Cutaneous Malignant Melanoma with Testicular Metastases in a Wild Rabbit (Oryctolagus cuniculus)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
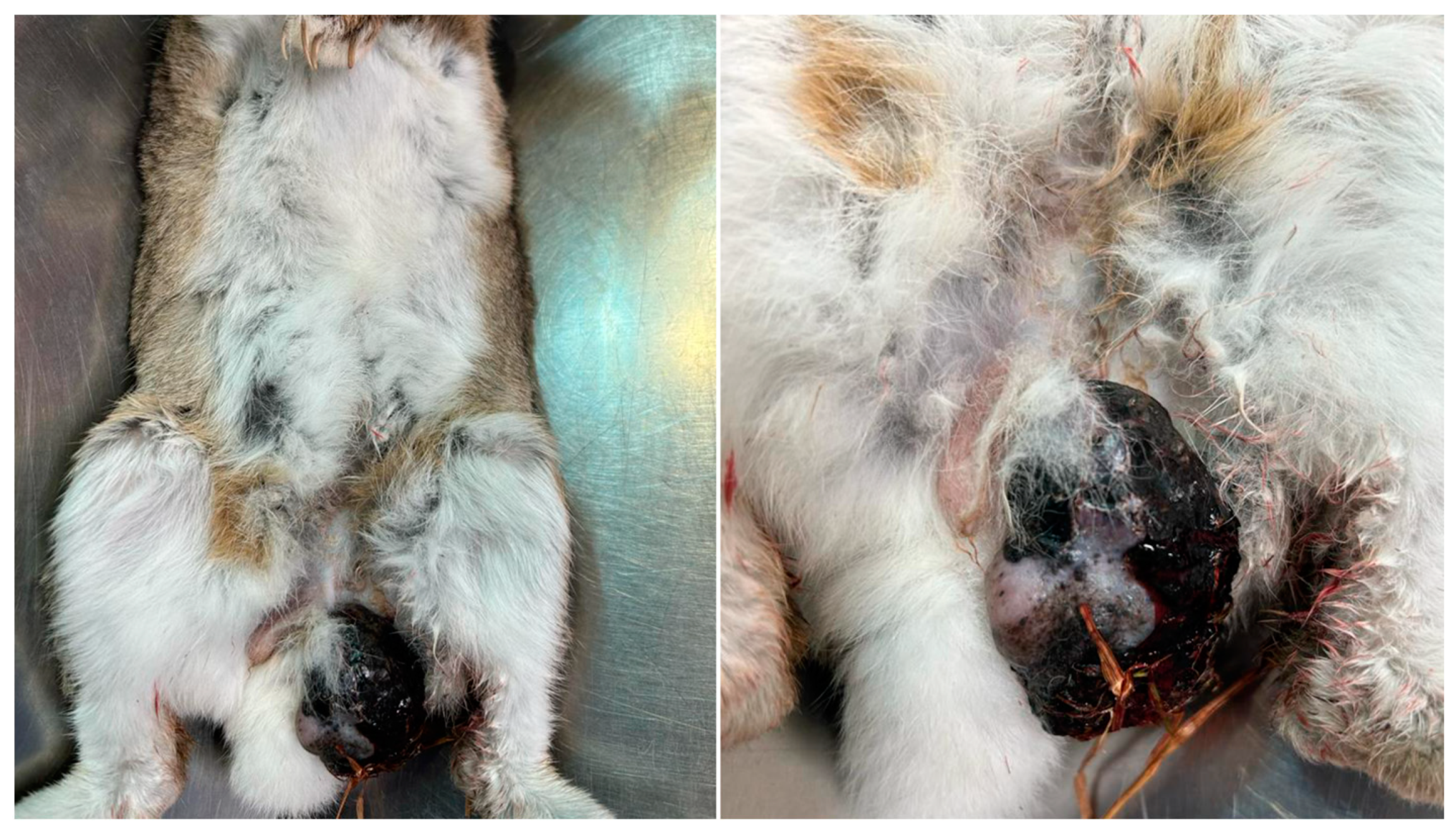
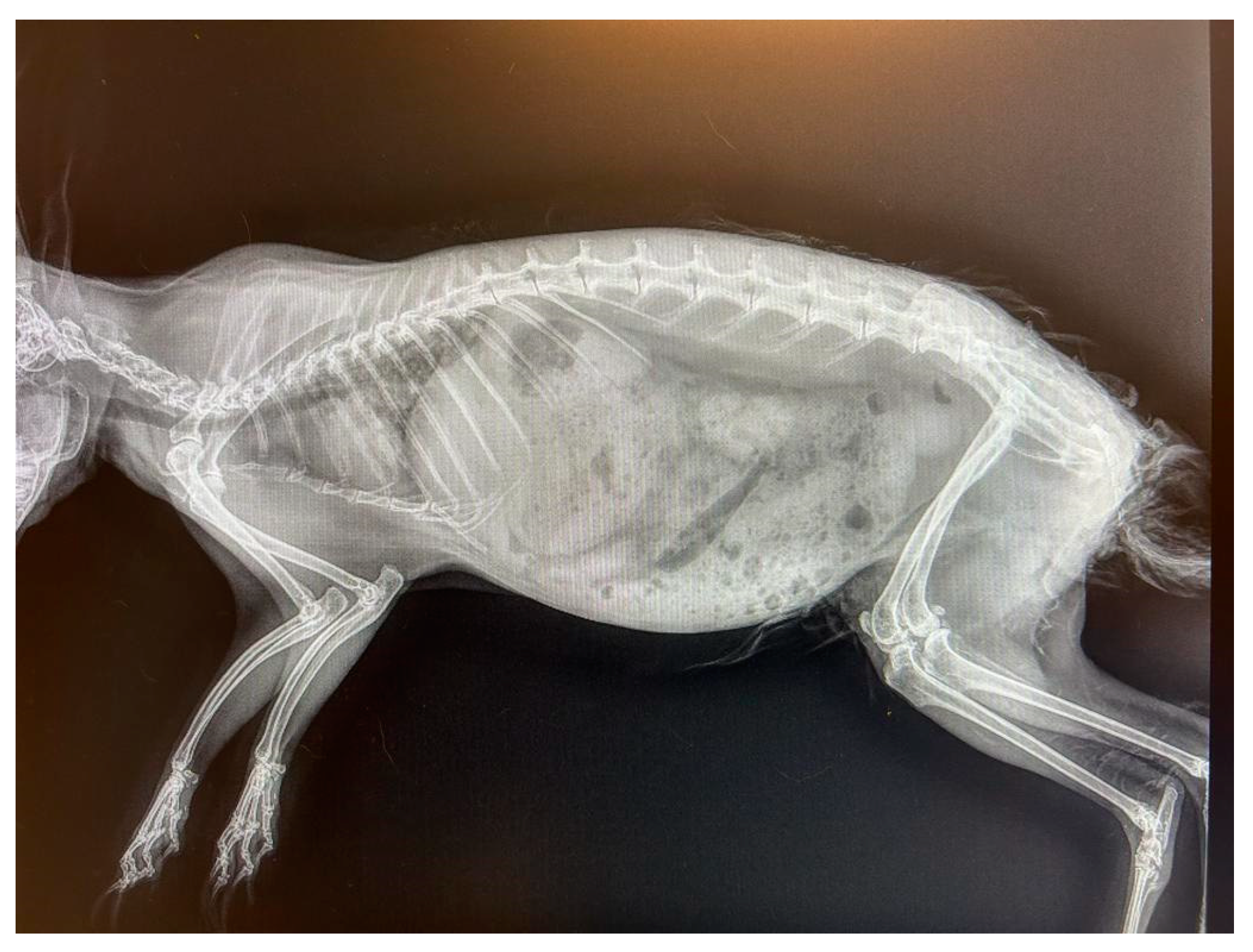
2.1. Case Presentation
2.2. Pathological Examination
2.3. Immunohistochemistry
3. Results
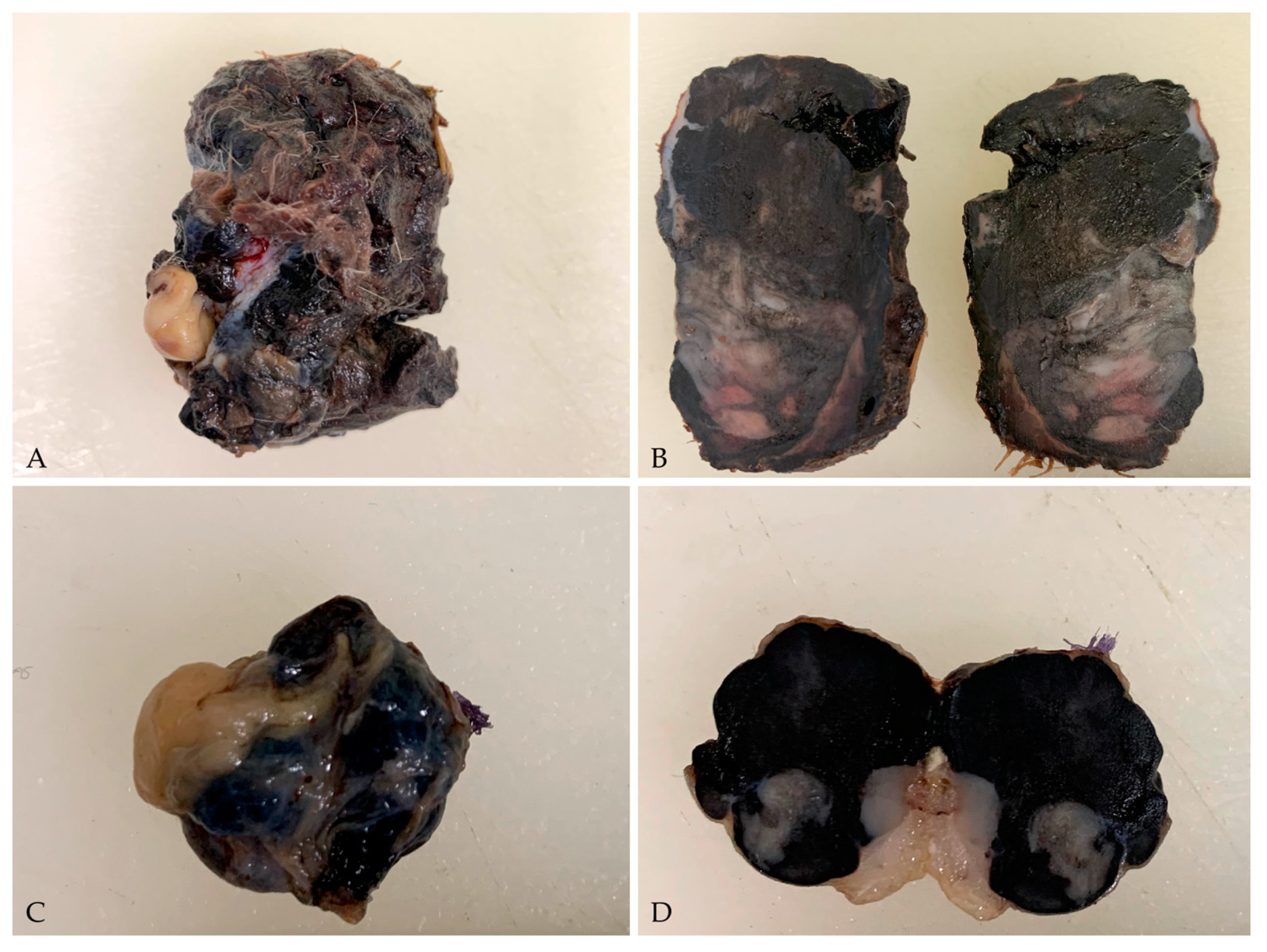
3.1. Gross Examination
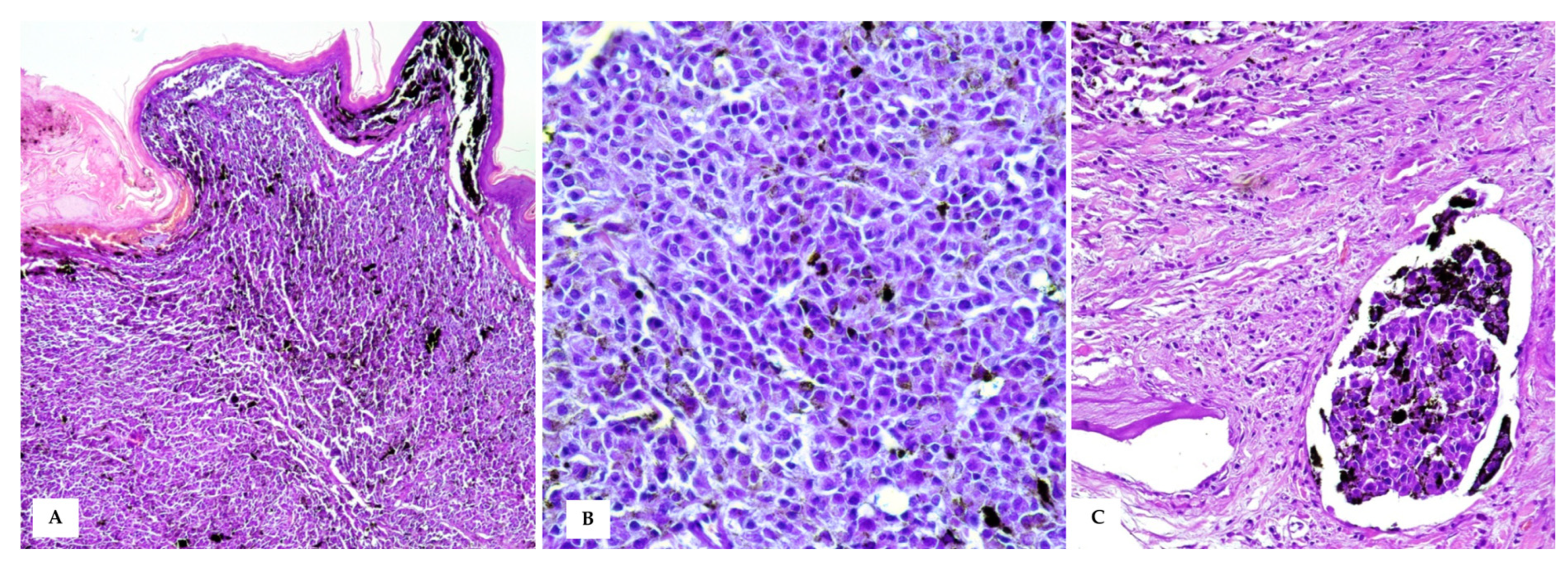
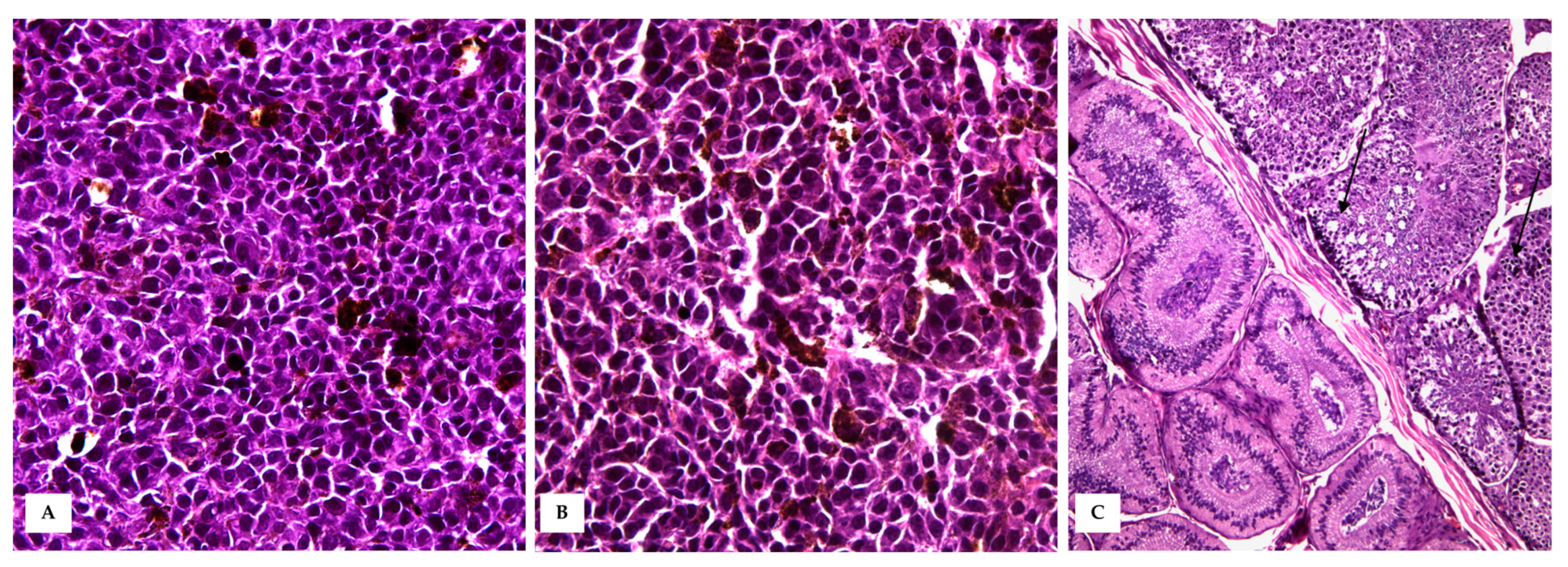
3.2. Histopathology
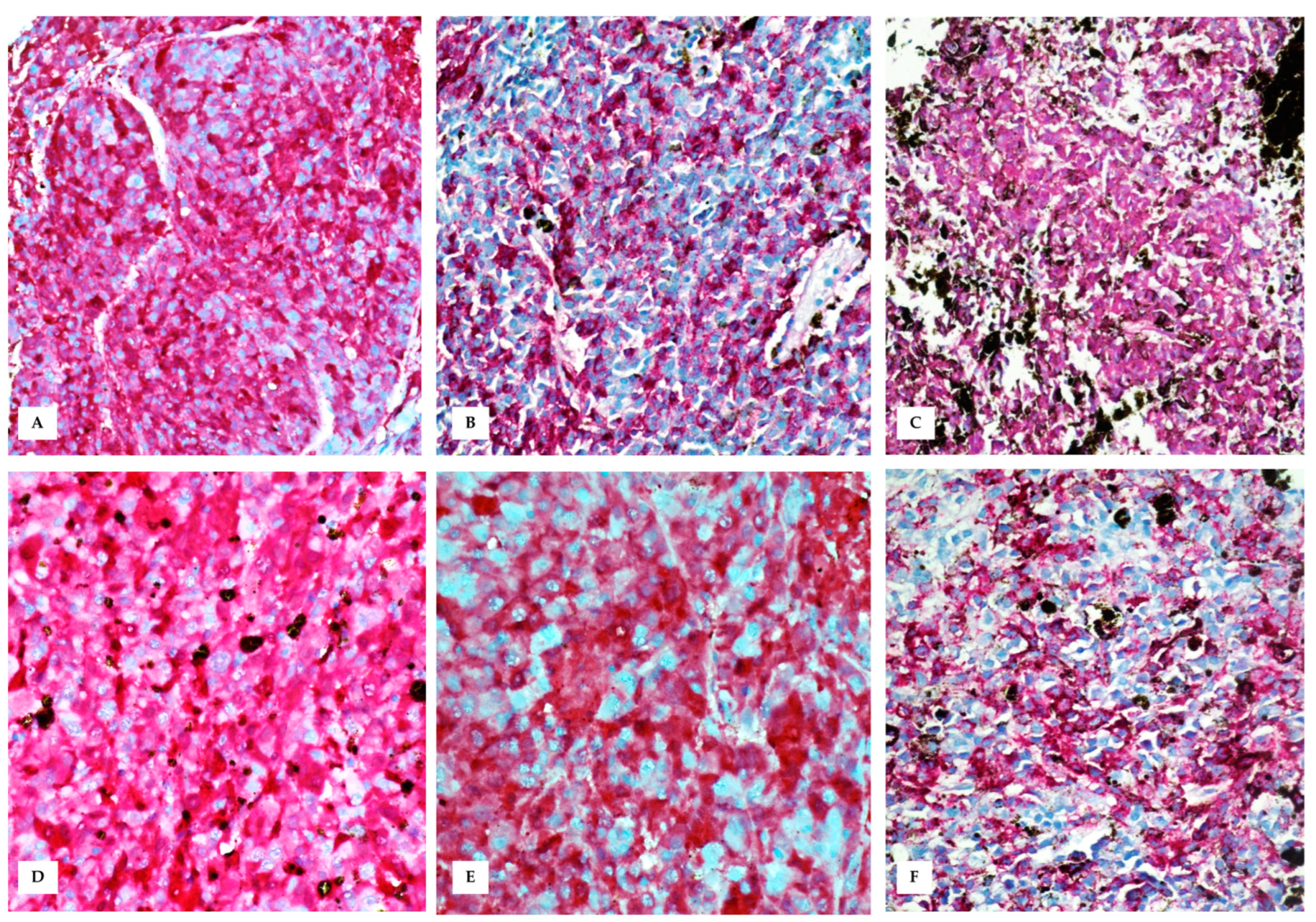
3.3. Immunohistochemistry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baum, B. Not just Uterine Adenocarcinoma-neoplastic and non-neoplastic masses in Domestic Pet rabbits (Oryctolagus cuniculi): A Review. Vet. Pathol. 2021, 58, 890–900. [Google Scholar] [CrossRef]
- Bertram, C.A.; Bertram, B.; Bartel, A.; Ewringmann, A.; Fragoso-Garcia, M.A.; Erickson, N.A.; Müller, K.; Klopfleisch, R. Neoplasia and Tumor-Like Lesions in Pet Rabbits (Oryctolagus cuniculus): A Retrospective Analysis of Cases between 1995 and 2019. Vet. Pathol. 2021, 58, 901–911. [Google Scholar] [CrossRef] [PubMed]
