. In Proceedings of the Agriculture for Life, Life for Agriculture, Bucharest, Romania, 7–9 June 2018. [Google Scholar]
- Garcia, E.F.V.; Loughin, C.A.; Marino, D.J.; Sackman, J.; Umbaugh, S.E.; Fu, J.; Subedi, S.; Lesser, M.L.; Akerman, M.; Schossler, J.E.W. Medical infrared imaging and orthostatic analysis to determine lameness in the pelvic limbs of dogs. Open Vet. J. 2017, 7, 342–348. [Google Scholar] [CrossRef]
- Baxter, G.M.; Stashak, T.S.; Keegan, K.G. Examination for lameness. In Adams and Stashak’s Lameness in Horses, 6th ed.; Wiley-Blackwell Publishing Ltd.: Chichester, UK, 2011; pp. 67–185. [Google Scholar] [CrossRef]
- Landman, M.A.A.M.; de Blaauw, J.A.; Hofland, L.J.; van Weenren, P.R. Field study of the prevalence of lameness in horses with back problems. Vet. Rec. 2004, 155, 165–168. [Google Scholar] [CrossRef]
- Haussler, K.V. Review of the examination and treatment of back and pelvic disorders. In Proceedings of the Focus Meeting AAEP—Lameness and Imaging, Fort Collins, CO, USA, 31 July 2007; pp. 151–158. [Google Scholar]
- Russell, L.; Tucker, M.S.; Ronald, D.S. Computed Tomography and Magnetic Resonance Imaging in Equine Musculoskeletal Conditions. Vet. Clin. N. Am. Equine Pract. 2001, 17, 145–157. [Google Scholar] [CrossRef]
- Whitton, R.C.; Buckley, C.; Donovan, T.; Wales, A.D.; Dennis, R. The diagnosis of lameness associated with distal limbpathology in a horse: A comparison of radiography, computed tomography and magnetic resonance imaging. Vet. J. 1998, 155, 223–229. [Google Scholar] [CrossRef]
- Keegan, K.G. Evidence-Based Lameness Detection and Quantification. Vet. Clin. Equine 2007, 23, 403–423. [Google Scholar] [CrossRef] [PubMed]
