Cooling Methods Used to Manage Heat-Related Illness in Dogs Presented to Primary Care Veterinary Practices during 2016–2018 in the UK
Abstract
Simple Summary
Abstract
1. Introduction
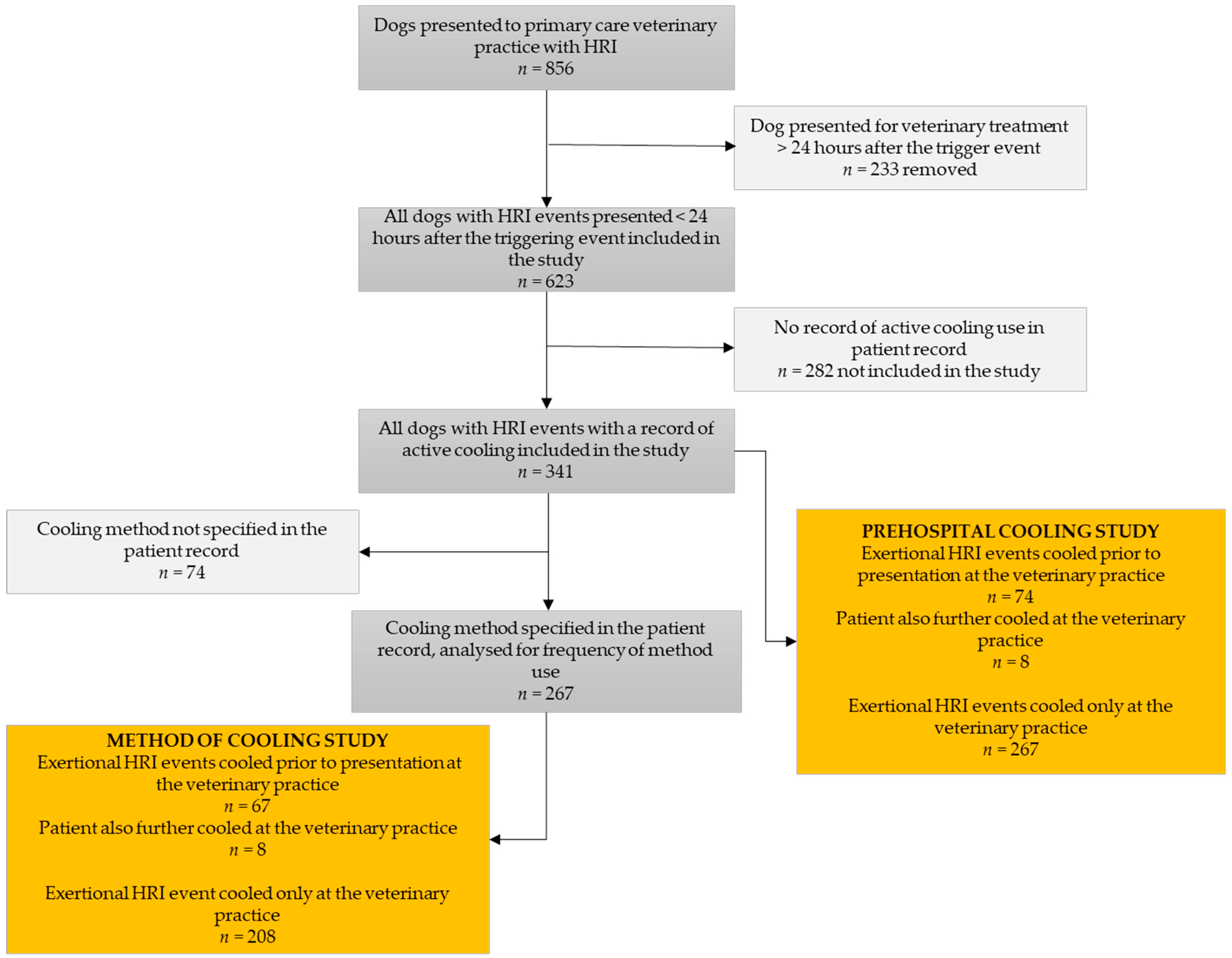
2. Materials and Methods
3. Results
3.1. Prehospital Cooling
3.2. Review of Cooling Methods Used
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bouchama, A.; Knochel, J.P. Heat Stroke. N. Engl. J. Med. 2002, 346, 1978–1988. [Google Scholar] [CrossRef] [PubMed]
- Bruchim, Y.; Horowitz, M.; Aroch, I. Pathophysiology of Heatstroke in Dogs–Revisited. Temperature 2017, 4, 356–370. [Google Scholar] [CrossRef]
- Hall, E.J.; Carter, A.J.; Bradbury, J.; Barfield, D.; O’Neill, D.G. Proposing the VetCompass Clinical Grading Tool for Heat-Related Illness in Dogs. Sci. Rep. 2021, 11, 6828. [Google Scholar] [CrossRef]
- Flournoy, S.W.; Wohl, J.S.; Macintire, D.K. Heatstroke in Dogs: Pathophysiology and Predisposing Factors. Compend. Contin. Educ. Pract. Vet. 2003, 25, 410–418. [Google Scholar]
- Hall, E.J.; Carter, A.J.; O’Neill, D.G. Dogs Don’t Die Just in Hot Cars—Exertional Heat-Related Illness (Heatstroke) Is a Greater Threat to UK Dogs. Animals 2020, 10, 1324. [Google Scholar] [CrossRef] [PubMed]
- Duncan, A. On Heat Stroke. Edinb. Med. J. 1903, 13, 217–224. [Google Scholar]
- Shapiro, Y.; Seidman, D.S. Field and Clinical Observations of Exertional Heat Stroke Patients. Med. Sci. Sport. Exerc. 1990, 22, 6–14. [Google Scholar] [CrossRef]
- Klous, L.; van Diemen, F.; Ruijs, S.; Gerrett, N.; Daanen, H.; de Weerd, M.; Veenstra, B.; Levels, K. Efficiency of Three Cooling Methods for Hyperthermic Military Personnel Linked to Water Availability. Appl. Ergon. 2022, 102, 103700. [Google Scholar] [CrossRef]
- Bouchama, A.; Dehbi, M.; Chaves-Carballo, E. Cooling and Hemodynamic Management in Heatstroke: Practical Recommendations. Crit. Care 2007, 11, R54. [Google Scholar] [CrossRef]
