Quantitative Analysis of Brain CT Perfusion in Healthy Beagle Dogs: A Pilot Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Preparation for CT
2.3. CT Image Acquisition
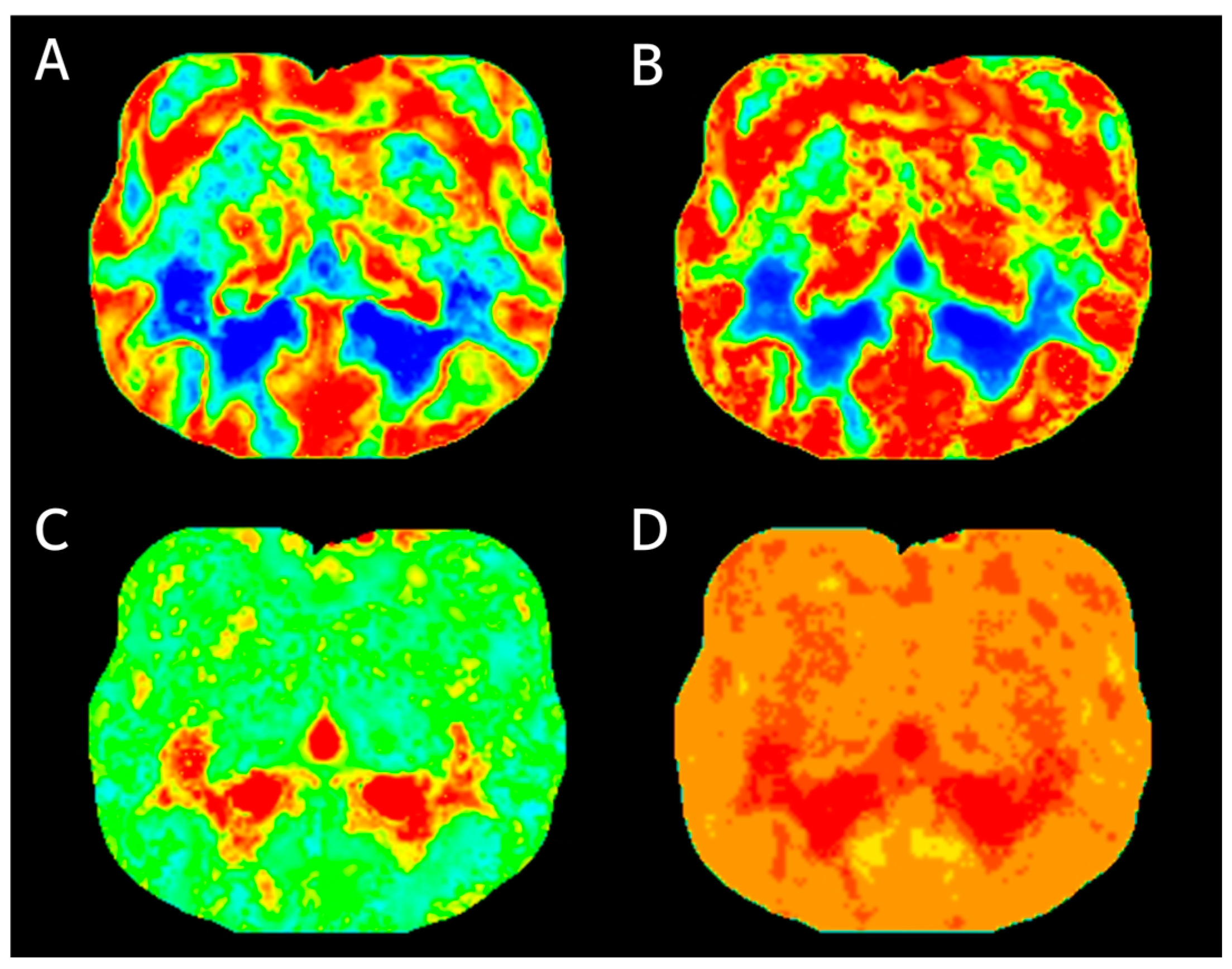
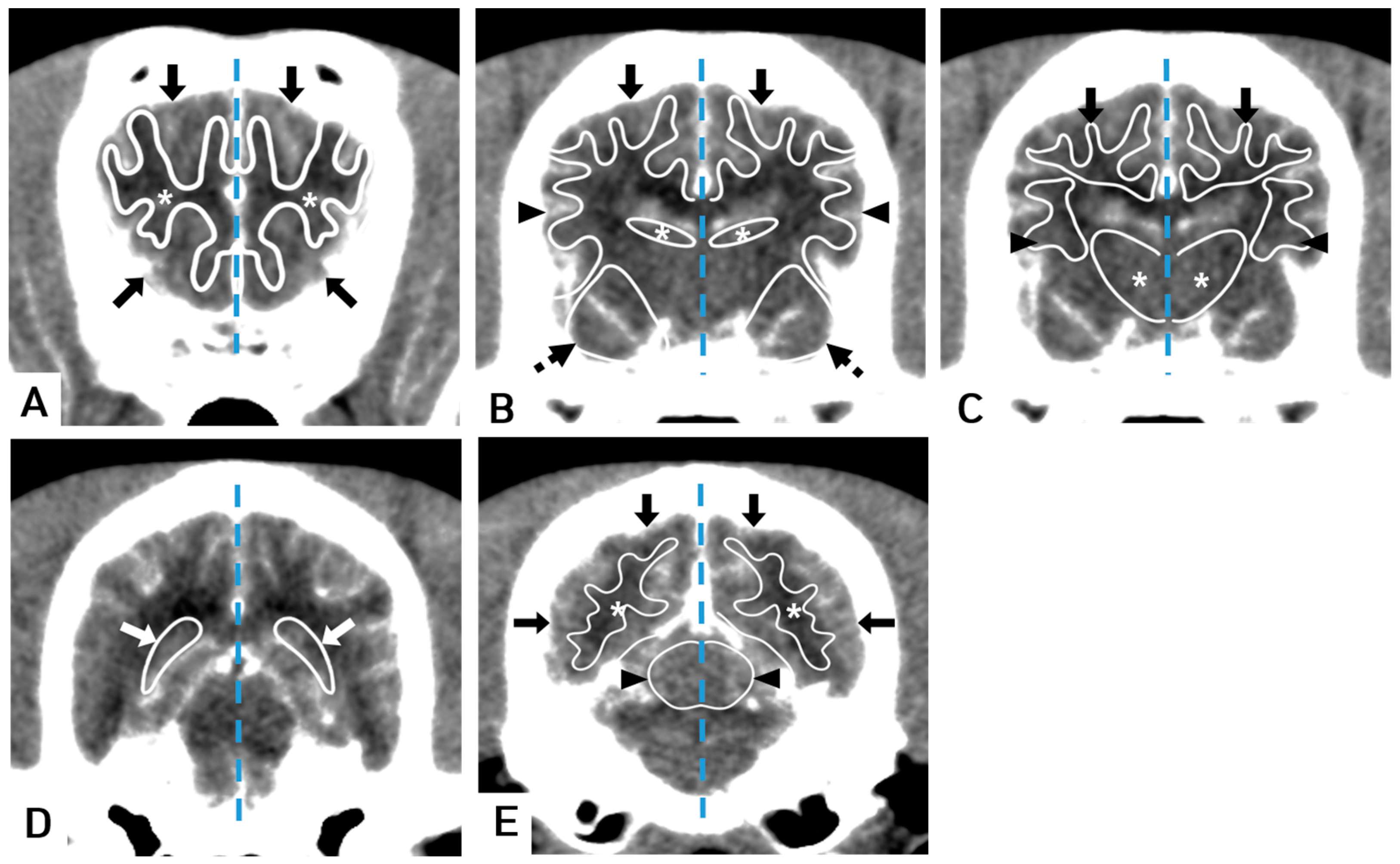
2.4. Perfusion Data Analysis
2.5. Statistical Analysis
3. Results
3.1. Comparison of CT Perfusion Measurements between White Matter and Gray Matter
3.2. Comparison of CT Perfusion Measurements by Aging
3.3. Comparison of CT Perfusion Measurements by the Side of Hemisphere
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Konstas, A.A.; Goldmakher, G.V.; Lee, T.Y.; Lev, M.H. Theoretic basis and technical implementations of CT perfusion in acute ischemic stroke, part 1: Theoretic basis. Am. J. Neuroradiol. 2009, 30, 662–668. [Google Scholar] [CrossRef]
- Jeon, Y.W.; Kim, S.H.; Lee, J.Y.; Whang, K.; Kim, M.S.; Kim, Y.J.; Lee, M.S. Dynamic CT perfusion imaging for the detection of crossed cerebellar diaschisis in acute ischemic stroke. Korean J. Radiol. 2012, 13, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Edwards, L.S.; Cappelen-Smith, C.; Cordato, D.; Bivard, A.; Churilov, L.; Parsons, M.W. Review of CT perfusion and current applications in posterior circulation stroke. Vessel Plus 2021, 5, 42. [Google Scholar] [CrossRef]
- Konstas, A.A.; Goldmakher, G.V.; Lee, T.Y.; Lev, M.H. Theoretic basis and technical implementations of CT perfusion in acute ischemic stroke, part 2: Technical implementations. Am. J. Neuroradiol. 2009, 30, 885–892. [Google Scholar] [CrossRef]
- Axel, L. Cerebral blood flow determination by rapid-sequence computed tomography: Theoretical analysis. Radiology 1980, 137, 679–686. [Google Scholar] [CrossRef]
- Nabavi, D.G.; Cenic, A.; Craen, R.A.; Gelb, A.W.; Bennett, J.D.; Kozak, R.; Lee, T.Y. CT assessment of cerebral perfusion: Experimental validation and initial clinical experience. Radiology 1999, 213, 141–149. [Google Scholar] [CrossRef]
- Esteban, J.M.; Cervera, V. Perfusion CT and angio CT in the assessment of acute stroke. Neuroradiology 2004, 46, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Gobbel, G.T.; Cann, C.E.; Fike, J.R. Measurement of regional cerebral blood flow using ultrafast computed tomography: Theoretical aspects. Stroke 1991, 22, 768–771. [Google Scholar] [CrossRef]
- Schmidt, M.J.; Kolecka, M.; Kirberger, R.; Hartmann, A. Dynamic susceptibility contrast perfusion magnetic resonance imaging demonstrates reduced periventricular cerebral blood flow in dogs with ventriculomegaly. Front. Vet. Sci. 2017, 4, 5–7. [Google Scholar] [CrossRef]