- Back, W.; MacAllister, C.G.; van Heel, M.C.V.; Pollmeier, M.; Hanson, P.D. Vertical Frontlimb Ground Reaction Forces of Sound and Lame Warmbloods Differ from Those in Quarter Horses. J. Equine Vet. Sci. 2007, 27, 123–129. [Google Scholar] [CrossRef]
- Radaelli, V.; Bergero, D.; Zucca, E.; Ferrucci, F.; Costa, L.N.; Crosta, L.; Luzi, F. Use of Thermography Techniques in Equines: Principles and Applications. J. Equine Vet. Sci. 2014, 34, 345–350. [Google Scholar] [CrossRef]
- Turner, T.A. Diagnostic Thermography. Vet. Clin. N. Am. Equine Pract. 2001, 17, 95–105. [Google Scholar] [CrossRef]
- Weil, M.; Litzke, L.F.; Fritsch, R. Diagnostic validity of thermography of lameness in horses. Tierarztl. Prax. Ausg. G Grosstiere Nutztiere 1998, 26, 346–354. [Google Scholar]
- Soroko, M.; Howell, K.; Zielinska, P. Application of thermography in racehorse performance. In Proceedings of the XV Congress of the European Association of Thermology, Wroclaw, Poland, 1–4 September 2021. [Google Scholar] [CrossRef]
- Purohit, R.C.; McCoy, M.D. Thermography in the diagnosis of inflammatory processes in the horse. Am. J. Vet. Res. 1980, 41, 1167–1174. [Google Scholar]
- Kastberger, G.; Stachl, R. Infrared Imaging technology and biological applications. Behav. Res. Methods Instrum. Comput. 2003, 35, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Turner, T.A. Thermography as an aid to the clinical lameness evaluation. Vet. Clin. N. Am. Equine Pract. 1991, 7, 311–318. [Google Scholar] [CrossRef]
- Masko, M.; Krajewska, A.; Zdrojkowski, L.; Domino, M.; Gajewski, Z. An application of temperature mapping of horse’s back for leisure horse-rider-matching. Anim. Sci. J. 2019, 90, 1396–1406. [Google Scholar] [CrossRef]
