Assessment of Vitality, Blood Profile, and Degree of Meconium Staining on the Skin in Newborn Dogs According to Its Birth Weight
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Facilities
2.2. Study Population
2.3. Blood Sampling
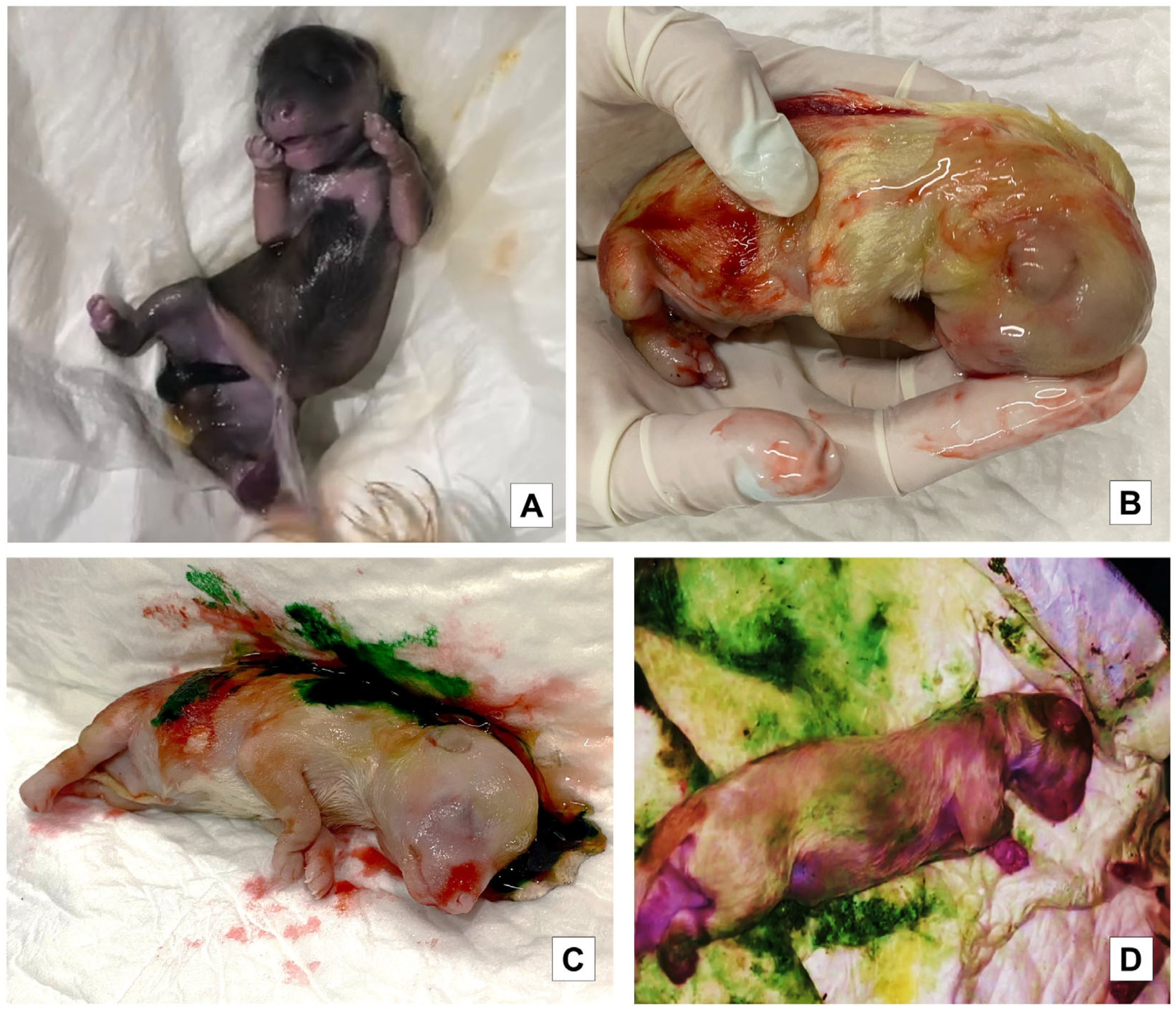
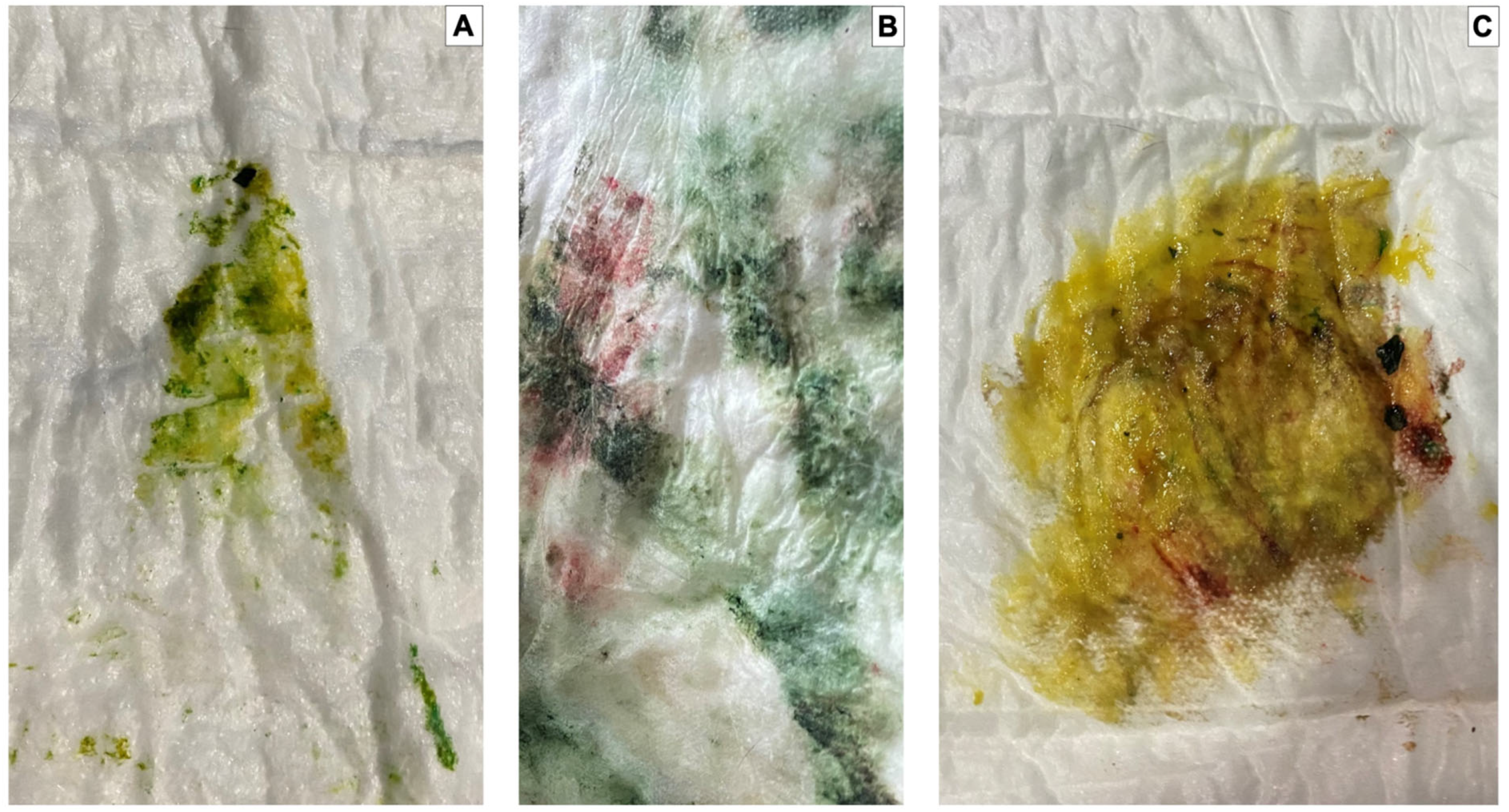
2.4. Meconium Staining Degree on Skin
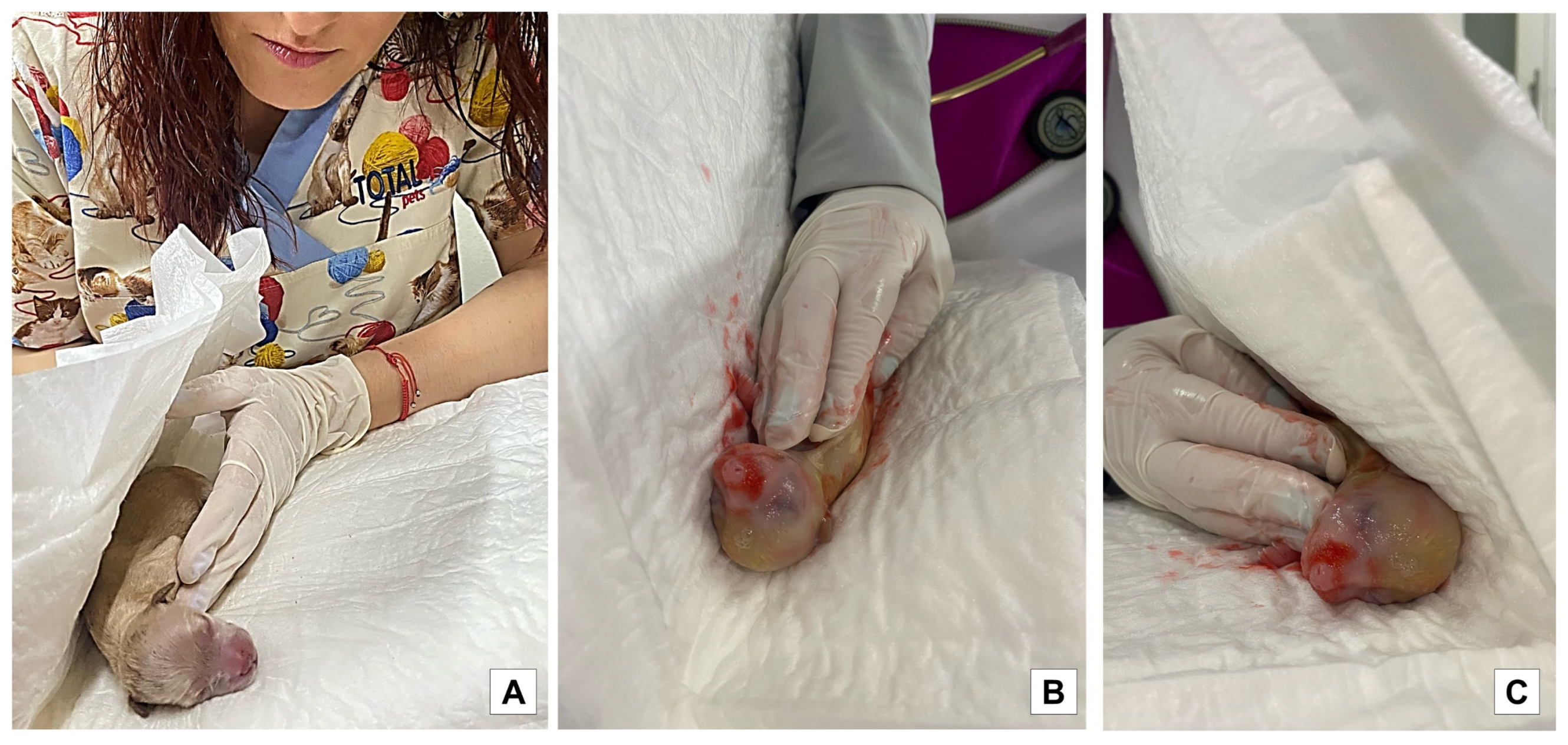
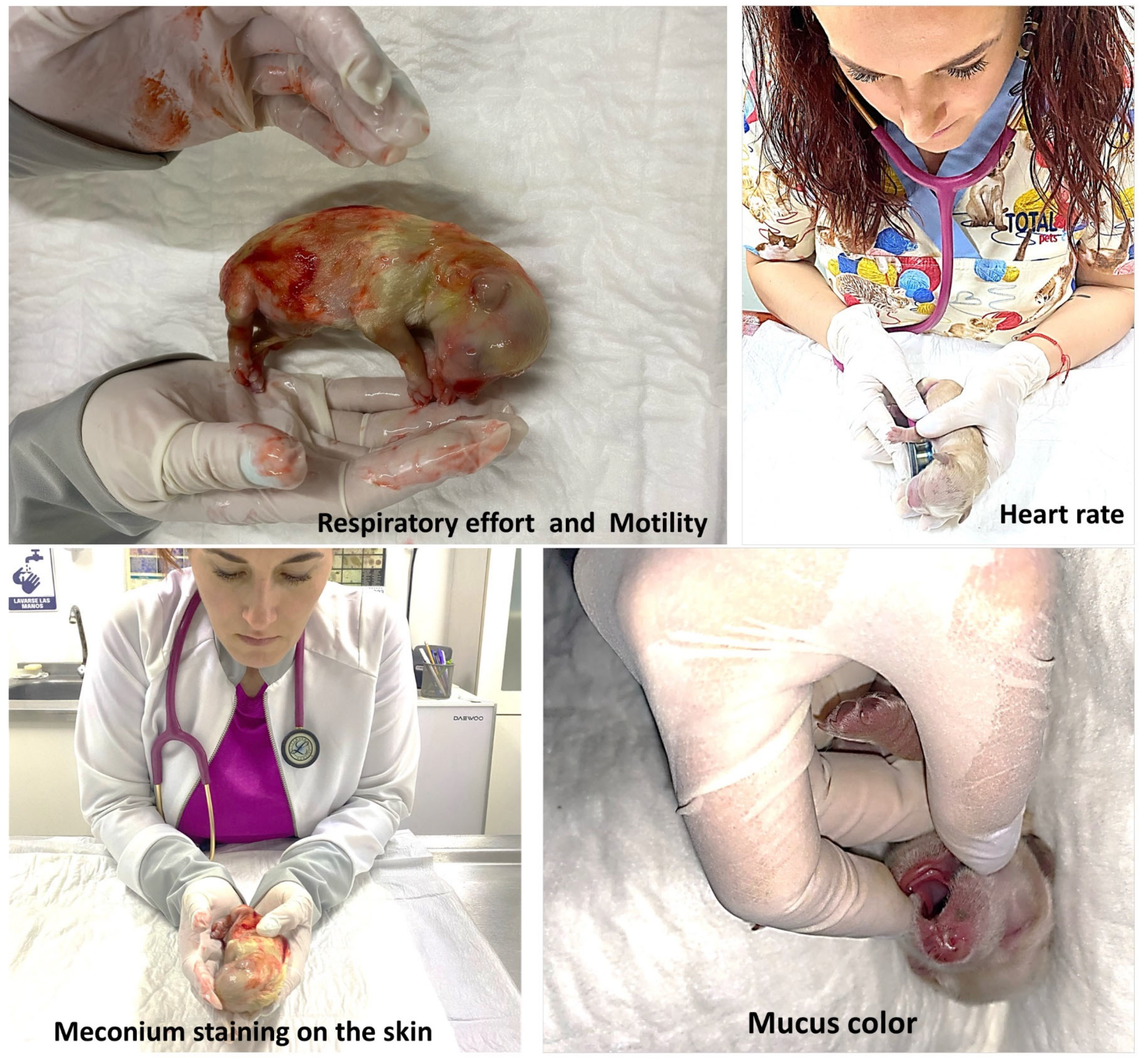
2.5. Vitality Score
2.6. Statistical Analysis
2.7. Ethical Statement
3. Results
4. Discussion
4.1. Blood Profile Values
4.2. Vitality Score
4.3. Meconium Staining Degree
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Veronesi, M. Assessment of canine neonatal viability-the Apgar score. Reprod. Domest. Anim. 2016, 51, 46–50. [Google Scholar] [CrossRef]
- Veronesi, M.C.; Panzani, S.; Faustini, M.; Rota, A. An Apgar scoring system for routine assessment of newborn puppy viability and short-term survival prognosis. Theriogenology 2009, 72, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Chastant-Maillard, S.; Guillemot, C.; Feugier, A.; Mariani, C.; Grellet, A.; Mila, H. Reproductive performance and pre-weaning mortality: Preliminary analysis of 27,221 purebred female dogs and 204,537 puppies in France. Reprod. Domest. Anim. 2017, 52, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Lezama-García, K.; Martínez-Burnes, J.; Marcet-Rius, M.; Gazzano, A.; Olmos-Hernández, A.; Mora-Medina, P.; Domínguez-Oliva, A.; Pereira, A.M.F.; Hernández-Ávalos, I.; Baqueiro-Espinosa, U.; et al. Is the weight of the newborn puppy related to its thermal balance? Animals 2022, 12, 3536. [Google Scholar] [CrossRef]
- Veronesi, M.C.; Fusi, J. Neonatal severity indicators in dogs. Rev. Bras. Reprodução Anim. 2021, 45, 525–532. [Google Scholar] [CrossRef]
- Kirkden, R.D.; Broom, D.M.; Andersen, I.L. Invited review: Piglet mortality: Management solutions. J. Anim. Sci. 2013, 91, 3361–3389. [Google Scholar] [CrossRef]
- Tønnessen, R.; Borge, K.S.; Nødtvedt, A.; Indrebø, A. Canine perinatal mortality: A cohort study of 224 breeds. Theriogenology 2012, 77, 1788–1801. [Google Scholar] [CrossRef] [PubMed]
- Vassalo, F.G.; Simões, C.R.B.; Sudano, M.J.; Prestes, N.C.; Lopes, M.D.; Chiacchio, S.B.; Lourenço, M.L.G. topics in the routine assessment of newborn puppy viability. Top. Companion Anim. Med. 2015, 30, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Fusi, J.; Faustini, M.; Bolis, B.; Veronesi, M.C. Apgar score or birthweight in Chihuahua dogs born by elective Caesarean section: Which is the best predictor of the survival at 24 h after birth? Acta Vet. Scand. 2020, 62, 39. [Google Scholar] [CrossRef]
- Gill, M.A. Perinatal and late neonatal mortality in the dog. J. Am. Vet. Med. Assoc. 2001, 230, 1463–1464. [Google Scholar]
- Fix, J. Relationship of Piglet Birth Weight with Growth, Efficiency, Composition, and Mortality. Ph.D. Thesis, University of North Carolina State, Charlotte, NC, USA, 2010. [Google Scholar]
- Manani, M.; Jegatheesan, P.; DeSandre, G.; Song, D.; Showalter, L.; Govindaswami, B. Elimination of admission hypothermia in preterm very low-birth-weight infants by standardization of delivery room management. Perm. J. 2013, 17, 8–13. [Google Scholar] [CrossRef]
