Investigation of Trehalose Supplementation Impacting Campylobacter jejuni and Clostridium perfringens from Broiler Farming
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Treatments
2.2. Preparation of C. jejuni, C. perfringens and L. johnsonii
2.3. Experiment 1: The Tolerance Test of Trehalose on Broilers
2.4. Experiment 2-1: The Antibacterial Tests of Trehalose on C. jejuni
2.5. Experiment 2-2: The Antibacterial Tests of Trehalose on C. perfringens
2.6. Sample Collection
2.7. Isolation and Identification of Bacteria from Chyme and Feces by Real-Time PCR
2.8. Statistical Analysis
3. Results
3.1. Experiment 1: The Tolerance Test of Trehalose on Broilers
3.2. Experiment 2-1: The Antibacterial Tests of Trehalose on C. jejuni
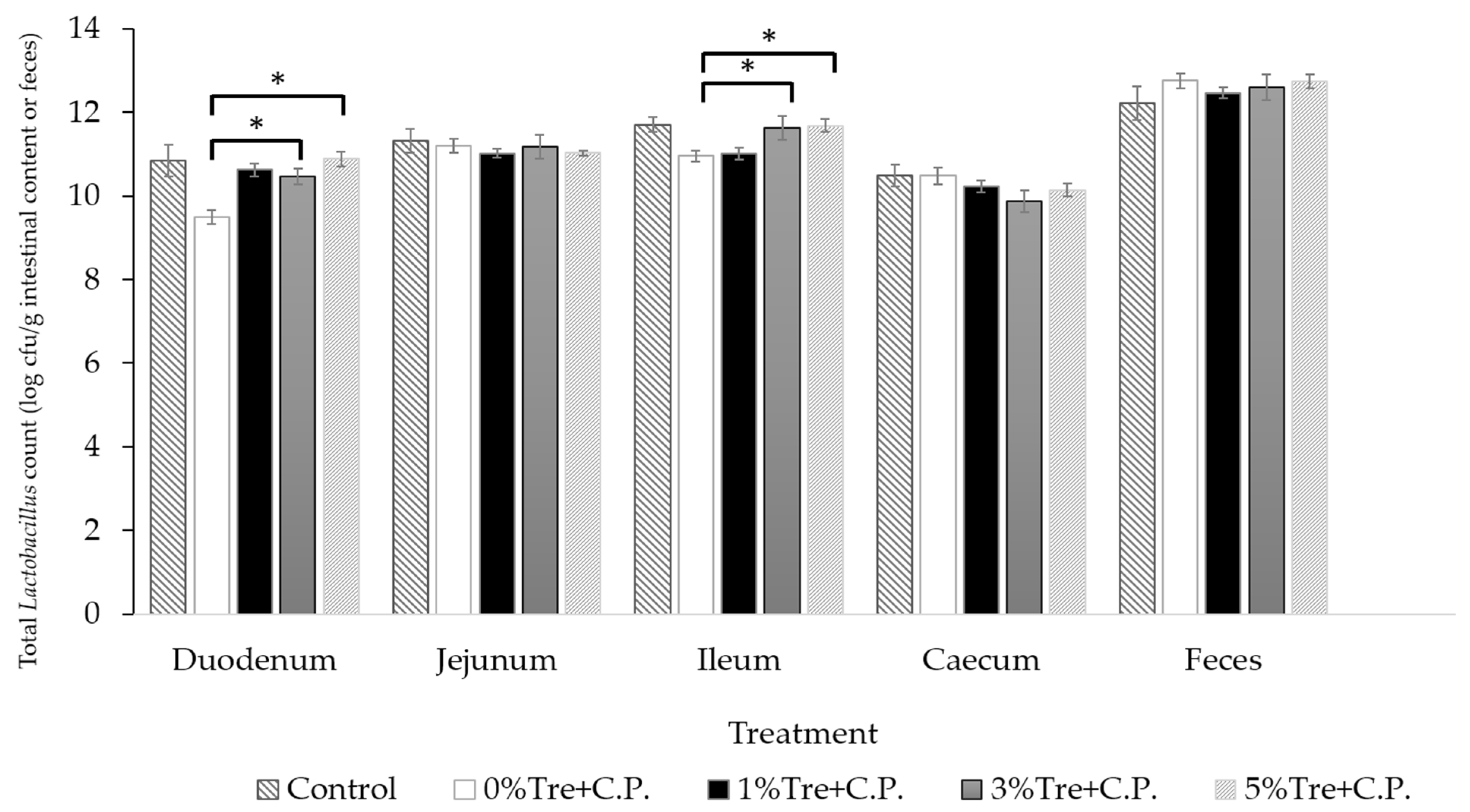
3.3. Experiment 2-2: The Antibacterial Tests of Trehalose on C. perfringens
4. Discussion
4.1. Experiment 1: The Tolerance Test of Trehalose on Broilers
4.2. Experiment 2: The Antibacterial Tests of Trehalose on C. jejuni and C. perfringens
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campylobacter. Available online: https://www.who.int/news-room/fact-sheets/detail/campylobacter (accessed on 1 May 2020).