- Urakov, A.L.; Nikityuk, D.; Kasatkin, A.; Lukoyanov, I. Infrared plantography as a method to evaluate the functional anatomy of the human foot. In Proceedings of the 13th International Conference on Quantitative Infrared Thermography, Gdańsk, Poland, 4–8 July 2016. [Google Scholar]
- Clayton, H.M.; Gray, S.; Kraiser, L.J.; Bowker, R.M. Effects of barefoot trimming on hoof morphology. Aust. Vet. J. 2011, 89, 305–3011. [Google Scholar] [CrossRef]
- Keegan, K.G.; Wilson, D.A. Comparison of a body-mounted inertial sensor system-based method with subjective evaluation for detection of lameness in horses. Am. J. Vet. Res. 2013, 74, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Davidson, E.J. Lameness Evaluation of the Athletic Horse. Vet. Clin. N. Am. Equine Pract. 2018, 34, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Greve, L.; Dyson, S. What can we learn from visual and objective assessment of non-lame and lame horses in straight lines, on the lunge and ridden? Equine Vet. Educ. 2018, 32, 479–491. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Q.; Li, D.; Hu, M.; Yao, N.; Zhai, G. Estimating Departure Time Using Thermal Camera and Heat Traces Tracking Technique. Sensors 2020, 20, 782. [Google Scholar] [CrossRef]
- Hardeman, A.M.; Egenvall, A.; Braganca, F.M.S.; Swagemakers, J.M.; Koene, M.H.; Roepstorff, L.; van Weeren, R.; Bystrom, A. Visual lameness assessment in comparison to quantitative gait analysis data in horses. Equine Vet. J. 2022, 54, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Clayton, H.; Nauwelaerts, S. Effect of blindfolding of centre of pressure variables in healthy horses during quiet standing. Vet. J. 2013, 199, 365–369. [Google Scholar] [CrossRef]
- Yanmaz, L.E.; Okumus, Z.; Dogan, E. Instrumentation of Thermography and its Applications in Horses. J. Anim. Vet. Adv. 2007, 6, 858–862. [Google Scholar]