- Pereira, K.H.N.P.; Fuchs, K.M.; Corrêa, J.V.; Chiacchio, S.B.; Lourenço, M.L.G. Neonatology: Topics on Puppies and Kittens Neonatal Management to Improve Neonatal Outcome. Animals 2022, 12, 3426. [Google Scholar] [CrossRef]
- Cavaliere, T.A. From fetus to neonate: A sensational journey. Newborn Infant Nurs. Rev. 2016, 16, 43–47. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Villanueva-García, D.; Hernández-González, R.; Roldan-Santiago, P.; Martínez-Rodríguez, R.; Mora-Medina, P.; Trujillo-Ortega, M.E. Assessment of the vitality of the newborn: An overview. Sci. Res. Essays 2012, 7, 712–718. [Google Scholar] [CrossRef]
- Massip, A. Relationship between pH, plasma cortisol and glucose concentrations in the calf at birth. Br. Vet. J. 1980, 136, 597–601. [Google Scholar] [CrossRef]
- Wiberg, N.; Källén, K.; Olofsson, P. Physiological development of a mixed metabolic and respiratory umbilical cord blood acidemia with advancing gestational age. Early Hum. Dev. 2006, 82, 583–589. [Google Scholar] [CrossRef]
- Nobre Pacífico Pereira, K.H.; Hibaru, V.Y.; Fuchs, K.M.; Cruz dos Santos Correia, L.E.; Lopes, M.D.; Ferreira, J.C.P.; Ferreira de Souza, F.; Machado, L.H.D.A.; Chiacchio, S.B.; Gomes Lourenço, M.L. Use of cardiac troponin I (cTnI) levels to diagnose severe hypoxia and myocardial injury induced by perinatal asphyxia in neonatal dogs. Theriogenology 2022, 180, 146–153. [Google Scholar] [CrossRef]
- Bleul, U.; Lejeune, B.; Schwantag, S.; Kähn, W. Blood gas and acid-base analysis of arterial blood in 57 newborn calves. Vet. Rec. 2007, 161, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Andres, R.L.R.L.; Saade, G.; Gilstrap, L.C.C.; Wilkins, I.; Witlin, A.; Zlatnik, F.; Hankins, G.V.V. Association between umbilical blood gas parameters and neonatal morbidity and death in neonates with pathologic fetal acidemia. Am. J. Obstet. Gynecol. 1999, 181, 867–871. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, L.; Stenson, B.J. Use of umbilical cord blood gas analysis in the assessment of the newborn. Arch. Dis. Child.-Fetal Neonatal Ed. 2007, 92, F430–F434. [Google Scholar] [CrossRef]
- Rodríguez Fernández, V.; López Ramón y Cajal, C.N.; Marín Ortiz, E.; Couceiro Naveira, E. Intrapartum and perinatal results associated with different degrees of staining of meconium stained amniotic fluid. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 228, 65–70. [Google Scholar] [CrossRef]
- Antończyk, A.; Kiełbowicz, Z.; Niżański, W.; Ochota, M. Comparison of 2 anesthetic protocols and surgical timing during cesarean section on neonatal vitality and umbilical cord blood parameters. BMC Vet. Res. 2023, 19, 48. [Google Scholar] [CrossRef]
- Rootwelt, V.; Reksen, O.; Farstad, W.; Framstad, T. Associations between intrapartum death and piglet, placental, and umbilical characteristics1. J. Anim. Sci. 2012, 90, 4289–4296. [Google Scholar] [CrossRef] [PubMed]
- Le Cozler, Y.; Guyomarc’h, C.; Pichodo, X.; Quinio, P.-Y.Y.; Pellois, H.H. Factors associated with stillborn and mummified piglets in high-prolific sows. Anim. Res. 2002, 51, 261–268. [Google Scholar] [CrossRef]