- Hartmann, A.; Driesen, A.; Lautenschläger, I.E.; Scholz, V.B.; Schmidt, M.J. Quantitative analysis of brain perfusion in healthy dogs by means of magnetic resonance imaging. Am. J. Vet. Res. 2016, 77, 1227–1235. [Google Scholar] [CrossRef]
- Hartmann, A.; von Klopmann, C.; Lautenschläger, I.E.; Scholz, V.B.; Schmidt, M.J. Quantitative analysis of brain perfusion parameters in dogs with idiopathic epilepsy by use of magnetic resonance imaging. Am. J. Vet. Res. 2018, 79, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Seidel, G.; Algermissen, C.; Christoph, A.; Katzer, T.; Kaps, M. Visualization of brain perfusion with harmonic gray scale and power doppler technology. Stroke 2000, 31, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Peremans, K.; Audenaert, K.; Blanckaert, P.; Jacobs, F.; Coopman, F.; Verschooten, F.; Van Bree, H.; Van Heeringen, C.; Mertens, J.; Slegers, G.; et al. Effects of aging on brain perfusion and serotonin-2A receptor binding in the normal canine brain measured with single photon emission tomography. Prog. Neuropsychopharmacol. Biol. Psychiatry 2002, 26, 1393–1404. [Google Scholar] [CrossRef] [PubMed]
- Peremans, K.; De Bondt, P.; Audenaert, K.; Van Laere, K.; Gielen, I.; Koole, M.; Versijpt, J.; Van Bree, H.; Verschooten, F.; Dierckx, R. Regional brain perfusion in 10 normal dogs measured using technetium-99m ethyl cysteinate dimer spect. Vet. Radiol. Ultrasound 2001, 42, 562–568. [Google Scholar] [CrossRef]
- Jokinen, T.S.; Haaparanta-Solin, M.; Viitmaa, R.; Grönroos, T.J.; Johansson, J.; Bergamasco, L.; Snellman, M.; Metsähonkala, L. FDG-PET in healthy and epileptic lagotto romagnolo dogs and changes in brain glucose uptake with Age. Vet. Radiol. Ultrasound 2014, 55, 331–341. [Google Scholar] [CrossRef]
- Costantino, S.; Paneni, F.; Cosentino, F. Ageing, Metabolism and cardiovascular disease. J. Physiol. 2016, 594, 2061–2073. [Google Scholar] [CrossRef]
- Wintermark, M.; Lepori, D.; Cotting, J.; Roulet, E.; Van Melle, G.; Meuli, R.; Maeder, P.; Regli, L.; Verdun, F.R.; Deonna, T.; et al. Brain perfusion in children: Evolution with age assessed by quantitative perfusion computed tomography. Pediatrics 2004, 113, 1642–1652. [Google Scholar] [CrossRef]
- Petit-Taboué, M.C.; Landeau, B.; Desson, J.F.; Desgranges, B.; Baron, J.C. Effects of healthy aging on the regional cerebral metabolic rate of glucose assessed with statistical parametric mapping. Neuroimage 1998, 7, 176–184. [Google Scholar] [CrossRef]
- Su, M.; Head, E.; Brooks, W.M.; Wang, Z.; Muggenburg, B.A.; Adam, G.E.; Sutherland, R.; Cotman, C.W.; Nalcioglu, O. Magnetic resonance imaging of anatomic and vascular characteristics in a canine model of human aging. Neurobiol. Aging 1998, 19, 479–485. [Google Scholar] [CrossRef]