- Waguespack, R.; Hanson, R.R. Navicular syndrome in equine patients anatomy, causes, and diagnosis. Compend. Contin. Educ. Vet. 2010, 32, E7. [Google Scholar]
- Colles, C.M.; Hickman, J. The arterial supply of the navicular bone and its variations in navicular disease. Equine Vet. J. 1977, 9, 150–154. [Google Scholar] [CrossRef]
- Sievers, H.; Hiebl, B.; Hunigen, H.; Hirschberg, R.M. Pododermal angioarchitecture in the equine hoof wall: A light and scanning electron microscopic study of the wall proper. Clin. Hemorheol. Microcirc. 2020, 74, 21–44. [Google Scholar] [CrossRef]
- Zaha, C.; Schuszler, L.; Dascalu, R.; Nistor, P.; Florea, T.; Kalman, I.; Rujescu, C.; Sicoe, B.; Igna, C. Evaluation of thermal changes of the sole surface in horses with Palmar Foot Pain: A pilot Study. Biology 2023, 12, 423. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.F.; Fugler, L.A.; Eades, S.C. The management of equine acute laminitis. Vet. Med. 2015, 6, 39–47. [Google Scholar] [CrossRef]
- Kim, S.M.; Cho, G.J. Evaluation of Heat Distribution for the Diagnosis of the Hoof with Abscess by Infrared Thermography in Horses. Open Agric. J. 2021, 15, 48–53. [Google Scholar] [CrossRef]
- Jones, E.; Vinuela-Fernandez, I.; Eager, R.A.; Delaney, A.; Anderson, H.; Patel, A.; Robertson, D.C.; Allchorne, A.; Sirinathsinghji, E.C.; Milne, E.M.; et al. Neuropathic changes in equine laminitis pain. Pain 2007, 132, 231–331. [Google Scholar] [CrossRef] [PubMed]
- Van Eps, A.W.; Pollitt, C.C. Digital Hypotermia. In Equine Laminitis, 1st ed.; Belknap, J.K., Geor, R.J., Eds.; John Wiley & Sons: Chichester, UK, 2017; pp. 306–315. [Google Scholar] [CrossRef]