- Lucia, T.; Corrêa, M.N.; Deschamps, J.C.; Bianchi, I.; Donin, M.A.; Machado, A.C.; Meincke, W.; Matheus, J.E.M. Risk factors for stillbirths in two swine farms in the south of Brazil. Prev. Vet. Med. 2002, 53, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Sotelo, B.; Mota-Rojas, D.; Mora-Medina, P.; Ogi, A.; Mariti, C.; Olmos-Hernández, A.; Martínez-Burnes, J.; Hernández-Ávalos, I.; Sánchez-Millán, J.; Gazzano, A. Blood biomarker profile alterations in newborn canines: Effect of the mother’s weight. Animals 2021, 11, 2307. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Sotelo, B.; Ogi, A.; Mora-Medina, P.; Mariti, C.; Olmos-Hernández, A.; Hernández-Ávalos, I.; Domínguez-Oliva, A.; Rosas, M.E.; Verduzco-Mendoza, A.; Gazzano, A. Early blood analysis and gas exchange monitoring in the canine neonate: Effect of dam’s size and birth order. Animals 2022, 12, 1508. [Google Scholar] [CrossRef]
- Ogi, A.; Naef, V.; Santorelli, F.M.; Mariti, C.; Gazzano, A. Oxytocin receptor gene polymorphism in lactating dogs. Animals 2021, 11, 3099. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Martinez-Burnes, J.; Alonso-Spilsbury, M.L.; Lopez, A.; Ramirez-Necoechea, R.; Trujillo-Ortega, M.E.; Medina-Hernandez, F.J.; de la Cruz, N.I.; Albores-Torres, V.; Loredo-Osti, J. Meconium staining of the skin and meconium aspiration in porcine intrapartum stillbirths. Livest. Sci. 2006, 102, 155–162. [Google Scholar] [CrossRef]
- Martínez-Burnes, J.; Mota-Rojas, D.; Villanueva-García, D.; Ibarra-Ríos, D.; Lezama-García, K.; Barrios-García, H.; López-Mayagoitia, A. Meconium aspiration syndrome in mammals. CAB Rev. 2019, 14, 1–11. [Google Scholar] [CrossRef]
- Chiruvolu, A.; Miklis, K.K.; Chen, E.; Petrey, B.; Desai, S. Delivery room management of meconium-stained newborns and respiratory support. Pediatrics 2018, 142, e20181485. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Villanueva-García, D.; Mota-Reyes, A.; Orihuela, A.; Hernández-Ávalos, I.; Domínguez-Oliva, A.; Casas-Alvarado, A.; Flores-Padilla, K.; Jacome-Romero, J.; Martínez-Burnes, J. Meconium aspiration syndrome in animal models: Inflammatory process, apoptosis, and surfactant inactivation. Animals 2022, 12, 3310. [Google Scholar] [CrossRef] [PubMed]
- Monfredini, C.; Cavallin, F.; Villani, P.E.; Paterlini, G.; Allais, B.; Trevisanuto, D. Meconium aspiration syndrome: A narrative review. Children 2021, 8, 230. [Google Scholar] [CrossRef]
- Kirimi, E.; Tuncer, O.; Kösem, M.; Ceylan, E.; Tas, A.; Tasal, I.; Balahoroǧlu, R.; Caksen, H.; Balahoroğlu, R.; Caksen, H. The effects of prednisolone and serum malondialdehyde levels in puppies with experimentally induced meconium aspiration syndrome. J. Int. Med. Res. 2003, 31, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Swarnam, K.; Soraisham, A.S.; Sivanandan, S. Advances in the management of meconium aspiration syndrome. Int. J. Pediatr. 2012, 2012, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Castagnetti, C.; Pirrone, A.; Mariella, J.; Mari, G. Venous blood lactate evaluation in equine neonatal intensive care. Theriogenology 2010, 73, 343–357. [Google Scholar] [CrossRef]