- Catafau, A.M.; Lomeña, F.J.; Pavia, J.; Parellada, E.; Bernardo, M.; Setoain, J.; Tolosa, E. Regional cerebral blood flow pattern in normal young and aged volunteers: A99mTc-HMPAO SPET study. Eur. J. Nucl. Med. 1996, 23, 1329–1337. [Google Scholar] [CrossRef]
- Campbell, B.C.V.; Christensen, S.; Levi, C.R.; Desmond, P.M.; Donnan, G.A.; Davis, S.M.; Parsons, M.W. Cerebral blood flow is the optimal CT perfusion parameter for assessing infarct core. Stroke 2011, 42, 3435–3440. [Google Scholar] [CrossRef]
- Boutelier, T.; Kudo, K.; Pautot, F.; Sasaki, M. Bayesian hemodynamic parameter estimation by bolus tracking perfusion weighted imaging. IEEE Trans. Med. Imaging 2012, 31, 1381–1395. [Google Scholar] [CrossRef]
- Eastwood, J.D.; Lev, M.H.; Azhari, T.; Lee, T.-Y.; Barboriak, D.P.; Delong, D.M.; Fitzek, C.; Herzau, M.; Wintermark, M.; Meuli, R.; et al. CT perfusion scanning with deconvolution analysis: Pilot study in patients with acute middle cerebral artery stroke. Radiology 2002, 222, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Wintermark, M.; Reichhart, M.; Thiran, J.-P.; Maeder, P.; Chalaron, M.; Schnyder, P.; Bogousslavsky, J.; Meuli, R. Prognostic accuracy of cerebral blood flow measurement by perfusion computed tomography, at the time of emergency room admission, in acute stroke patients. Ann. Neurol. 2002, 51, 417–432. [Google Scholar] [CrossRef]
- Roberts, H.C.; Roberts, T.P.L.; Lee, T.-Y.; Dillon, W.P. Dynamic, contrast-enhanced CT of human brain tumors: Quantitative assessment of blood volume, blood flow, and microvascular permeability: Report of two cases. Am. J. Neuroradiol. 2002, 23, 828–832. [Google Scholar] [PubMed]
- Jain, R. Perfusion CT imaging of brain tumors: An overview. Am. J. Neuroradiol. 2011, 32, 1570–1577. [Google Scholar] [CrossRef] [PubMed]
- Zwingenberger, A.L.; Pollard, R.E.; Taylor, S.L.; Chen, R.X.; Nunley, J.; Kent, M.S. Perfusion and volume response of canine brain tumors to stereotactic radiosurgery and radiotherapy. J. Vet. Intern. Med. 2016, 30, 827–835. [Google Scholar] [CrossRef]
- Peterson, K.L.; MacLeod, A.G.; Wisner, E.R.; Larson, R.F.; Pollard, R.E. Quantitative assessment of blood volume, blood flow, and permeability of the brain of clinically normal dogs by use of dynamic contrast-enhanced computed tomography. Am. J. Vet. Res. 2008, 69, 45–50. [Google Scholar] [CrossRef]
- Zhao, Q.; Lee, S.; Kent, M.; Schatzberg, S.; Platt, S. Dynamic contrast-enhanced magnetic resonance imaging of canine brain tumors. Vet. Radiol. Ultrasound 2010, 51, 122–129. [Google Scholar] [CrossRef]
- MacLeod, A.G.; Dickinson, P.J.; LeCouteur, R.A.; Higgins, R.J.; Pollard, R.E. Quantitative assessment of blood volume and permeability in cerebral mass lesions using dynamic contrast-enhanced computed tomography in the dog. Acad. Radiol. 2009, 16, 1187–1195. [Google Scholar] [CrossRef]