- Peroni, J.F.; Moore, J.N.; Noschka, E.; Grafton, M.E.; Aceves-Avila, M.; Levis, S.J.; Robertson, T.P. Predisposition for venoconstriction in the equine laminar dermis: Implications in equine laminitis. J. Appl. Psychol. 2006, 103, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Keeegan, K.G.; MacAllister, C.G.; Wilson, D.A.; Gedon, C.A.; Kramer, J.; Yonezawa, Y.; Maki, H.; Pai, P.F. Comparison of an inertial sensor system with a stationary force plate for evaluation of horses with bilateral forelimb lameness. Am. J. Vet. Res. 2012, 73, 368–372. [Google Scholar] [CrossRef]
- Bell, R.P.; Reed, S.K.; Schoonover, M.J.; Whitfield, C.T.; Yonezawa, Y.; Maki, H.; Keegan, K.G. Associations of force plate and body-mounted inertial sensor measurements for identification of hind limb lameness in horses. Am. J. Vet. Res. 2016, 77, 337–345. [Google Scholar] [CrossRef]
- Hobbs, S.J.; Clayton, H.M. Sagittal plane ground reaction forces, centre of pressure and centre of mass in trotting horses. Vet. J. 2013, 198, 14–19. [Google Scholar] [CrossRef]
- Ovnicek, G.D.; Page, B.T.; Trotter, G.W. Natural balance trimming and shoening: Its theory and application. Vet. Clin. N. Am. Equine Pract. 2003, 19, 353–377. [Google Scholar] [CrossRef]
- Souza, A.F.; Kunz, J.R.; Laus, R.; Moreira, M.A.; Muller, T.R.; Fonteque, J.H. Biometrics of hoof balance in equids. Arq. Bras. Med. Vet. Zootec. 2016, 68, 825–831. [Google Scholar] [CrossRef]



| Examination Method | Positive Response to Hoof Tester | Positive Reaction Joint Flexion | Vertical Movement of the Head during Walking | Rotation of the Pelvis | Gluteal Muscle and Sacral Tuberosities Asymmetries | Tendinitis of the Flexors | Alteration in Limb Positioning | Unique Condition | Multiple Condition |
|---|---|---|---|---|---|---|---|---|---|