- von Bomhard, W.; Goldschmidt, M.H.; Shofer, F.S.; Perl, L.; Rosenthal, K.L.; Mauldin, E.A. Cutaneous Neoplasms in Pet Rabbits: A Retrospective Study. Vet. Pathol. 2007, 44, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Weisbroth, S.H. Neoplastic diseases. In The Biology of the Laboratory Rabbit; Weisbroth, S.H., Flatt, R.E., Kraus, A.L., Eds.; Academic Press: Cambridge, MA, USA, 1974; pp. 259–292. [Google Scholar]
- Barthold, S.W.; Griffey, S.M.; Percy, D.H. Pathology of Laboratory Rodents and Rabbits, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; Chapter 6; pp. 253–323. [Google Scholar]
- Strayer, D.S.; Skaletsky, E.; Cabirac, G.F.; Sharp, P.A.; Corbeil, L.B.; Sell, S.; Leibowitz, J.L. Malignant rabbit fibroma virus causes secondary immunosuppression in rabbits. J. Immunol. 1983, 130, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Petterino, C.; Modesto, P.; Strata, D.; Vascellari, M.; Mutinelli, F.; Ferrari, A.; Ratto, A. A case of interscapular fibrosarcoma in a dwarf rabbit (Oryctolagus cuniculus). J. Vet. Diagn. Investig. 2009, 21, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Miwa, Y.; Nakata, M.; Takimoto, H.; Chambers, J.K.; Uchida, K. Spontaneous oral tumours in 18 rabbits (2005–2015). J. Small Anim. Pract. 2021, 62, 156–160. [Google Scholar] [CrossRef]