- Fantini, S.; Sassaroli, A.; Tgavalekos, K.T.; Kornbluth, J. Cerebral blood flow and autoregulation: Current measurement techniques and prospects for noninvasive optical methods. Neurophotonics 2016, 3, 031411. [Google Scholar] [CrossRef] [PubMed]
- Detre, J.A.; Leigh, J.S.; Williams, D.S.; Koretsky, A.P. Perfusion imaging. Magn. Reson. Med. 1992, 23, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Hendrich, K.S.; Kochanek, P.M.; Melick, J.A.; Schiding, J.K.; Statler, K.D.; Williams, D.S.; Marion, D.W.; Ho, C. Cerebral perfusion during anesthesia with fentanyl, isoflurane, or pentobarbital in normal rats studied by arterial spin-labeled MRI. Magn. Reson. Med. 2001, 46, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Matta, B.F.; Heath, K.J.; Tipping, K.; Summors, A.C. Direct cerebral vasodilatory effects of sevoflurane and isoflurane. Anesthesiology 1999, 91, 677–680. [Google Scholar] [CrossRef]
- Martin, A.J.; Friston, K.J.; Colebatch, J.G.; Frackowiak, R.S. Decreases in regional cerebral blood flow with normal aging. J. Cereb Blood. Flow. Metab. 1991, 11, 684–689. [Google Scholar] [CrossRef]
- Jefferson, A.L.; Cambronero, F.E.; Liu, D.; Moore, E.E.; Neal, J.E.; Terry, J.G.; Nair, S.; Pechman, K.R.; Rane, S.; Davis, L.T.; et al. Higher aortic stiffness is related to lower cerebral blood flow and preserved cerebrovascular reactivity in older adults. Circulation 2018, 138, 1951–1962. [Google Scholar] [CrossRef]
- Shin, W.; Horowitz, S.; Ragin, A.; Chen, Y.; Walker, M.; Carroll, T.J. Quantitative cerebral perfusion using dynamic susceptibility contrast MRI: Evaluation of reproducibility and age- and gender-dependence with fully automatic image postprocessing algorithm. Magn. Reson. Med. 2007, 58, 1232–1241. [Google Scholar] [CrossRef]
- Creevy, K.E.; Grady, J.; Little, S.E.; Moore, G.E.; Groetzinger Strickler, B.; Thompson, S.; Webb, J.A. 2019 AAHA Canine life stage guidelines. J. Am. Anim. Hosp. Assoc. 2019, 55, 267–290. [Google Scholar] [CrossRef]
- London, E.D.; Ohata, M.; Takei, H.; French, A.W.; Rapoport, S.I. Regional cerebral metabolic rate for glucose in beagle dogs of different ages. Neurobiol. Aging 1983, 4, 121–126. [Google Scholar] [CrossRef]
- Martlé, V.; Peremans, K.; Van Ham, L.; Vermeire, S.; Waelbers, T.; Dobbeleir, A.; Gielen, I.; Boon, P.; Claes, K.; Bhatti, S. High-resolution micro-SPECT to evaluate the regional brain perfusion in the adult beagle dog. Res. Vet. Sci. 2013, 94, 701–706. [Google Scholar] [CrossRef]