| Number of affected horses | 7 | 9 | 8 | 6 | 11 | 7 | 4 | 8 | 18 |
| Position | Number | Variable | Mean | Std Dev | Minimum | Maximum | Median | Range | Lower 95% CL for Mean | Upper 95% CL for Mean |
|---|---|---|---|---|---|---|---|---|---|---|
| Forelimb (T) | 120 | Toe | 20.44 | 2.72 | 16.20 | 25.90 | 20.25 | 9.70 | 19.94 | 20.93 |
| Sole | 20.30 | 2.80 | 16.40 | 25.60 | 19.95 | 9.20 | 19.79 | 20.81 | ||
| Frog | 20.63 | 2.79 | 16.40 | 28.65 | 19.90 | 12.25 | 20.12 | 21.13 | ||
| Frog apex | 20.43 | 2.81 | 16.500 | 25.70 | 20.25 | 9.20 | 19.92 | 20.94 | ||
| Hoof wall | 20.27 | 2.84 | 16.20 | 25.20 | 20.15 | 9.00 | 19.76 | 20.78 | ||
| Heel | 20.14 | 2.79 | 16.40 | 24.70 | 19.65 | 8.30 | 19.63 | 20.64 |
| Position | Number | Variable | Mean | Std Dev | Minimum | Maximum | Median | Range | Lower 95% CL for Mean | Upper 95% CL for Mean |
|---|---|---|---|---|---|---|---|---|---|---|
| Hindlimb (H) | 120 | Toe | 20.15 | 2.52 | 17.10 | 27.30 | 18.90 | 10.20 | 19.70 | 20.61 |
| Sole | 20.04 | 2.62 | 16.70 | 27.30 | 18.60 | 10.60 | 19.57 | 20.52 | ||
| Frog | 20.25 | 2.52 | 17.00 | 27.40 | 19.15 | 10.40 | 19.79 | 20.71 | ||
| Frog apex | 20.09 | 2.58 | 16.70 | 27.30 | 18.70 | 10.60 | 19.63 | 20.56 | ||
| Hoof wall | 19.96 | 2.63 | 16.40 | 27.40 | 18.60 | 11.00 | 19.49 | 20.44 | ||
| Heel | 19.88 | 2.58 | 16.50 | 27.10 | 18.50 | 10.60 | 19.41 | 20.34 |
| Group Comparison | Toe | Sole | Frog | Frog Apex | Hoff Wall | Heels |
|---|---|---|---|---|---|---|
| Left forelimb versus right forelimb | 1 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 |
| Left hindlimb versus right hindlimb | 0.98 | 0.99 | 0.99 | 0.99 | 0.97 | 0.98 |
| Left forelimb versus right hindlimb | 0.87 | 0.88 | 0.75 | 0.89 | 0.80 | 0.84 |