- van der Weyden, L.; Brenn, T.; Patton, E.E.; Wood, G.A.; Adams, D.J. Spontaneously occurring melanoma in animals and their relevance to human melanoma. J. Pathol. 2020, 252, 4–21. [Google Scholar] [CrossRef]
- Ueda, K.; Ueda, A.; Ozaki, K. Cutaneous malignant melanoma in two rabbits (Oryctolagus cuniculus). J. Vet. Med. Sci. 2018, 80, 973–976. [Google Scholar] [CrossRef]
- Zerfas, P.M.; Brinster, L.R.; Starost, M.F.; Burkholder, T.H.; Raffeld, M.; Eckhaus, M.A. Amelanotic melanoma in a New Zealand White Rabbit (Oryctolagus cuniculus). Vet. Pathol. 2010, 47, 977–981. [Google Scholar] [CrossRef]
- Fisher, P.; Graham, J.E. Rabbits. In Carpenter’s Exotic Animal Formulary, 6th ed.; Carpenter, J., Harms, C., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2023; Volume 10, pp. 574–625. [Google Scholar] [CrossRef]
- Smedley, R.C.; Sebastian, K.; Kiupel, M. Diagnosis and Prognosis of Canine Melanocytic Neoplasms. Vet. Sci. 2022, 9, 175. [Google Scholar] [CrossRef]