- Wang, X.; Zhuo, Q.; Hong, Y.; Wu, Y.; Gu, Q.; Yuan, D.; Dong, Q.; Shao, J. Correlation between multilocus sequence typing and antibiotic resistance, virulence potential of Campylobacter jejuni isolates from poultry meat. Foods 2022, 11, 1768. [Google Scholar] [CrossRef]
- Popa, S.A.; Morar, A.; Ban-Cucerzan, A.; Tirziu, E.; Herman, V.; Sallam, K.I.; Morar, D.; Acaroz, U.; Imre, M.; Florea, T.; et al. Occurrence of Campylobacter spp. and phenotypic antimicrobial resistance profiles of Campylobacter jejuni in slaughtered broiler chickens in north-western Romania. Antibiotics 2022, 11, 1713. [Google Scholar] [CrossRef]
- Lima, L.M.P.G.; Borges, K.A.; Furian, T.Q.; Salle, C.T.P.; Moraes, H.L.D.; do Nascimento, V. Prevalence and distribution of pathogenic genes in Campylobacter jejuni isolated from poultry and human sources. J. Infect. Dev. Ctries. 2022, 16, 1466–1472. [Google Scholar] [CrossRef] [PubMed]
- Bendary, M.M.; Abd El-Hamid, M.I.; El-Tarabili, R.M.; Hefny, A.A.; Algendy, R.M.; Elzohairy, N.A.; Ghoneim, M.M.; Al-Sanea, M.M.; Nahari, M.H.; Moustafa, W.H. Clostridium perfringens associated with foodborne infections of animal origins: Insights into prevalence, antimicrobial resistance, toxin genes profiles, and toxinotypes. Biology 2022, 11, 551. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Konkel, M.E.; Lu, X. Antimicrobial resistance gene transfer from Campylobacter jejuni in mono- and dual-species biofilms. Appl. Environ. Microbiol. 2021, 87, e0065921. [Google Scholar] [CrossRef] [PubMed]
- Anju, K.; Karthik, K.; Divya, V.; Mala Priyadharshini, M.L.; Sharma, R.K.; Manoharan, S. Toxinotyping and molecular characterization of antimicrobial resistance in Clostridium perfringens isolated from different sources of livestock and poultry. Anaerobe 2021, 67, 102298. [Google Scholar] [CrossRef]
- Mora, Z.V.; Macías-Rodríguez, M.E.; Arratia-Quijada, J.; Gonzalez-Torres, Y.S.; Nuño, K.; Villarruel-López, A. Clostridium perfringens as foodborne pathogen in broiler production: Pathophysiology and potential strategies for controlling necrotic enteritis. Animals 2020, 10, 1718. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2016. EFSA J. 2017, 15, e05077. [Google Scholar] [CrossRef]
- Hermans, D.; Van Deun, K.; Messens, W.; Martel, A.; Van Immerseel, F.; Haesebrouck, F.; Rasschaert, G.; Heyndrickx, M.; Pasmans, F. Campylobacter control in poultry by current intervention measures ineffective: Urgent need for intensified fundamental research. Vet. Microbiol. 2011, 152, 219–228. [Google Scholar] [CrossRef]