- Park, S.; Jang, M.; Lee, K.; Choi, H.; Lee, Y.; Park, I.; Choi, S. Optimal placement of the region of interest for bolus tracking on brain computed tomography angiography in beagle dogs. J. Vet. Med. Sci. 2021, 83, 1196–1201. [Google Scholar] [CrossRef] [PubMed]
- Cenic, A.; Nabavi, D.G.; Craen, R.A.; Gelb, A.W.; Lee, T.Y. Dynamic CT measurement of cerebral blood flow: A validation study. Am. J. Neuroradiol. 1999, 20, 63–73. [Google Scholar] [PubMed]
| ROI (mm²) | HU Value | rCBV (mL/100 g) | rCBF (mL/100 g/min) | MTT (s) | TTP (s) | |
|---|---|---|---|---|---|---|
| Frontal white matter | 1.59 ± 0.39 | 44.2 ± 6.95 | 9.63 ± 2.48 | 118.64 ± 27.46 | 4.35 ± 1.15 | 22.97 ± 5.85 |
| Frontal gray matter | 2.53 ± 0.56 | 61.93 ± 12.42 | 13.11 ± 3.42 | 142.27 ± 14.09 | 4.03 ± 0.71 | 22.33 ± 5.44 |
| Temporal white matter | 1.20 ± 0.21 | 43.62 ± 6.95 | 9.54 ± 2.57 | 117.53 ± 28.06 | 4.32 ± 1.11 | 22.98 ± 5.85 |
| Temporal gray matter | 1.72 ± 0.56 | 57.27 ± 10.05 | 12.94 ± 3.2 | 141.43 ± 14.13 | 4.11 ± 0.75 | 22.47 ± 5.46 |
| Parietal white matter | 0.91 ± 0.15 | 41.61 ± 6.69 | 8.67 ± 2.72 | 102.77 ± 34.23 | 5.27 ± 1.8 | 23.72 ± 6.38 |
| Parietal gray matter | 1.27 ± 0.19 | 63.38 ± 8.9 | 13.58 ± 3.21 | 141.93 ± 14.5 | 4.19 ± 0.73 | 22.44 ± 5.52 |
| Occipital white matter | 1.66 ± 0.32 | 44.8 ± 5.65 | 9.04 ± 2.71 | 108.84 ± 33.08 | 5.03 ±1.61 | 23.48 ± 6.18 |
| Occipital gray matter | 2.36 ± 0.72 | 66.51 ± 9.69 | 13.41 ± 3.64 | 140.46 ± 16.21 | 4.26 ± 0.8 | 22.31 ± 5.39 |
| Caudate nucleus | 0.28 ± 0.04 | 42.96 ± 11.78 | 10.72 ± 3.29 | 108.49 ± 22.25 | 6.15 ± 1.84 | 23.89 ± 5.97 |
| Thalamus | 0.68 ± 0.13 | 50.47 ± 8.32 | 12.98 ± 3.1 | 136.37 ± 18.61 | 4.46 ± 0.94 | 22.79 ± 5.63 |
| Piriform lobe | 1.63 ± 0.37 | 56.31 ± 7.35 | 12.48 ± 3.06 | 138.27 ± 17.83 | 4.11 ± 0.77 | 22.51 ± 5.52 |
| Hippocampus | 1.04 ± 0.15 | 55.87 ± 6.16 | 13.19 ± 2.49 | 139.44 ± 13.53 | 4.36 ± 0.77 | 22.7 ± 5.51 |
| Cerebellum | 1.81 ± 0.35 | 56.26 ± 6.1 | 13.22 ± 3.18 | 137.71 ± 20.82 | 4.37 ± 0.87 | 22.73 ± 5.66 |
| Frontal White vs. Gray Matter | Temporal White vs. Gray Matter | Parietal White vs. Gray Matter | Occipital White vs. Gray Matter | |
|---|---|---|---|---|
| rCBV (mL/100 g) | 0.003 | 0.003 | <0.0001 | 0.001 |
| rCBF (mL/100 g/min) | <0.0001 | 0.001 | <0.0001 | 0.001 |
| MTT (s) | 0.962 | 0.788 | 0.159 | 0.079 |
| TTP (s) | 0.506 | 0.506 | 0.547 | 0.447 |
| ROI (mm²) | HU value | rCBV (mL/100 g) | rCBF (mL/100 g/min) | MTT (s) | TTP (s) | ||
|---|---|---|---|---|---|---|---|
| Frontal white matter | Young | 1.78 ± 0.33 | 46.53 ± 1.35 | 10.48 ± 2.64 | 120.96 ± 26.95 | 4.45 ± 1.09 | 24.26 ± 5.63 |
| Old | 1.44 ± 0.39 | 42.34 ± 9.02 | 8.96 ± 2.25 | 116.79 ± 29.18 | 4.27 ± 1.25 | 21.93 ± 6.1 | |
| p value | 0.021 | 0.515 | 0.315 | 0.762 | 0.762 | 0.633 | |