- Hadad, E.; Rav-Acha, M.; Heled, Y.; Epstein, Y.; Moran, D.S. Heat Stroke A Review of Cooling Methods. Sports Med. 2004, 34, 501–511. [Google Scholar] [CrossRef]
- Kielblock, A.J.; Van Rensburg, J.P.; Franz, R.M. Body Cooling as a Method for Reducing Hyperthermia. An Evaluation of Techniques. S. Afr. Med. J. 1986, 69, 378–380. [Google Scholar] [PubMed]
- Casa, D.J.; McDermott, B.P.; Lee, E.C.; Yeargin, S.W.; Armstrong, L.E.; Maresh, C.M. Cold Water Immersion: The Gold Standard for Exertional Heatstroke Treatment. Exerc. Sport Sci. Rev. 2007, 35, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Demartini, J.K.; Casa, D.J.; Stearns, R.; Belval, L.; Crago, A.; Davis, R.; Jardine, J. Effectiveness of Cold Water Immersion in the Treatment of Exertional Heat Stroke at the Falmouth Road Race. Med. Sci. Sports Exerc. 2015, 47, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Binkley, H.M.; Beckett, J.; Casa, D.J.; Kleiner, D.M.; Plummer, P.E. National Athletic Trainers’ Association Position Statement: Exertional Heat Illnesses. J. Athl. Train. 2002, 37, 329–343. [Google Scholar]
- McDermott, B.P.; Casa, D.J.; Ganio, M.S.; Lopez, R.M.; Yeargin, S.W.; Armstrong, L.E.; Maresh, C.M. Acute Whole-Body Cooling for Exercise-Induced Hyperthermia: A Systematic Review. J. Athl. Train. 2009, 44, 84–93. [Google Scholar] [CrossRef]
- Gaudio, F.G.; Grissom, C.K. Cooling Methods in Heat Stroke. J. Emerg. Med. 2016, 50, 607–616. [Google Scholar] [CrossRef]
- Kanda, J.; Nakahara, S.; Nakamura, S.; Miyake, Y.; Shimizu, K.; Yokobori, S.; Yaguchi, A.; Sakamoto, T. Association between Active Cooling and Lower Mortality among Patients with Heat Stroke and Heat Exhaustion. PLoS ONE 2021, 16, e0259441. [Google Scholar] [CrossRef]
- Davis, M.S.; Marcellin-Little, D.J.; O’Connor, E. Comparison of Postexercise Cooling Methods in Working Dogs. J. Spec. Oper. Med. 2019, 19, 56–60. [Google Scholar] [CrossRef]
- Magazanik, A.; Epstein, Y.; Udassin, R.; Shapiro, Y.; Sohar, E. Tap Water, an Efficient Method for Cooling Heatstroke Victims--a Model in Dogs. Aviat. Space. Environ. Med. 1980, 51, 864–866. [Google Scholar]