- Stern, N.J.; Cox, N.A.; Musgrove, M.T.; Park, C.M. Incidence and levels of Campylobacter in broilers after exposure to an inoculated seeder bird. J. Appl. Poult. Res. 2001, 10, 315–318. [Google Scholar] [CrossRef]
- Al Hakeem, W.G.; Fathima, S.; Shanmugasundaram, R.; Selvaraj, R.K. Campylobacter jejuni in poultry: Pathogenesis and control strategies. Microorganisms 2022, 10, 2134. [Google Scholar] [CrossRef]
- Taha-Abdelaziz, K.; Singh, M.; Sharif, S.; Sharma, S.; Kulkarni, R.R.; Alizadeh, M.; Yitbarek, A.; Helmy, Y.A. Intervention strategies to control Campylobacter at different stages of the food chain. Microorganisms 2023, 11, 113. [Google Scholar] [CrossRef]
- Al-Surrayai, T.; Al-Khalaifah, H. Dietary supplementation of fructooligosaccharides enhanced antioxidant activity and cellular immune response in broiler chickens. Front. Vet. Sci. 2022, 9, 857294. [Google Scholar] [CrossRef]
- Smialek, M.; Kowalczyk, J.; Koncicki, A. The use of probiotics in the reduction of Campylobacter spp. prevalence in poultry. Animals 2021, 11, 1355. [Google Scholar] [CrossRef]
- Taha-Abdelaziz, K.; Astill, J.; Kulkarni, R.R.; Read, L.R.; Najarian, A.; Farber, J.M.; Sharif, S. In vitro assessment of immunomodulatory and anti-Campylobacter activities of probiotic lactobacilli. Sci. Rep. 2019, 9, 17903. [Google Scholar] [CrossRef]
- Saint-Cyr, M.J.; Haddad, N.; Taminiau, B.; Poezevara, T.; Quesne, S.; Amelot, M.; Daube, G.; Chemaly, M.; Dousset, X.; Guyard-Nicodeme, M. Use of the potential probiotic strain Lactobacillus salivarius SMXD51 to control Campylobacter jejuni in broilers. Int. J. Food Microbiol. 2017, 247, 9–17. [Google Scholar] [CrossRef]
- Manes-Lazaro, R.; Van Diemen, P.M.; Pin, C.; Mayer, M.J.; Stevens, M.P.; Narbad, A. Administration of Lactobacillus johnsonii FI9785 to chickens affects colonisation by Campylobacter jejuni and the intestinal microbiota. Br. Poult. Sci. 2017, 58, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Rana, E.A.; Nizami, T.A.; Islam, M.S.; Barua, H.; Islam, M.Z. Phenotypical identification and toxinotyping of Clostridium perfringens isolates from healthy and enteric disease-affected chickens. Vet. Med. Int. 2023, 2023, 2584171. [Google Scholar] [CrossRef]
- Mohiuddin, M.; Song, Z.F.; Liao, S.Q.; Qi, N.S.; Li, J.; Lv, M.; Lin, X.H.; Cai, H.M.; Hu, J.J.; Liu, S.B.; et al. Animal model studies, antibiotic resistance and toxin gene profile of NE reproducing Clostridium perfringens type A and type G strains isolated from commercial poultry farms in China. Microorganisms 2023, 11, 622. [Google Scholar] [CrossRef] [PubMed]