- Randall, G.C. The relationship of arterial blood pH and pCO2 to the viability of the newborn piglet. Can. J. Comp. Med. Rev. Can. Med. Comp. 1971, 35, 141–146. [Google Scholar]
- Zaleski, H.M.; Hacker, R.R. Comparison of viability scoring and blood gas analysis as measures of piglet viability. Can. J. Anim. Sci. 1993, 73, 649–653. [Google Scholar] [CrossRef]
- De Roth, L.; Downie, H.G. Evaluation of viability of neonatal swine. Can. Vet. J. Rev. Vet. Can. 1976, 17, 275–279. [Google Scholar]
- Groppetti, D.; Ravasio, G.; Bronzo, V.; Pecile, A. The role of birth weight on litter size and mortality within 24 h of life in purebred dogs: What aspects are involved? Anim. Reprod. Sci. 2015, 163, 112–119. [Google Scholar] [CrossRef]
- Mila, H.; Grellet, A.; Feugier, A.; Chastant-Maillard, S. Differential impact of birth weight and early growth on neonatal mortality in puppies1,2. J. Anim. Sci. 2015, 93, 4436–4442. [Google Scholar] [CrossRef] [PubMed]
- Mugnier, A.; Mila, H.; Guiraud, F.; Brévaux, J.; Lecarpentier, M.; Martinez, C.; Mariani, C.; Adib-Lesaux, A.; Chastant-Maillard, S.; Saegerman, C.; et al. Birth weight as a risk factor for neonatal mortality: Breed-specific approach to identify at-risk puppies. Prev. Vet. Med. 2019, 171, 104746. [Google Scholar] [CrossRef] [PubMed]
- Tesi, M.; Miragliotta, V.; Scala, L.; Aronica, E.; Lazzarini, G.; Fanelli, D.; Abramo, F.; Rota, A. Relationship between placental characteristics and puppies’ birth weight in toy and small sized dog breeds. Theriogenology 2020, 141, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Boyko, A.R.; Quignon, P.; Li, L.; Schoenebeck, J.J.; Degenhardt, J.D.; Lohmueller, K.E.; Zhao, K.; Brisbin, A.; Parker, H.G.; VonHoldt, B.M.; et al. A simple genetic architecture underlies morphological variation in dogs. PLoS Biol. 2010, 8, e1000451. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Nava-Ocampo, A.A.; Trujillo, M.E.; Velázquez-Armenta, Y.; Ramírez-Necoechea, R.; Martínez-Burnes, J.; Alonso-Spilsbury, A. Dose minimization study of oxytocin in early labor in sows: Uterine activity and fetal outcome. Reprod. Toxicol. 2005, 20, 255–259. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Rosales, A.M.; Trujillo, M.E.; Orozco, H.; Ramírez, R.; Alonso-Spilsbury, M. The effects of vetrabutin chlorhydrate and oxytocin on stillbirth rate and asphyxia in swine. Theriogenology 2005, 64, 1889–1897. [Google Scholar] [CrossRef]
- Sherwin, C.M.; Christiansen, S.B.; Duncan, I.J.; Erhard, H.W.; Lay, D.C.; Mench, J.A.; O’Connor, C.E.; Petherick, J.C. Guidelines for the ethical use of animals in applied ethology studies. Appl. Anim. Behav. Sci. 2003, 81, 291–305. [Google Scholar] [CrossRef]
- Lezama-García, K.; Martínez-Burnes, J.; Pérez-Jiménez, J.C.; Domínguez-Oliva, A.; Mora-Medina, P.; Olmos-Hernández, A.; Hernández-Ávalos, I.; Mota-Rojas, D. Relation between the dam’s weight on superficial temperature of her puppies at different stages of the post-partum. Vet. Sci. 2022, 9, 673. [Google Scholar] [CrossRef]
- Manning, M. Electrolyte disorders. Vet. Clin. N. Am.-Small Anim. Pract. 2001, 17, 8–13. [Google Scholar] [CrossRef]