- Gorman, C. Heatstroke. Companion Anim. 2011, 16, 40–45. [Google Scholar] [CrossRef]
- Newfield, A. Providing Care for Dogs with Heatstroke. Today’s Vet. Nurse 2019, 2, 1–11. [Google Scholar]
- Flournoy, S.; Macintire, D.; Wohl, J. Heatstroke in Dogs: Clinical Signs, Treatment, Prognosis, and Prevention. Compend. Contin. Educ. Vet. 2003, 25, 422–431. [Google Scholar]
- RSPCA Treatment for Heatstroke. Available online: https://www.rspca.org.uk/adviceandwelfare/pets/dogs/health/heatstroke (accessed on 28 April 2023).
- Hemmelgarn, C.; Gannon, K. Heatstroke: Clinical Signs, Diagnosis, Treatment, and Prognosis. Compend. Contin. Educ. Vet. 2013, 35, E3. [Google Scholar]
- Shapiro, Y.; Rosenthal, T.; Sohar, E. Experimental Heatstroke a Model in Dogs. Arch. Intern. Med. 1973, 131, 688–692. [Google Scholar] [CrossRef]
- Bruchim, Y.; Klement, E.; Saragusty, J.; Finkeilstein, E.; Kass, P.; Aroch, I. Heat Stroke in Dogs: A Retrospective Study of 54 Cases (1999-2004) and Analysis of Risk Factors for Death. J. Vet. Intern. Med. 2006, 20, 38–46. [Google Scholar] [CrossRef]
- Casa, D.J.; Armstrong, L.E.; Ganio, M.S.; Yeargin, S.W. Exertional Heat Stroke in Competitive Athletes. Curr. Sports Med. Rep. 2005, 4, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Monseau, A.J.; Hurlburt, G.A.; Balcik, B.J.; Oppenlander, K.E.; Chill, N.M.; Martin, P.S. Status of US Emergency Medical Service Protocols Regarding Pre-Transfer Cooling for Exertional Heat Stroke. Cureus 2021, 13, e19505. [Google Scholar] [CrossRef] [PubMed]
- Hanel, R.M.; Palmer, L.; Baker, J.; Brenner, J.-A.A.; Crowe, D.T.T.; Dorman, D.; Gicking, J.C.; Gilger, B.; Otto, C.M.; Robertson, S.A.; et al. Best Practice Recommendations for Prehospital Veterinary Care of Dogs and Cats. J. Vet. Emerg. Crit. Care 2016, 26, 166–233. [Google Scholar] [CrossRef]
- Royal Veterinary College About VetCompass. Available online: https://www.rvc.ac.uk/vetcompass/about (accessed on 28 July 2019).