- Brandão, J.; Blair, R.; Kelly, A.; Fowlkes, N.; Shiomitsu, K.; Gomes, F.E.; Rich, G.; Tully, T.N. Amelanotic Melanoma in the Rabbit: A case Report with an Overview of Immunohistochemical Characterization. J. Exot. Pet. Med. 2015, 24, 193–200. [Google Scholar] [CrossRef]
- Hotchkiss, C.E.; Norden, H.; Collins, B.R.; Ginn, P.E. Malignant melanoma in two rabbits. Lab. Anim. Sci. 1994, 44, 377–379. [Google Scholar] [PubMed]
- Hammer, M.; Weigner, F.; Klopfleisch, R. Cutaneous melanomas in rabbits: Rare but often fatal. Vet. Sci. Dev. 2011, 1, e9. [Google Scholar] [CrossRef]
- Goldschmidt, M.H.; Goldschmidt, K.H. Epithelial and Melanocytic Tumors of the Skin. In Tumors in Domestic Animals, 5th ed.; Meuten, D.J., Ed.; John Wiley & Sons Inc.: Ames, IA, USA, 2017. [Google Scholar]
- Trappler, M.C.; Popovitch, C.A.; Goldschmidt, M.H.; Goldschmidt, K.H.; Risbon, R.E. Scrotal tumors in dogs: A retrospective study of 676 cases (1986–2010). Can. Vet. J. 2014, 55, 1229–1233. [Google Scholar] [PubMed]
- Patel, S.R.; Richardson, R.L.; Kvols, L. Metastatic cancer to the testes: A report of 20 cases and review of the literature. J. Urol. 1989, 142, 1003–1005. [Google Scholar] [CrossRef]