| Frontal gray matter | Young | 2.9 ± 0.51 | 64.49 ± 2.14 | 14.21 ± 3.37 | 144.45 ± 9.75 | 4.14 ± 0.58 | 23.45 ± 5.28 |
| Old | 2.24 ± 0.42 | 59.89 ± 16.65 | 12.22 ± 3.36 | 140.53 ± 17.13 | 3.95 ± 0.83 | 21.43 ± 5.67 | |
| p value | 0.021 | 0.515 | 0.203 | 0.237 | 0.696 | 0.696 | |
| Temporal white matter | Young | 1.28 ± 0.19 | 46.01 ± 1.96 | 10.25 ± 2.69 | 120.48 ± 27.93 | 4.38 ± 1.04 | 24.28 ± 5.64 |
| Old | 1.14 ± 0.21 | 41.71 ± 8.9 | 8.97 ± 2.46 | 115.17 ± 29.44 | 4.28 ± 1.22 | 21.94 ± 6.09 | |
| p value | 0.122 | 0.315 | 0.573 | 0.696 | 0.762 | 0.762 | |
| Temporal gray matter | Young | 2.18 ± 0.51 | 62.49 ± 4.37 | 14.0 ± 3.41 | 143.34 ± 9.97 | 4.24 ± 0.64 | 23.64 ± 5.26 |
| Old | 1.36 ± 0.25 | 53.1 ± 11.51 | 12.1 ± 2.91 | 139.9 ± 17.15 | 4.01 ± 0.85 | 21.53 ± 5.71 | |
| p value | 0.001 | 0.203 | 0.237 | 0.762 | 0.633 | 0.696 | |
| Parietal white matter | Young | 0.95 ± 0.18 | 44.28 ± 3.77 | 9.61 ± 3.08 | 108.34 ± 33.25 | 5.16 ± 1.48 | 24.81 ± 6.09 |
| Old | 0.88 ± 0.12 | 39.48 ± 7.88 | 7.92 ± 2.28 | 98.32 ± 36.11 | 22.84 ± 6.79 | 22.84 ± 6.79 | |
| p value | 0.315 | 0.237 | 0.408 | 0.460 | 0.965 | 0.762 | |
| Parietal gray matter | Young | 1.4 ± 0.13 | 66.61 ± 3.51 | 14.69 ± 3.31 | 144.3 ± 9.61 | 4.33 ± 0.7 | 23.65 ± 5.34 |
| Old | 1.16 ± 0.16 | 60.79 ± 11.11 | 12.69 ± 2.99 | 140.03 ± 17.79 | 4.09 ± 0.77 | 21.48 ± 5.75 | |
| p value | 0.006 | 0.360 | 0.315 | 0.762 | 0.408 | 0.696 | |
| Occipital white matter | Young | 1.83 ± 0.09 | 46.29 ± 1.43 | 10.11 ± 2.81 | 112.59 ± 29.72 | 5.18 ± 1.1 | 24.71 ± 5.78 |
| Old | 1.52 ± 0.37 | 43.61 ± 7.43 | 8.19 ± 2.42 | 105.85 ± 36.84 | 4.92 ± 1.98 | 22.5 ± 6.62 | |
| p value | 0.034 | 0.515 | 0.203 | 0.897 | 1.000 | 0.762 | |
| Occipital gray matter | Young | 2.48 ± 0.71 | 71.95 ± 5.41 | 15.06 ± 3.75 | 143.94 ± 9.78 | 4.54 ± 0.69 | 23.76 ± 5.46 |
| Old | 2.26 ± 0.75 | 62.16 ± 10.36 | 12.08 ± 3.1 | 137.68 ± 20.06 | 4.04 ± 0.85 | 24.65 ± 5.7 | |
| p value | 0.173 | 0.034 | 0.173 | 0.360 | 0.173 | 0.633 | |
| Caudate nucleus | Young | 0.3 ± 0.0 | 38.7 ± 6.85 | 9.61 ± 1.36 | 102.94 ± 22.76 | 6.4 ± 1.66 | 25.41 ± 5.4 |
| Old | 0.26 ± 0.05 | 46.36 ± 14.02 | 11.61 ± 4.13 | 112.93 ± 21.98 | 5.95 ± 2.04 | 22.68 ± 6.4 | |
| p value | 0.173 | 0.315 | 0.146 | 0.573 | 0.573 | 0.633 | |
| Thalamus | Young | 0.68 ± 0.16 | 50.79 ± 3.7 | 13.33 ± 3.37 | 136.64 ± 15.92 | 4.46 ± 0.65 | 23.98 ± 5.33 |
| Old | 0.68 ± 0.1 | 50.22 ± 10.95 | 12.71 ± 3.02 | 136.16 ± 21.37 | 4.45 ± 1.15 | 21.84 ± 5.96 | |
| p value | 0.762 | 0.897 | 0.696 | 0.762 | 0.829 | 0.515 | |
| Piriform lobe | Young | 1.58 ± 0.27 | 57.6 ± 2.3 | 13.24 ± 3.39 | 139.21 ± 15.93 | 4.23 ± 0.64 | 23.74 ± 5.35 |