- Ausvet Epitools-Confidence Limits for a Proportion. Available online: https://epitools.ausvet.com.au/ciproportion (accessed on 5 May 2023).
- O’Neill, D.G.; Corah, C.H.; Church, D.B.; Brodbelt, D.C.; Rutherford, L. Lipoma in Dogs under Primary Veterinary Care in the UK: Prevalence and Breed Associations. Canine Genet. Epidemiol. 2018, 5, 9. [Google Scholar] [CrossRef]
- Smith, J.E. Cooling Methods Used in the Treatment of Exertional Heat Illness * Commentary. Br. J. Sports Med. 2005, 39, 503–507. [Google Scholar] [CrossRef]
- Hall, E.J.; Carter, A.J.; Chico, G.; Bradbury, J.; Gentle, L.K.; Barfield, D.; O’Neill, D.G. Risk Factors for Severe and Fatal Heat-Related Illness in UK Dogs—A VetCompass Study. Vet. Sci. 2022, 9, 231. [Google Scholar] [CrossRef]
- Goncalves Caldas, G.; Oliveira Barbosa da Silva, D.; Barauna Junior, D. Heat Stroke in Dogs: Literature Review. Rev. Vet. Med. 2022, 67, 354–364. [Google Scholar] [CrossRef]
- White, J.; Kamath, R.; Nucci, R.; Johnson, C.; Shepherd, S. Evaporation versus Iced Peritoneal Lavage Treatment of Heatstroke: Comparative Efficacy in a Canine Model. Am. J. Emerg. Med. 1993, 11, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Blue Cross Top Tips for Keeping Your Dog Cool–and Safe–In Summer. Available online: https://www.bluecross.org.uk/advice/dog/health-and-injuries/top-tips-for-keeping-your-dog-cool-and-safe-in-summer#:~:text=Wet%2Ccooltowels (accessed on 17 April 2023).
- Foreman, J.H.; Benson, G.J.; Foreman, M.H. Effects of a Pre-Moistened Multilayered Breathable Fabric in Promoting Heat Loss during Recovery after Exercise under Hot Conditions. Equine Vet. J. 2006, 38, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, S.; Yang, X.; Zhao, J.; Zhang, Y.; Feng, X. Association between Cooling Temperature and Outcomes of Patients with Heat Stroke. Intern. Emerg. Med. 2023. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.J.; Carter, A.J.; O’Neill, D.G. Incidence and Risk Factors for Heat-Related Illness (Heatstroke) in UK Dogs under Primary Veterinary Care in 2016. Sci. Rep. 2020, 10, 9128. [Google Scholar] [CrossRef] [PubMed]
- Ebi, K.L.; Vanos, J.; Baldwin, J.W.; Bell, J.E.; Hondula, D.M.; Errett, N.A.; Hayes, K.; Reid, C.E.; Saha, S.; Spector, J.; et al. Extreme Weather and Climate Change: Population Health and Health System Implications. Annu. Rev. Public Health 2020, 42, 293–315. [Google Scholar] [CrossRef]
- Dee, S.G.; Nabizadeh, E.; Nittrouer, C.L.; Baldwin, J.W.; Li, C.; Gaviria, L.; Guo, S.; Lu, K.; Saunders-Shultz, B.M.; Gurwitz, E.; et al. Increasing Health Risks During Outdoor Sports Due To Climate Change in Texas: Projections Versus Attitudes. GeoHealth 2022, 6, e2022GH000595. [Google Scholar] [CrossRef]
| Year of Study | Number of Dogs Cooled Prior to Presentation for Veterinary Treatment/Total Number of Dogs Cooled | Percentage of Dogs Cooled Prior to Presentation (95% Confidence Interval) |
|---|---|---|
| 2016 | 33/167 | 19.76 (14.43–26.45) |
| 2017 | 20/96 | 20.83 (13.91–30.00) |
| 2018 | 21/78 | 26.92 (18.34–37.68) |