- Dusaud, M.; Adjadj, L.; Debelmas, A.; Souraud, J.B.; Durand, X. Malignant melanoma revealed by testicular metatasi. Int. J. Surg. Case Rep. 2015, 12, 102–105. [Google Scholar] [CrossRef]
- Camparo, P.; Durand, X.; Avances, C.; Culine, S.; Segui, B.; Rigaud, J. Histological features and principles of treating testicle tumors in the elderly subject. Prog. Urol. 2009, 19, 142–146. [Google Scholar] [CrossRef]
- Tiltman, A.J. Metastatic tumors in the testis. Histopathology 1979, 3, 21–37. [Google Scholar] [CrossRef]
- Anderson, W.I.; Car, B.D.; Kenny, K.; Schlafer, D.H. Bilateral testicular seminoma in a New Zealand white rabbit (Oryctolagus cuniculus). Lab. Anim. Sci. 1990, 40, 420–421. [Google Scholar]
- Webb, J.K.; Reavill, D.R.; Garner, M.M.; Kiupel, M.; Graham, J.E. Characterization of Testicular Granular Cell Tumors in Domestic Rabbits (Oryctolagus cuniculus). J. Exot. Pet. Med. 2019, 29, 159–165. [Google Scholar] [CrossRef]
- Banco, B.; Binanti, D.; Penna, V.; Grieco, V. Sertoli cell tumor in a pet rabbit (Oryctolagus cuniculus): Histological and immunohistochemical characterization. Open Vet. J. 2018, 8, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Ozaki, M.; Ano, N.; Nomura, K.; Ozaki, K.; Narama, I. Testicular gonadoblastoma in two pet domestic rabbits (Oryctolagus cuniculus domesticus). J. Vet. Diagn. Invest. 2011, 23, 1028–1032. [Google Scholar] [CrossRef]
- Kunzel, F.; Hittmair, K.M.; Hassan, J.; Dupré, G.; Russold, E.; de Arespachochaga, A.G.; Fuchs-Baumgartinger, A.; Bilek, A. Thymomas in Rabbits: Clinical evaluations, diagnosis, and treatment. J. Am. Anim. Hosp. Assoc. 2012, 48, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Morrisey, J.; Mcentee, M. Therapeutic Options of Thymoma in the Rabbit. Semin. Avian Exot. Pet. Med. 2005, 14, 175–181. [Google Scholar] [CrossRef]
- Meredith, A.; Lord, B. BSAVA Manual of Rabbit Medicine; Meredith, A., Lord, B., Eds.; British Small Animal Veterinary Association: Quedgeley, UK, 2014. [Google Scholar]
- Song, S.L.; Deng, C.; Wen, L.F.; Liu, J.J.; Wang, H.; Feng, D.; Wong, C.O.; Huang, G. 18F-FDG PET/CT-related metabolic parameters and their value in early prediction of chemotherapy response in a VX2 