| Right forelimb versus left hindlimb | 0.97 | 0.99 | 0.94 | 0.91 | 0.98 | 0.99 |
| Forelimbs versus hindlimbs | 0.83 | 0.89 | 0.73 | 0.81 | 0.81 | 0.86 |
| All four limbs | 0.60 | 0.70 | 1 | 0.81 | 0.61 | 0.58 |
| Comparison | Toe | Sole | Frog | Frog Apex | Hoof Wall | Heels |
|---|---|---|---|---|---|---|
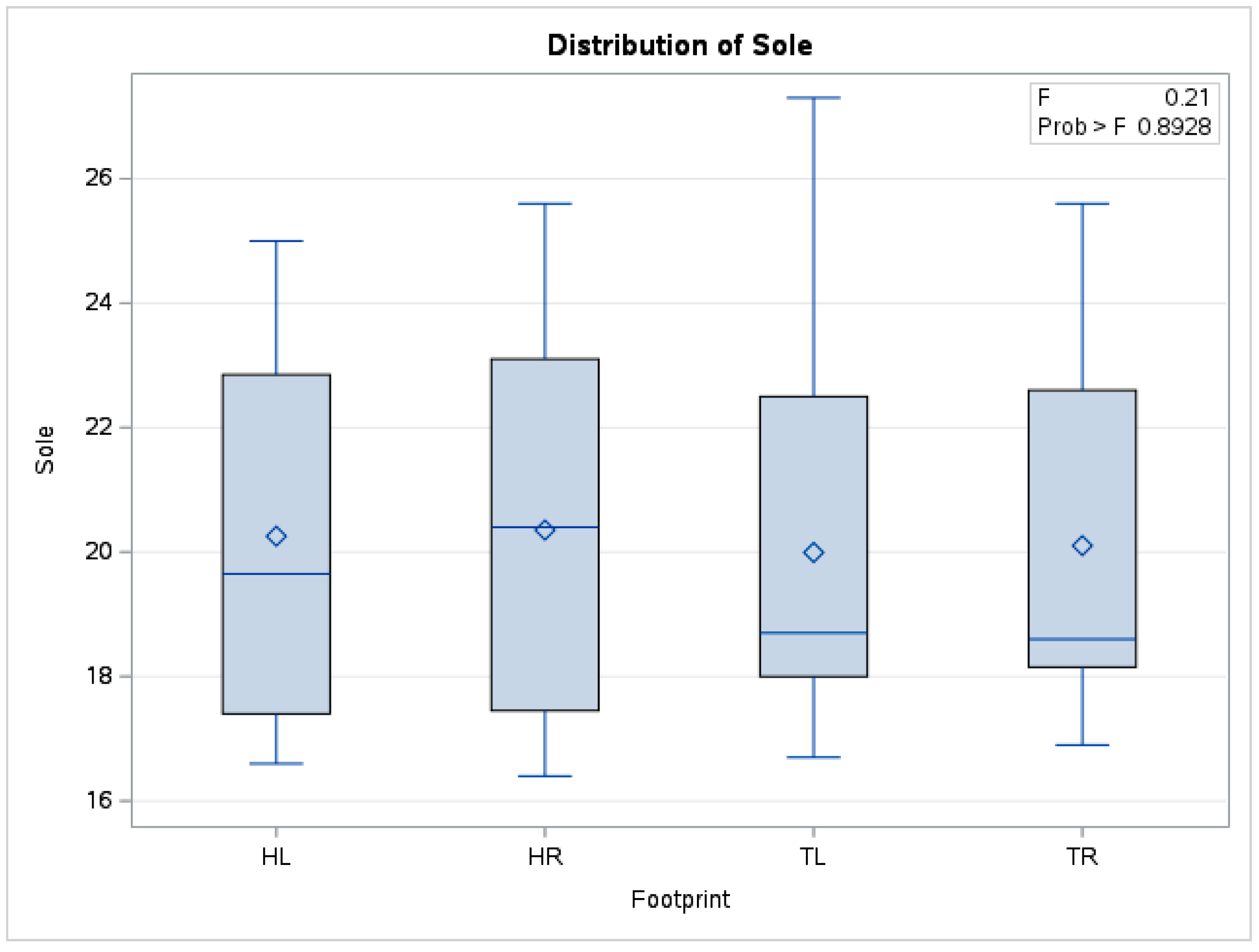
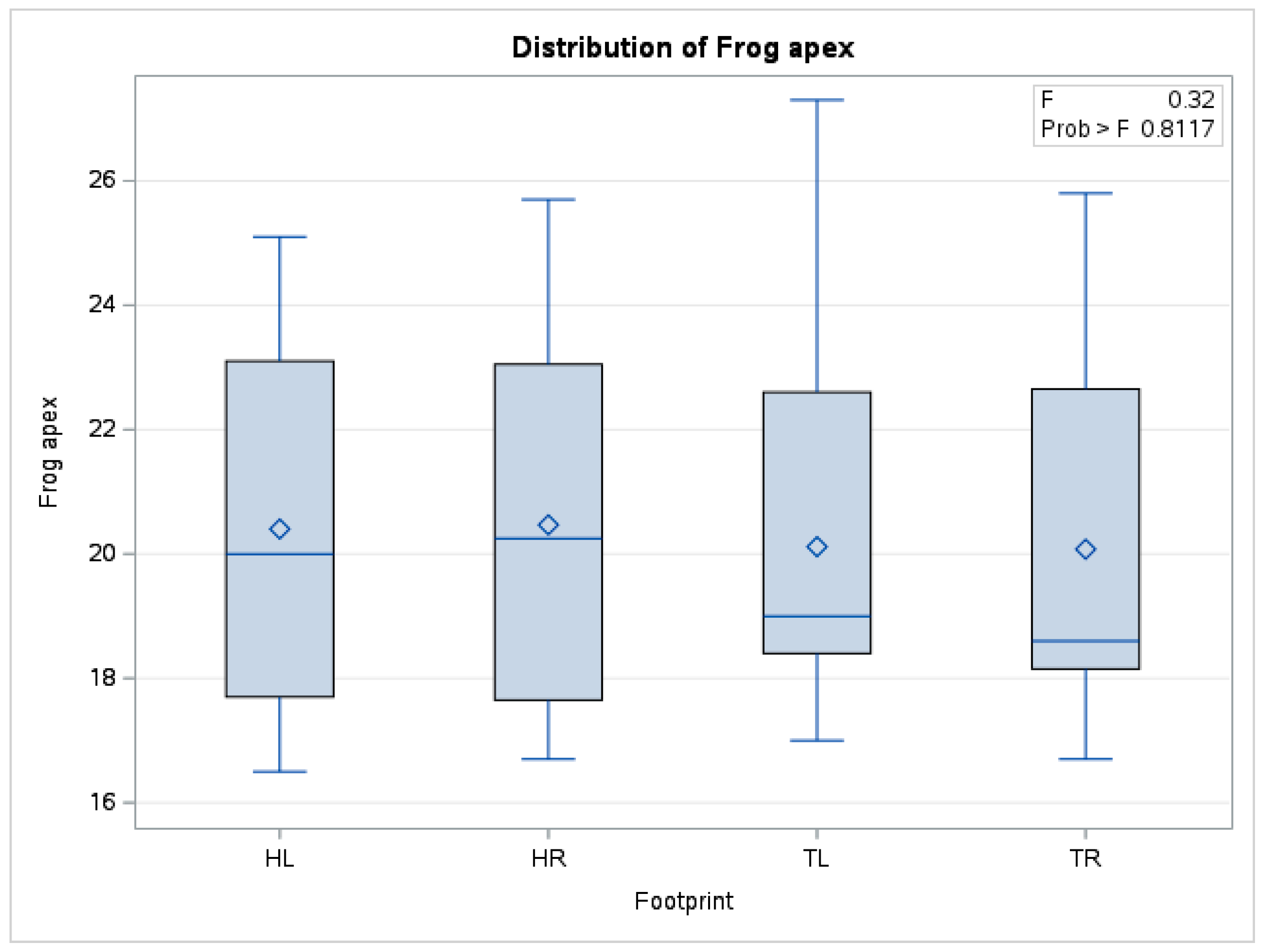
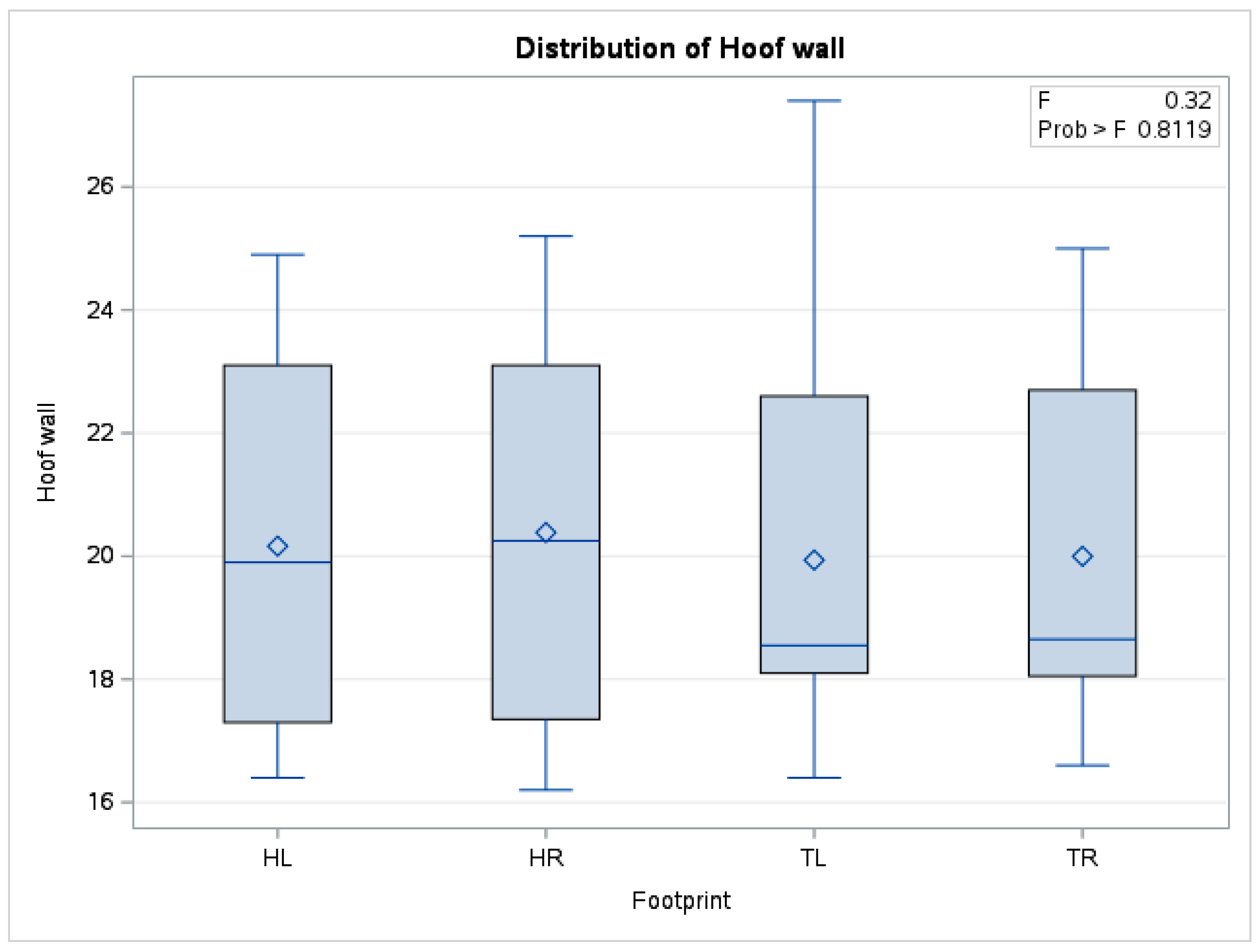
| Leisure and cross-country horses | 0.17 | 0.20 | 0.12 | 0.24 | 0.64 | 0.89 |
| Location A, Location B and Location C | 0.35 | 0.31 | 0.33 | 0.34 | 0.31 | 0.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaha, C.; Schuszler, L.; Dascalu, R.; Nistor, P.; Florea, T.; Rujescu, C.; Sicoe, B.; Igna, C. Thermographic Image of the Hoof Print in Leisure and Cross-Country Warmblood Horses: A Pilot Study. Vet. Sci. 2023, 10, 470. https://doi.org/10.3390/vetsci10070470
Zaha C, Schuszler L, Dascalu R, Nistor P, Florea T, Rujescu C, Sicoe B, Igna C. Thermographic Image of the Hoof Print in Leisure and Cross-Country Warmblood Horses: A Pilot Study. Veterinary Sciences. 2023; 10(7):470. https://doi.org/10.3390/vetsci10070470
Chicago/Turabian StyleZaha, Cristian, Larisa Schuszler, Roxana Dascalu, Paula Nistor, Tiana Florea, Ciprian Rujescu, Bogdan Sicoe, and Cornel Igna. 2023. "Thermographic Image of the Hoof Print in Leisure and Cross-Country Warmblood Horses: A Pilot Study" Veterinary Sciences 10, no. 7: 470. https://doi.org/10.3390/vetsci10070470
APA StyleZaha, C., Schuszler, L., Dascalu, R., Nistor, P., Florea, T., Rujescu, C., Sicoe, B., & Igna, C. (2023). Thermographic Image of the Hoof Print in Leisure and Cross-Country Warmblood Horses: A Pilot Study. Veterinary Sciences, 10(7), 470. https://doi.org/10.3390/vetsci10070470