| Year of Study | Number of Dogs Cooled Using a Recommended Method/Total Number of Dogs Cooled | Percentage of Dogs Cooled Using a Recommended Method (95% Confidence Interval) |
|---|---|---|
| 2016 | 35/130 | 26.92 (20.04–35.13) |
| 2017 | 17/70 | 24.29 (15.75–35.5) |
| 2018 | 12/67 | 17.91 (10.55–28.75) |
| Cooling Method Used | Number of Cases Cooled with This Method (%, 95% CI of All Cooled Cases) n = 267 | Number of Cases Cooled Prior to Presentation with This Method (%, 95% CI of Cases Cooled Prehospital) n = 67 | Number of Cases Cooled with This Method by the Veterinary Practice (%, 95% CI of Veterinary Cooled Cases) n = 208 | |
|---|---|---|---|---|
| Vet-COT-recommended cooling method used: | Cold-water immersion or evaporative cooling | 64 (23.97, 19.24–29.44) | 17 (25.37, 16.49–36.93) | 47 (22.60, 17.44–28.75) |
| Cold-water immersion | 45 (16.85, 12.84–21.81) | 15 (22.39, 14.06–33.71) | 30 (14.42, 10.29–19.84) | |
| Evaporative cooling | 20 (7.49, 4.90–11.29) | 2 (2.99, 0.82–10.25) | 18 (8.65, 5.54–13.26) | |
| Other cooling method used | Water-soaked towel | 137 (51.31, 45.34–57.24) | 26 (38.81, 28.05–50.78) | 111 (53.37, 46.59–60.02) |
| Air movement | 84 (31.46, 26.19–37.26) | 11 (16.42, 9.42–27.06) | 73 (35.10, 28.93–41.80) | |
| Water spray (no air movement) | 62 (23.22, 18.56–28.64) | 19 (28.36, 18.97–40.09) | 43 (20.67, 15.73–26.68) | |
| Ice/ice packs | 46 (17.23, 13.17–22.21) | 4 (5.97, 2.35–14.37) | 42 (20.19, 15.3–26.17) | |
| Cold intravenous fluids | 26 (9.74, 6.73–13.89) | 0 | 26 (12.50, 8.67–17.69) | |
| Alcohol application | 21 (7.87, 5.20–11.72) | 0 | 21 (10.10, 6.70–14.94) | |
| Cold-water enema | 8 (3.00, 1.53–5.80) | 8 (3.85, 1.96–7.40) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hall, E.J.; Carter, A.J.; Bradbury, J.; Beard, S.; Gilbert, S.; Barfield, D.; O’Neill, D.G. Cooling Methods Used to Manage Heat-Related Illness in Dogs Presented to Primary Care Veterinary Practices during 2016–2018 in the UK. Vet. Sci. 2023, 10, 465. https://doi.org/10.3390/vetsci10070465
Hall EJ, Carter AJ, Bradbury J, Beard S, Gilbert S, Barfield D, O’Neill DG. Cooling Methods Used to Manage Heat-Related Illness in Dogs Presented to Primary Care Veterinary Practices during 2016–2018 in the UK. Veterinary Sciences. 2023; 10(7):465. https://doi.org/10.3390/vetsci10070465
Chicago/Turabian StyleHall, Emily J., Anne J. Carter, Jude Bradbury, Sian Beard, Sophie Gilbert, Dominic Barfield, and Dan G. O’Neill. 2023. "Cooling Methods Used to Manage Heat-Related Illness in Dogs Presented to Primary Care Veterinary Practices during 2016–2018 in the UK" Veterinary Sciences 10, no. 7: 465. https://doi.org/10.3390/vetsci10070465
APA StyleHall, E. J., Carter, A. J., Bradbury, J., Beard, S., Gilbert, S., Barfield, D., & O’Neill, D. G. (2023). Cooling Methods Used to Manage Heat-Related Illness in Dogs Presented to Primary Care Veterinary Practices during 2016–2018 in the UK. Veterinary Sciences, 10(7), 465. https://doi.org/10.3390/vetsci10070465