tumor model. Nucl. Med. Biol. 2010, 37, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Heatley, J.J.; Smith, A.N. Spontaneous neoplasms of lagomorphs. Vet. Clin. N. Am. Exot. Anim. Pract. 2004, 7, 561–577. [Google Scholar] [CrossRef]
| Parameters | Values | Reference Ranges |
|---|---|---|
| Red Blood Cells (RBC) (×106/μL) | 5.9 | 4.5–6.9 |
| Haematocrit (%) | 40.2 | 31.3–43.3 |
| Haemoglobin (Hgb) (g/dL) | 12.3 | 11.0–14.4 |
| Platelets (PLT) (×103/μL) | 244.8 | 134–567 |
| White Blood Cells (WBC) (×103/μL) | 9.7 | 4.1–10.8 |
| Heterophils (%) | 61.2 | 20–75 |
| Lymphocytes (%) | 36.6 | 30–85 |
| Eosinophils (%) | 0.4 | 0–5 |
| Basophils (%) | 0.1 | 0–10 |
| Monocytes (%) | 1.6 | 0–10 |
| Glucose (mg/dL) | 145 | 109–161 |
| Blood Urea Nitrogen (BUN) (mg/dL) | 23.2 | 9–29 |
| Creatinine (mg/dL) | 1.3 | 1.0–2.2 |
| Total Protein (g/dL) | 7.1 | 6.1–7.7 |
| Albumin (g/dL) | 3.3 | 2.8–4.0 |
| Globulin (g/dL) | 3.8 | 2.1–3.7 |
| Alanine Aminotransferase (ALT) (U/L) | 36 | 14–80 |
| Alkaline Phosphatase (ALP) (U/L) | 24 | 10–140 |
| Gamma-Glutamyl Transferase (GGT) (U/L) | 7.7 | 0–7 |
| Total Bilirubin (mg/dL) | 0.1 | 0.1–0.5 |