- Vannucchi, C.I.; Rodrigues, J.A.; Silva, L.C.G.; Lúcio, C.F.; Veiga, G.A.L. A clinical and hemogasometric survey of neonatal lambs. Small Rumin. Res. 2012, 108, 107–112. [Google Scholar] [CrossRef]
- Nowak, R.; Poindron, P. From birth to colostrum: Early steps leading to lamb survival. Reprod. Nutr. Dev. 2006, 46, 431–446. [Google Scholar] [CrossRef] [PubMed]
- Herpin, P.; Le Dividich, J.; Hulin, J.C.; Fillaut, M.; De Marco, F.; Bertin, R. Effects of the level of asphyxia during delivery on viability at birth and early postnatal vitality of newborn pigs. J. Anim. Sci. 1996, 74, 2067. [Google Scholar] [CrossRef] [PubMed]
- Tuchscherer, M.; Puppe, B.; Tuchscherer, A.; Tiemann, U. Early identification of neonates at risk: Traits of newborn piglets with respect to survival. Theriogenology 2000, 54, 371–388. [Google Scholar] [CrossRef]
- Alonso-Spilsbury, M.; Mota-Rojas, D.; Villanueva-García, D.; Martínez-Burnes, J.; Orozco, H.; Ramírez-Necoechea, R.; Mayagoitia, A.L.; Trujillo, M.E. Perinatal asphyxia pathophysiology in pig and human: A review. Anim. Reprod. Sci. 2005, 90, 1–30. [Google Scholar] [CrossRef]
- Zamora-Moran, E. Metabolic blood gas disorders. In Acid-Base and Electrolyte Handbook for Veterinary Technicians; Randels-Thorp, A., Liss, D., Eds.; John Wiley & Sons: Chichester, UK, 2017; pp. 121–135. [Google Scholar]
- Okere, C.; Hacker, R.R.; Werchola, G. Relationships between serum IGF-I concentrations and piglet development or neonatal viability following porcine somatotropin (pST) and insulin administration to gestating gilts. Theriogenology 1997, 47, 1403–1412. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Ortega, M.E.; Mota-Rojas, D.; Juárez, O.; Villanueva-García, D.; Roldán-Santiago, P.; Becerril-Herrera, M.; Hernández-González, R.; Mora-Medina, P.; Alonso-Spilsbury, M.; Rosales, A.M.; et al. Porcine neonates failing vitality score: Physio-metabolic profile and latency to the first teat contact. Czech J. Anim. Sci. 2011, 56, 499–508. [Google Scholar] [CrossRef]
- Schrank, M.; Mollo, A.; Contiero, B.; Romagnoli, S. Bodyweight at birth and growth rate during the neonatal period in three canine breeds. Animals 2019, 10, 8. [Google Scholar] [CrossRef]
- Veronesi, M.C.; Faustini, M.; Probo, M.; Rota, A.; Fusi, J. Refining the APGAR score cutoff values and viability classes according to breed body size in newborn dogs. Animals 2022, 12, 1664. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Wang, D.D.-H.; Titto, C.G.; Martínez-Burnes, J.; Villanueva-García, D.; Lezama, K.; Domínguez, A.; Hernández-Avalos, I.; Mora-Medina, P.; Verduzco, A.; et al. Neonatal infrared thermography images in the hypothermic ruminant model: Anatomical-morphological-physiological aspects and mechanisms for thermoregulation. Front. Vet. Sci. 2022, 9, 63205. [Google Scholar] [CrossRef]
- Røsvik, A.; Øymar, K.; Kvaløy, J.T.; Berget, M. Oxygen saturation in healthy newborns; influence of birth weight and mode of delivery. J. Perinat. Med. 2009, 37, 403–406. [Google Scholar] [CrossRef]