- Charlebois, A.; Parent, E.; Létourneau-Montminy, M.; Boulianne, M. Persistence of a Clostridium perfringens strain in a broiler chicken farm over a three-year period. Avian Dis. 2020, 64, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Fathima, S.; Hakeem, W.G.A.; Shanmugasundaram, R.; Selvaraj, R.K. Necrotic enteritis in broiler chickens: A review on the pathogen, pathogenesis, and prevention. Microorganisms 2022, 10, 1958. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Salem, H.M.; El-Tahan, A.M.; Soliman, M.M.; Youssef, G.B.A.; Taha, A.E.; Soliman, S.M.; Ahmed, A.E.; El-Kott, A.F.; et al. Alternatives to antibiotics for organic poultry production: Types, modes of action and impacts on bird’s health and production. Poult. Sci. 2022, 101, 101696. [Google Scholar] [CrossRef]
- Calik, A.; Omara, I.I.; White, M.B.; Evans, N.P.; Karnezos, T.P.; Dalloul, R.A. Dietary non-drug feed additive as an alternative for antibiotic growth promoters for broilers during a necrotic enteritis challenge. Microorganisms 2019, 7, 257. [Google Scholar] [CrossRef]
- Kim, G.B.; Seo, Y.M.; Kim, C.H.; Paik, I.K. Effect of dietary prebiotic supplementation on the performance, intestinal microflora, and immune response of broilers. Poult. Sci. 2011, 90, 75–82. [Google Scholar] [CrossRef]
- Ruvalcaba-Gómez, J.M.; Villagrán, Z.; Valdez-Alarcón, J.J.; Martínez-Núñez, M.; Gomez-Godínez, L.J.; Ruesga-Gutiérrez, E.; Anaya-Esparza, L.M.; Arteaga-Garibay, R.I.; Villarruel-López, A. Non-antibiotics strategies to control Salmonella infection in poultry. Animals 2022, 12, 102. [Google Scholar] [CrossRef]
- Arsene, M.M.J.; Davares, A.K.L.; Andreevna, S.L.; Vladimirovich, E.A.; Carime, B.Z.; Marouf, R.; Khelifi, I. The use of probiotics in animal feeding for safe production and as potential alternatives to antibiotics. Vet. World 2021, 14, 319–328. [Google Scholar] [CrossRef]
- Figueroa-Gonzalez, I.; Quijano, G.; Ramirez, G.; Cruz-Guerrero, A. Probiotics and prebiotics—Perspectives and challenges. J. Sci. Food Agric. 2011, 91, 1341–1348. [Google Scholar] [CrossRef]
- Wu, Y.T.; Yang, W.Y.; Samuel Wu, Y.H.; Chen, J.W.; Chen, Y.C. Modulations of growth performance, gut microbiota, and inflammatory cytokines by trehalose on Salmonella Typhimurium-challenged broilers. Poult. Sci. 2020, 99, 4034–4043. [Google Scholar] [CrossRef] [PubMed]