| Total Cholesterol (mg/dL) | 18 | 6–65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbate, J.M.; Palazzolo, S.; Ieni, A.; Rapisarda, G.S.; Lanteri, G. Cutaneous Malignant Melanoma with Testicular Metastases in a Wild Rabbit (Oryctolagus cuniculus). Vet. Sci. 2023, 10, 471. https://doi.org/10.3390/vetsci10070471
Abbate JM, Palazzolo S, Ieni A, Rapisarda GS, Lanteri G. Cutaneous Malignant Melanoma with Testicular Metastases in a Wild Rabbit (Oryctolagus cuniculus). Veterinary Sciences. 2023; 10(7):471. https://doi.org/10.3390/vetsci10070471
Chicago/Turabian StyleAbbate, Jessica Maria, Simone Palazzolo, Antonio Ieni, Giuseppe Santi Rapisarda, and Giovanni Lanteri. 2023. "Cutaneous Malignant Melanoma with Testicular Metastases in a Wild Rabbit (Oryctolagus cuniculus)" Veterinary Sciences 10, no. 7: 471. https://doi.org/10.3390/vetsci10070471
APA StyleAbbate, J. M., Palazzolo, S., Ieni, A., Rapisarda, G. S., & Lanteri, G. (2023). Cutaneous Malignant Melanoma with Testicular Metastases in a Wild Rabbit (Oryctolagus cuniculus). Veterinary Sciences, 10(7), 471. https://doi.org/10.3390/vetsci10070471