- Trujillo-Ortega, M.E.; Mota-Rojas, D.; Olmos-Hernández, A.; Alonso-Spilsbury, M.; González, M.; Orozco, H.; Ramírez-Necoechea, R.; Nava-Ocampo, A.A. A study of piglets born by spontaneous parturition under uncontrolled conditions: Could this be a naturalistic model for the study of intrapartum asphyxia? Acta Biomed. 2007, 78, 29–35. [Google Scholar]
- Marchant, J.; Rudd, A.; Mendl, M.; Broom, D.M.; Meredith, M.J.; Corning, S.; Simmins, P.H. Timing and causes of piglet mortality in alternative and conventional farrowing systems. Vet. Rec. 2000, 147, 209–214. [Google Scholar] [CrossRef]
- Stanton, H.C.; Carroll, J.K. Potential mechanisms responsible for prenatal and perinatal mortality or low viability of swine. J. Anim. Sci. 1974, 38, 1037–1044. [Google Scholar] [CrossRef]
- Cleary, G.M.; Wiswell, T.E. Meconium-stained amniotic fluid and the meconium aspiration syndrome. Pediatr. Clin. N. Am. 1998, 45, 511–529. [Google Scholar] [CrossRef] [PubMed]
- Wiswell, T.E.; Bent, R.C. Meconium staining and the Meconium Aspiration Syndrome: Unresolved issues. Pediatr. Clin. N. Am. 1993, 40, 955–981. [Google Scholar] [CrossRef]
- Mitchell, S.; Chandraharan, E. Meconium-stained amniotic fluid. Obstet. Gynaecol. Reprod. Med. 2018, 28, 120–124. [Google Scholar] [CrossRef]
- Olicker, A.L.; Raffay, T.M.; Ryan, R.M. Neonatal respiratory distress secondary to Meconium Aspiration Syndrome. Children 2021, 8, 246. [Google Scholar] [CrossRef] [PubMed]
- Kima, V.; Roy, I.; Saikia, N. Correlation of umbilical cord arterial blood pH with meconium-stained liquor. Indian J. Obstet. Gynecol. Res. 2023, 10, 27–31. [Google Scholar] [CrossRef]
- Groppetti, D.; Pecile, A.; Del Carro, A.P.; Copley, K.; Minero, M.; Cremonesi, F. Evaluation of newborn canine viability by means of umbilical vein lactate measurement, apgar score and uterine tocodynamometry. Theriogenology 2010, 74, 1187–1196. [Google Scholar] [CrossRef]
- Forsberg, C.L. Pregnancy diagnosis, normal pregnancy and parturition in the bitch. In BSAVA Manual of Canine and Feline Reproduction and Neonatology; England, G.C., von Heimendahl, A., Eds.; British Small Animal Veterinary Association: Gloucester, UK, 2010; pp. 89–97. [Google Scholar]
- Bienboire-Frosini, C.; Muns, R.; Marcet-Rius, M.; Gazzano, A.; Villanueva-García, D.; Martínez-Burnes, J.; Domínguez-Oliva, A.; Lezama-García, K.; Casas-Alvarado, A.; Mota-Rojas, D. Vitality in Newborn Farm Animals: Adverse Factors, Physiological Responses, Pharmacological Therapies, and Physical Methods to Increase Neonate Vigor. Animals 2023, 13, 1542. [Google Scholar] [CrossRef]
- Uchanska, O.; Ochota, M.; Eberhardt, M.; Nizanski, W. Dead or Alive? A Review of Perinatal Factors That Determine Canine Neonatal Viability. Animals 2022, 12, 1402. [Google Scholar] [CrossRef] [PubMed]
- Szenci, O. Importance of Monitoring Fetal and Neonatal Vitality in Bovine Practices. Animals 2023, 13, 1081. [Google Scholar] [CrossRef] [PubMed]
- Plavec, T.; Knific, T.; Slapšak, A.; Raspor, S.; Lukanc, B.; Pipan, M.Z. Canine Neonatal Assessment by Vitality Score, Amniotic Fluid, Urine, and Umbilical Cord Blood Analysis of Glucose, Lactate, and Cortisol: Possible Influence of Parturition Type? Animals 2022, 12, 1247. [Google Scholar] [CrossRef] [PubMed]