- Ruangpanit, Y.; Matsushita, K.; Mukai, K.; Kikusato, M. Effect of trehalose supplementation on growth performance and intestinal morphology in broiler chickens. Vet. Anim. Sci. 2020, 10, 100142. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Srionnual, S.; Onda, T.; Yanagida, F. Effects of prebiotic oligosaccharides and trehalose on growth and production of bacteriocins by lactic acid bacteria. Lett. Appl. Microbiol. 2007, 45, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Chotinsky, D.; Toncheva, E.; Profirov, Y. Development of disaccharidase activity in the small intestine of broiler chickens. Br. Poult. Sci. 2001, 42, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Olkowski, A.A.; Wojnarowicz, C.; Chirino-Trejo, M.; Drew, M.D. Responses of broiler chickens orally challenged with Clostridium perfringens isolated from field cases of necrotic enteritis. Res. Vet. Sci. 2006, 81, 99–108. [Google Scholar] [CrossRef]
- Line, J.; Hiett, K.; Conlan, A. Comparison of challenge models for determining the colonization dose of Campylobacter jejuni in broiler chicks. Poult. Sci. 2008, 87, 1700–1706. [Google Scholar] [CrossRef] [PubMed]
- Sails, A.D.; Fox, A.J.; Bolton, F.J.; Wareing, D.R.; Greenway, D.L. A real-time PCR assay for the detection of Campylobacter jejuni in foods after enrichment culture. Appl. Environ. Microbiol. 2003, 69, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.F.; Cao, W.W.; Franklin, W.; Campbell, W.; Cerniglia, C.E. A 16S rDNA-based PCR method for rapid and specific detection of Clostridium perfringens in food. Mol. Cell. Probes 1994, 8, 131–137. [Google Scholar] [CrossRef]
- Haarman, M.; Knol, J. Quantitative real-time PCR analysis of fecal Lactobacillus species in infants receiving a prebiotic infant formula. Appl. Environ. Microbiol. 2006, 72, 2359–2365. [Google Scholar] [CrossRef]
- Hond, E.D.; Geypens, B.; Ghoos, Y. Effect of high performance chicory inulin on constipation. Nutr. Res. 2000, 20, 731–736. [Google Scholar] [CrossRef]
- Savaiano, D.A. Lactose digestion from yogurt: Mechanism and relevance. Am. J. Clin. Nutr. 2014, 99, 1251S–1255S. [Google Scholar] [CrossRef]
- Hermans, D.; Van Deun, K.; Messens, W.; Martel, A.; Van Immerseel, F.; Haesebrouck, F.; Pasmans, F. Colonization factors of Campylobacter jejuni in the chicken gut. Vet. Res. 2011, 42, 82. [Google Scholar] [CrossRef]
- Khan, S.; Moore, R.J.; Stanley, D.; Chousalkar, K.K. The gut microbiota of laying hens and its manipulation with prebiotics and probiotics to enhance gut health and food safety. Appl. Environ. Microbiol. 2020, 86, e00600-20. [Google Scholar] [CrossRef]
- Bereswill, S.; Ekmekciu, I.; Escher, U.; Fiebiger, U.; Stingl, K.; Heimesaat, M.M. Lactobacillus johnsonii ameliorates intestinal, extra-intestinal and systemic pro-inflammatory immune responses following murine Campylobacter jejuni infection. Sci. Rep. 2017, 7, 2138. [Google Scholar] [CrossRef]