| Old | 1.68 ± 0.44 | 55.27 ± 9.76 | 11.88 ± 2.8 | 137.52 ± 20.04 | 4.02 ± 0.88 | 21.53 ± 5.74 | |
| p value | 0.515 | 0.696 | 0.408 | 0.897 | 0.829 | 0.633 | |
| Hippocampus | Young | 1.1 ± 0.13 | 55.3 ± 4.48 | 13.7 ± 2.23 | 140.66 ± 9.35 | 4.5 ± 0.75 | 24.0 ± 5.16 |
| Old | 1.0 ± 0.15 | 56.33 ± 7.45 | 12.79 ± 2.65 | 138.47 ± 16.59 | 4.25 ± 0.82 | 21.66 ± 5.83 | |
| p value | 0.237 | 0.573 | 0.633 | 0.633 | 0.573 | 0.762 | |
| Cerebellum | Young | 1.83 ± 0.21 | 56.35 ± 1.65 | 14.16 ± 3.43 | 139.51 ± 18.9 | 4.43 ± 0.76 | 23.98 ± 5.47 |
| Old | 1.8 ± 0.44 | 56.19 ± 8.26 | 12.46 ± 2.92 | 136.26 ± 23.15 | 4.33 ± 0.99 | 21.73 ± 5.9 | |
| p value | 0.633 | 0.965 | 0.315 | 0.633 | 0.762 | 0.762 |
| ROI (mm²) | HU value | rCBV (mL/100 g) | rCBF (mL/100 g/min) | MTT (s) | TTP (s) | ||
|---|---|---|---|---|---|---|---|
| Frontal white matter | Right | 1.59 ± 0.4 | 44.49 ± 7.04 | 9.61 ± 2.45 | 119.03 ± 27.7 | 4.36 ± 1.17 | 22.96 ± 5.98 |
| Left | 1.59 ± 0.4 | 43.91 ± 7.29 | 9.66 ± 2.65 | 118.26 ± 28.9 | 4.34 ± 1.2 | 22.98 ± 6.07 | |
| p value | 1.000 | 0.931 | 0.931 | 1.000 | 1.000 | 1.000 | |
| Frontal gray matter | Right | 2.53 ± 0.58 | 62.37 ± 12.69 | 13.06 ± 3.42 | 142.41 ± 14.42 | 4.03 ± 0.71 | 22.38 ± 5.59 |
| Left | 2.53 ± 0.58 | 61.5 ± 12.89 | 13.16 ± 3.63 | 142.13 ± 14.62 | 4.03 ± 0.76 | 22.28 ± 5.62 | |
| p value | 1.000 | 0.931 | 0.863 | 0.863 | 1.000 | 0.931 | |
| Temporal white matter | Right | 1.2 ± 0.21 | 43.74 ± 7.2 | 9.6 ± 2.81 | 117.14 ± 29.8 | 4.38 ± 1.16 | 22.98 ± 6.05 |
| Left | 1.2 ± 0.21 | 43.5 ± 7.13 | 9.48 ± 2.49 | 117.91 ± 28.01 | 4.27 ± 1.13 | 22.98 ± 6.0 | |
| p value | 1.000 | 1.000 | 1.000 | 1.000 | 0.796 | 1.000 | |
| Temporal gray matter | Right | 1.72 ± 0.58 | 57.21 ± 10.24 | 12.9 ± 3.4 | 140.43 ± 14.73 | 4.17 ± 0.8 | 22.48 ± 5.67 |
| Left | 1.72 ± 0.58 | 57.33 ± 10.48 | 12.99 ± 3.18 | 142.42 ± 14.32 | 4.06 ± 0.74 | 22.46 ± 5.58 | |
| p value | 1.000 | 1.000 | 1.000 | 0.666 | 0.489 | 0.931 | |
| Parietal white matter | Right | 0.91 ± 0.15 | 41.98 ± 7.25 | 8.92 ± 3.0 | 102.9 ± 34.87 | 5.33 ± 1.79 | 23.7 ± 6.59 |
| Left | 0.91 ± 0.15 | 41.24 ± 6.49 | 8.42 ± 2.58 | 102.64 ± 35.69 | 5.21 ± 1.92 | 23.73 ± 6.57 | |
| p value | 1.000 | 0.730 | 0.605 | 0.863 | 1.000 | 1.000 | |
| Parietal gray matter | Right | 1.27 ± 0.19 | 63.29 ± 9.43 | 13.64 ± 3.33 | 142.02 ± 14.04 | 4.21 ± 0.75 | 22.44 ± 5.7 |
| Left | 1.27 ± 0.19 | 63.47 ± 8.92 | 13.51 ± 3.28 | 141.83 ± 15.8 | 4.18 ± 0.74 | 22.44 ± 5.69 | |
| p value | 1.000 | 0.796 | 0.796 | 0.863 | 0.863 | 1.000 | |
| Occipital white matter | Right | 1.66 ± 0.33 | 44.73 ± 5.44 | 9.2 ± 2.63 | 108.8 ± 32.41 | 5.04 ± 1.58 | 23.44 ± 6.34 |
| Left | 1.66 ± 0.33 | 44.87 ± 6.18 | 8.89 ± 2.93 | 108.89 ± 35.71 | 5.02 ± 1.73 | 23.52 ± 6.4 | |