| Blood Trait | Q1 n = 110 | Q2 n = 108 | Q3 n = 108 | Q4 n = 109 |
|---|---|---|---|---|
| pH | 7.49 ± 0.0126 a | 7.24 ± 0.0136 b | 7.25 ± 0.0116 b | 7.24 ± 0.0125 b |
| pCO2 (mmHg) | 46.9 ± 0.879 a | 56.5 ± 1.41 b | 58.8 ± 1.46 b | 64.0 ± 1.42 c |
| pO2 (mmHg) | 18.0 ± 0.325 a | 14.4 ± 0.323 b | 14.0 ± 0.302 b | 12.5 ± 0.306 c |
| Glucose (mg/dL) | 93.7 ± 1.83 a | 92.7 ± 2.44 a | 93.4 ± 2.76 a | 81.9 ± 2.99 b |
| Ca++ (mmol/L) | 1.59 ± 0.0196 a | 1.90 ± 0.0206 a | 1.90 ± 0.0200 a | 1.94 ± 0.0208 b |
| Lactate (mmol/L) | 4.74 ± 0.226 a | 6.86 ± 0.198 a,b | 7.54 ± 0.216 b | 8.29 ± 0.252 c |
| Hematocrit (%) | 45.3 ± 0.408 a | 48.7 ± 0.348 b | 50.0 ± 0.404 b | 51.8 ± 0.316 c |
| HCO3− (mmol/L) | 21.7 ± 0.357 a | 19.0 ± 0.322 b | 17.8 ± 0.305 b,c | 17.0 ± 0.321 c |
| Blood Trait | Q1 n = 100 | Q2 n = 108 | Q3 n = 108 | Q4 n = 109 |
|---|---|---|---|---|
| pH | 6.82 ± 0.0479 a | 6.80 ± 0.0449 a | 6.73 ± 0.0325 a | 6.82 ± 0.0176 a |
| pCO2 (mmHg) | 82.5 ± 2.37 a | 83.2 ± 1.34 ab | 91.7 ± 1.48 c | 95.1 ± 1.36 b,c |
| pO2 (mmHg) | 9.24 ± 0.531 a | 8.2 ± 0.932 a | 5.71 ± 0.486 b | 4.34 ± 0.386 b |
| Glucose (mg/dL) | 41.0 ± 6.06 a | 40.0 ± 4.34 a | 37.7 ± 2.10 a | 37.7 ± 2.26 a |
| Ca++ (mmol/L) | 2.12 ± 0.0630 a | 2.12 ± 0.0650 a | 2.26 ± 0.0474 a | 2.17 ± 0.0216 a |
| Lactate (mmol/L) | 12.3 ± 0.682 a | 12.1 ± 0.480 a | 14.3 ± 0.340 b | 14.7 ± 0.226 b |
| Hematocrit (%) | 59.9 ± 1.01 a | 60.5 ± 0.423 a | 59.3 ± 0.725 a | 59.1 ± 0.585 a |
| HCO3− (mmol/L) | 12.8 ± 0.685 a | 12.8 ± 0.685 a | 13.2 ± 0.571 a,b | 10.6 ± 0.343 b |
| Time | Q1 n = 110 | Q2 n = 108 | Q3 n = 108 | Q4 n = 109 |
|---|---|---|---|---|
| Minute 1 AB | 7.28 ± 0.173 a,1 | 6.80 ± 0.180 a,1 | 6.62 ± 0.187 a,b,1 | 5.77 ± 0.207 b,1 |
| Minute 5 AB | 7.64 ± 0.173 a,1,2 | 7.38 ± 0.180 a,2 | 7.28 ± 0.187 a,2 | 6.81 ± 0.207 a,2 |
| Minute 60 AB | 7.95 ± 0.173 a,2 | 7.73 ± 0.180 a,2 | 7.68 ± 0.187 a,2 | 7.48 ± 0.207 a,3 |
| Blood Trait | V 0–5 (Failed) n = 102 | V 6–7 (Medium) n = 105 | V 8–10 (High) n = 158 |
|---|---|---|---|
| Q1 | |||
| pH | 7.29 ± 0.0207 a,1 | 7.50 ± 0.0197 b,1 | 7.55 ± 0.0108 b,1 |
| pCO2 (mmHg) | 62.3 ± 2.30 a,1 | 42.5 ± 1.07 b,1 | 43.8 ± 0.362 b,1 |
| pO2 (mmHg) | 13.7 ± 0.578 a,1 | 18.7 ± 0.697 b,1 | 19.0 ± 0.306 b,1 |
| Glucose (mg/dL) | 62.8 ± 4.73 a,1 | 99.4 ± 2.55 b,1 | 101.0 ± 0.943 b,1 |
| Ca++ (mmol/L) | 1.92 ± 0.0519 a,1 | 1.56 ± 0.0259 b,1 | 1.51 ± 0.0108 b,1 |
| Lactate (mmol/L) | 8.09 ± 0.639 a,1 | 4.25 ± 0.325 b,1 | 3.91 ± 0.163 b,1 |
| Hematocrit (%) | 52.2 ± 0.858 a,1 | 43.6 ± 0.555 b,1 | 43.8 ± 0.273 b,1 |
| HCO3− (mmol/L) | 15.1 ± 0.665 a,1 | 22.2 ± 0.328 b,1 | 23.6 ± 0.189 b,1 |
| Q2 | |||
| pH | 7.09 ± 0.0214 a,2 | 7.27 ± 0.0159 b,2 | 7.31 ± 0.0196 b,2 |
| pCO2 (mmHg) | 75.3 ± 2.83 a,2 | 53.9 ± 1.84 b,2 | 48.6 ± 0.447 b,1,2 |
| pO2 (mmHg) | 11.0 ± 0.502 a,2 | 14.4 ± 0.443 b,2 | 16.4 ± 0.370 c,2 |
| Glucose (mg/dL) | 65.3 ± 4.67 a,1 | 95.1 ± 3.21 b,1 | 106.0 ± 2.59 b,1 |
| Ca++ (mmol/L) | 2.08 ± 0.0500 a,1 | 1.87 ± 0.0294 b,2 | 1.82 ± 0.0231 b,2 |
| Lactate (mmol/L) | 9.08 ± 0.416 a,1,2 | 6.96 ± 0.249 b,2 | 5.50 ± 0.145 c,2 |
| Hematocrit (%) | 51.5 ± 0.871 a,1 | 47.9 ± 0.519 b,2 | 47.8 ± 0.380 b,2 |