- Thomrongsuwannakij, T.; Chuanchuen, R.; Chansiripornchai, N. Identification of competitive exclusion and its ability to protect against Campylobacter jejuni in broilers. Thai J. Vet. Med. 2016, 46, 279–286. [Google Scholar]
- Arsi, K.; Donoghue, A.M.; Woo-Ming, A.; Blore, P.J.; Donoghue, D.J. The efficacy of selected probiotic and prebiotic combinations in reducing Campylobacter colonization in broiler chickens. J. Appl. Poultry Res. 2015, 24, 327–334. [Google Scholar] [CrossRef]
- Long, J.R.; Pettit, J.R.; Barnum, D.A. Necrotic enteritis in broiler chickens. II. pathology and proposed pathogenesis. Can. J. Comp. Med. 1974, 38, 467–474. [Google Scholar]
- Si, W.; Gong, J.; Han, Y.; Yu, H.; Brennan, J.; Zhou, H.; Chen, S. Quantification of cell proliferation and alpha-toxin gene expression of Clostridium perfringens in the development of necrotic enteritis in broiler chickens. Appl. Environ. Microbiol. 2007, 73, 7110–7113. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, J.P.; Wilkie, D.C.; Van Kessel, A.G.; Drew, M.D. Potential strategies for controlling necrotic enteritis in broiler chickens in post-antibiotic era. Anim. Feed Sci. Technol. 2006, 129, 60–88. [Google Scholar] [CrossRef]
- Mohiuddin, M.; Yuan, W.; Song, Z.; Liao, S.; Qi, N.; Li, J.; Lv, M.; Wu, C.; Lin, X.; Hu, J.; et al. Experimental induction of necrotic enteritis with or without predisposing factors using netB positive Clostridium perfringens strains. Gut. Pathog. 2021, 13, 68. [Google Scholar] [CrossRef]
- To, H.; Suzuki, T.; Kawahara, F.; Uetsuka, K.; Nagai, S.; Nunoya, T. Experimental induction of necrotic enteritis in chickens by a netB-positive Japanese isolate of Clostridium perfringens. J. Vet. Med. Sci. 2017, 79, 350–358. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, S.; Luo, Z.; Liu, D. Supplemental Bacillus subtilis PB6 improves growth performance and gut health in broilers challenged with Clostridium perfringens. J. Immunol. Res. 2021, 2021, 2549541. [Google Scholar] [CrossRef]
- Mora, Z.V.; Nuño, K.; Vazquez-Paulino, O.; Avalos, H.; Castro-Rosas, J.; Gomez-Aldapa, C.; Angulo, C.; Ascencio, F.; Villarruel-Lopez, A. Effect of a synbiotic mix on intestinal structural changes, and Salmonella Typhimurium and Clostridium perfringens colonization in broiler chickens. Animals 2019, 9, 777. [Google Scholar] [CrossRef]
- Vanaporn, M.; Titball, R.W. Trehalose and bacterial virulence. Virulence 2020, 11, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