| p value | 1.000 | 0.863 | 0.730 | 1.000 | 1.000 | 1.000 | |
| Occipital gray matter | Right | 2.36 ± 0.74 | 65.93 ± 10.6 | 13.24 ± 3.85 | 139.66 ± 16.6 | 4.3 ± 0.85 | 22.56 ± 5.77 |
| Left | 2.36 ± 0.74 | 67.09 ± 9.29 | 13.57 ± 3.63 | 141.27 ± 16.77 | 4.22 ± 0.81 | 22.06 ± 5.32 | |
| p value | 1.000 | 0.666 | 0.931 | 0.730 | 0.666 | 0.730 | |
| Caudate nucleus | Right | 0.28 ± 0.04 | 42.56 ± 13.22 | 10.51 ± 3.52 | 105.72 ± 24.55 | 6.29 ± 1.98 | 23.93 ± 6.21 |
| Left | 0.28 ± 0.04 | 43.36 ± 10.93 | 10.93 ± 3.24 | 111.26 ± 20.78 | 6.01 ± 1.8 | 23.86 ± 6.1 | |
| p value | 1.000 | 0.796 | 0.863 | 0.546 | 0.863 | 0.863 | |
| Thalamus | Right | 0.68 ± 0.13 | 49.81 ± 8.27 | 12.66 ± 3.12 | 135.21 ± 18.3 | 4.4 ± 0.77 | 22.74 ± 5.73 |
| Left | 0.68 ± 0.13 | 51.13 ± 8.81 | 13.31 ± 3.24 | 137.53 ± 19.94 | 4.51 ± 1.12 | 22.83 ± 5.87 | |
| p value | 1.000 | 0.796 | 0.666 | 0.387 | 1.000 | 0.796 | |
| Piriform lobe | Right | 1.7 ± 0.36 | 56.18 ± 7.97 | 12.37 ± 3.18 | 137.29 ± 19.92 | 4.12 ± 0.81 | 22.53 ± 5.74 |
| Left | 1.7 ± 0.36 | 56.43 ± 7.16 | 12.6 ± 3.12 | 139.26 ± 16.63 | 4.1 ± 0.77 | 22.49 ± 5.64 | |
| p value | 0.489 | 0.863 | 0.796 | 0.931 | 0.863 | 0.931 | |
| Hippocampus | Right | 1.04 ± 0.15 | 55.27 ± 6.36 | 12.91 ± 2.44 | 137.07 ± 14.29 | 4.43 ± 0.86 | 22.79 ± 5.69 |
| Left | 1.04 ± 0.15 | 56.48 ± 6.27 | 13.48 ± 2.65 | 141.82 ± 13.12 | 4.29 ± 0.72 | 22.61 ± 5.67 | |
| p value | 1.000 | 0.666 | 0.666 | 0.340 | 0.666 | 0.730 | |
| Cerebellum | Right | 1.81 ± 0.36 | 55.88 ± 6.26 | 13.19 ± 3.26 | 136.97 ± 23.08 | 4.39 ± 0.92 | 22.74 ± 5.86 |
| Left | 1.81 ± 0.36 | 56.64 ± 6.3 | 13.24 ± 3.3 | 138.44 ± 19.67 | 4.36 ± 0.87 | 22.71 ± 5.82 | |
| p value | 1.000 | 0.666 | 0.931 | 0.931 | 0.931 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, S.; Hwang, G.; Noh, S.A.; Lee, H.C.; Hwang, T.S. Quantitative Analysis of Brain CT Perfusion in Healthy Beagle Dogs: A Pilot Study. Vet. Sci. 2023, 10, 469. https://doi.org/10.3390/vetsci10070469
An S, Hwang G, Noh SA, Lee HC, Hwang TS. Quantitative Analysis of Brain CT Perfusion in Healthy Beagle Dogs: A Pilot Study. Veterinary Sciences. 2023; 10(7):469. https://doi.org/10.3390/vetsci10070469
Chicago/Turabian StyleAn, Soyon, Gunha Hwang, Seul Ah Noh, Hee Chun Lee, and Tae Sung Hwang. 2023. "Quantitative Analysis of Brain CT Perfusion in Healthy Beagle Dogs: A Pilot Study" Veterinary Sciences 10, no. 7: 469. https://doi.org/10.3390/vetsci10070469
APA StyleAn, S., Hwang, G., Noh, S. A., Lee, H. C., & Hwang, T. S. (2023). Quantitative Analysis of Brain CT Perfusion in Healthy Beagle Dogs: A Pilot Study. Veterinary Sciences, 10(7), 469. https://doi.org/10.3390/vetsci10070469