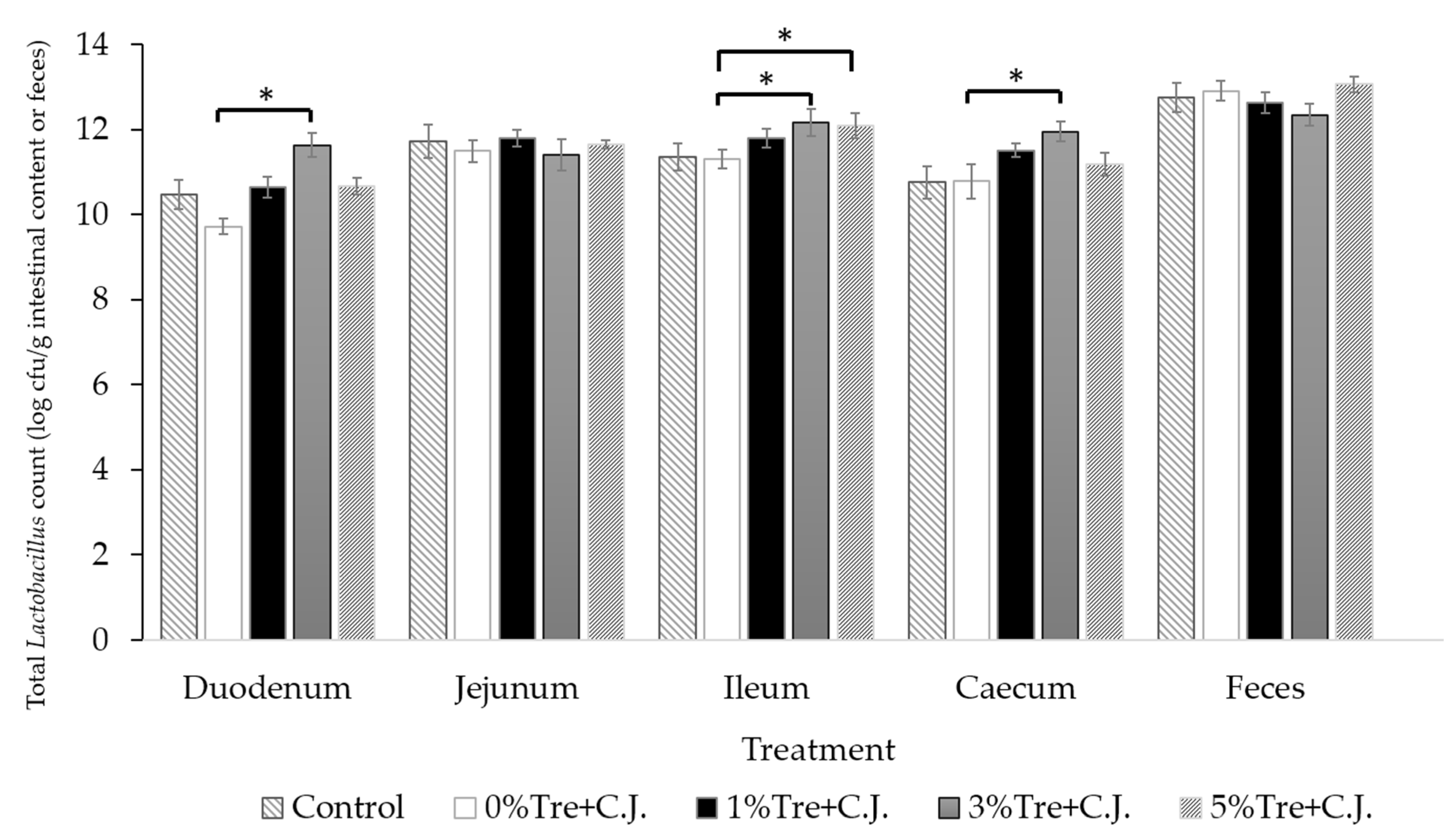
| Treatment | |||||
|---|---|---|---|---|---|
| Feeding Period (Day) | Control | 3% Tre | 5% Tre | 7% Tre | 10% Tre |
| 0–7 | 2 | 3 | 3 | 1 | 0 |
| 8–14 | 0 | 0 | 0 | 0 | 0 |
| 15–21 | 0 | 0 | 0 | 0 | 0 |
| 22–28 | 0 | 0 | 0 | 0 | 0 |
| 29–35 | 0 | 0 | 0 | 0 | 0 |
| Overall (0–35) | 2 | 3 | 3 | 1 | 0 |
| Intestinal | Treatment | ||||
|---|---|---|---|---|---|
| Segment/Feces | Control | 0%Tre + C.J. | 1%Tre + C.J. | 3%Tre + C.J. | 5%Tre + C.J. |
| C. jejuni Counts (log cfu/g Intestinal Content or Feces) | |||||
| Duodenum | N.D. | N.D. | N.D. | N.D. | N.D. |
| Jejunum | N.D. | N.D. | N.D. | N.D. | N.D. |
| Ileum | N.D. | N.D. | N.D. | N.D. | N.D. |
| Cecum | N.D. | 7.33 ± 0.16 | 7.42 ± 0.13 | 7.48 ± 0.16 | 7.30 ± 0.14 |
| Feces | N.D. | 4.65 ± 0.17 | 4.92 ± 0.36 | 4.55 ± 0.36 | 4.43 ± 0.25 |
| Intestinal | Treatment | ||||
|---|---|---|---|---|---|
| Segment/Feces | Control | 0%Tre + C.P. | 1%Tre + C.P. | 3%Tre + C.P. | 5%Tre + C.P. |
| C. perfringens Counts (log cfu/g Intestinal Content or Feces) | |||||
| Duodenum | N.D. | N.D. | N.D. | N.D. | N.D. |
| Jejunum | N.D. | N.D. | N.D. | N.D. | N.D. |
| Ileum | N.D. | N.D. | N.D. | N.D. | N.D. |
| Cecum | N.D. | 4.08 ± 0.16 | 4.28 ± 0.12 | 4.43 ± 0.15 | 4.13 ± 0.23 |
| Feces | N.D. | 5.82 ± 0.20 | 5.50 ± 0.32 | 5.24 ± 0.33 | 5.67 ± 0.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.-C.; Wu, Y.-T.; Wu, Y.-H.S.; Wang, C.-L.; Chou, C.-H.; Chen, Y.-C.; Tsai, H.-J. Investigation of Trehalose Supplementation Impacting Campylobacter jejuni and Clostridium perfringens from Broiler Farming. Vet. Sci. 2023, 10, 466. https://doi.org/10.3390/vetsci10070466
Fan Y-C, Wu Y-T, Wu Y-HS, Wang C-L, Chou C-H, Chen Y-C, Tsai H-J. Investigation of Trehalose Supplementation Impacting Campylobacter jejuni and Clostridium perfringens from Broiler Farming. Veterinary Sciences. 2023; 10(7):466. https://doi.org/10.3390/vetsci10070466
Chicago/Turabian StyleFan, Yang-Chi, Yi-Tei Wu, Yi-Hsieng Samuel Wu, Chia-Lan Wang, Chung-Hsi Chou, Yi-Chen Chen, and Hsiang-Jung Tsai. 2023. "Investigation of Trehalose Supplementation Impacting Campylobacter jejuni and Clostridium perfringens from Broiler Farming" Veterinary Sciences 10, no. 7: 466. https://doi.org/10.3390/vetsci10070466
APA StyleFan, Y.-C., Wu, Y.-T., Wu, Y.-H. S., Wang, C.-L., Chou, C.-H., Chen, Y.-C., & Tsai, H.-J. (2023). Investigation of Trehalose Supplementation Impacting Campylobacter jejuni and Clostridium perfringens from Broiler Farming. Veterinary Sciences, 10(7), 466. https://doi.org/10.3390/vetsci10070466