| HCO3− (mmol/L) | 14.6 ± 0.608 a,1 | 19.8 ± 0.385 b,2 | 20.6 ± 0.213 b,2 |
| Q3 | |||
| pH | 7.14 ± 0.0183 a,1,2 | 7.26 ± 0.0164 b,2 | 7.32 ± 0.0122 b,2 |
| pCO2 (mmHg) | 71.0 ± 2.70 a,1 | 57.1 ± 2.65 b,2 | 52.1 ± 1.28 b,2 |
| pO2 (mmHg) | 11.3 ± 0.472 a,1,2 | 14.2 ± 0.429 b,2 | 15.6 ± 0.371 b,2 |
| Glucose (mg/dL) | 71.3 ± 5.24 a,1 | 92.7 ± 4.69 b,1 | 108.0 ± 2.44 c,1 |
| Ca++ (mmol/L) | 2.05 ± 0.0427 a,1,2 | 1.89 ± 0.0336 b,2 | 1.81 ± 0.0171 b,2 |
| Lactate (mmol/L) | 9.75 ± 0.226 a,2 | 7.16 ± 0.377 b,2 | 6.36 ± 0.212 b,2 |
| Hematocrit (%) | 52.2 ± 0.976 a,1 | 48.9 ± 0.538 b,2 | 49.2 ± 0.498 b,2,3 |
| HCO3− (mmol/L) | 14.4 ± 0.382 a,1 | 18.6 ± 0.505 b,2,3 | 19.5 ± 0.204 b,2 |
| Q4 | |||
| pH | 7.17 ± 0.0150 a,2 | 7.28 ± 0.0147 b,2 | 7.33 ± 0.0169 b,2 |
| pCO2 (mmHg) | 71.4 ± 1.49 a,2 | 61.8 ± 3.13 b,2 | 51.9 ± 0.782 b,2 |
| pO2 (mmHg) | 11.2 ± 0.395 a,2 | 14.4 ± 0.507 b,2 | 13.3 ± 0.471 a,b,3 |
| Glucose (mg/dL) | 62.6 ± 1.82 a,1 | 89.8 ± 6.21 b,1 | 111.0 ± 1.96 c,1 |
| Ca++ (mmol/L) | 2.06 ± 0.0178 a,2 | 1.90 ± 0.0448 b,2 | 1.76 ± 0.0193 b,2 |
| Lactate (mmol/L) | 9.68 ± 0.228 a,2 | 8.26 ± 0.428 a,2 | 5.75 ± 0.226 b,2 |
| Hematocrit (%) | 53.1 ± 0.369 a,1 | 50.1 ± 0.546 b,2 | 50.6 ± 0.593 a,b,3 |
| HCO3− (mmol/L) | 15.4 ± 0.349 a,1 | 17.2 ± 0.690 a,3 | 19.7 ± 0.230 b,2 |
| Blood Trait | Absent | Mild | Moderate | Severe |
|---|---|---|---|---|
| Q1 | ||||
| pH | 7.54 ± 0.0098 a,1 | 7.47 ± 0.0323 a,1 | 7.11 ± 0.0919 b,1 | 7.21 ± 0.0476 b,1 |
| pCO2 (mmHg) | 43.5 ± 0.378 a,1 | 45.4 ± 2.45 a,1 | 69.8 ± 2.45 b,1 | 67.9 ± 3.79 b,1 |
| pO2 (mmHg) | 18.9 ± 0.314 a,1 | 18.5 ± 0.666 a,1 | 11.8 ± 0.869 b,1 | 12.2 ± 0.482 b,1 |
| Glucose (mg/dL) | 101.00 ± 0.877 a,1 | 95.4 ± 4.70 a,1 | 55.0 ± 5.59 b,1 | 51.0 ± 3.76 b,1 |
| Ca++ (mmol/L) | 1.51 ± 0.0102 a,1 | 1.60 ± 0.0485 a,1 | 2.04 ± 0.0371 b,1 | 1.98 ± 0.0659 b,1 |
| Lactate (mmol/L) | 3.87 ± 0.145 a,1 | 4.96 ± 0.604 a,1 | 8.95 ± 0.736 b,1,2 | 10.1 ± 0.749 b,1 |
| Hematocrit (%) | 43.8 ± 0.259 a,1 | 44.9 ± 1.49 a,1 | 55.0 ± 1.21 b,1 | 54.0 ± 1.15 b,1 |
| HCO3− (mmol/L) | 23.3 ± 0.189 a,1 | 21.9 ± 0.468 a,1 | 14.1 ± 0.435 b,1 | 13.5 ± 0.402 b,1 |
| Q2 | ||||
| pH | 7.29 ± 0.0154 a,2 | 7.26 ± 0.0224 a,1,2 | 7.14 ± 0.0280 a,1 | 6.90 ± 0.0380 b,2 |
| pCO2 (mmHg) | 49.6 ± 0.774 a,1,2 | 60.5 ± 3.56 a,b,1,2 | 68.2 ± 4.42 b,1 | 82.6 ± 1.31 c,2 |
| pO2 (mmHg) | 15.5 ± 0.371 a,2 | 13.8 ± 0.824 a,2 | 12.5 ± 0.484 a,b,1 | 9.04 ± 0.637 b,1,2 |
| Glucose (mg/dL) | 103.0 ± 1.99 a,1 | 87.6 ± 5.28 a,b,1 | 76.0 ± 6.78 b,1 | 44.3 ± 3.75 c,1 |
| Ca++ (mmol/L) | 1.82 ± 0.0176 a,2 | 1.95 ± 0.0453 a,b,2 | 1.99 ± 0.0560 b,1 | 2.15 ± 0.0595 b,1 |
| Lactate (mmol/L) | 6.02 ± 0.184 a,2 | 7.74 ± 0.373 a,1,2 | 8.18 ± 0.596 a,,1 | 11.0 ± 0.470 b,1 |
| Hematocrit (%) | 48.2 ± 0.435 a,2 | 49.0 ± 1.20 a,1,2 | 52.3 ± 1.14 a,b,1 | 54.2 ± 1.44 b,1 |
| HCO3− (mmol/L) | 20.6 ± 0.247 a,2 | 19.2 ± 0.469 a,1,2 | 14.8 ± 0.562 b,1 | 13.1 ± 0.380 b,1 |
| Q3 | ||||
| pH | 7.28 ± 0.0185 a,2 | 7.14 ± 0.0614 a,b,2,3 | 7.05 ± 0.0704 b,1 | 7.00 ± 0.0409 b,2 |
| pCO2 (mmHg) | 53.9 ± 1.58 a,2 | 64.9 ± 5.03 a,b,2 | 77.2 ± 4.34 b,1 | 77.7 ± 2.92 b,1,2 |
| pO2 (mmHg) | 14.9 ± 0.405 a,2,3 | 12.8 ± 0.976 a,b,2 | 9.75 ± 1.01 b,1 | 9.42 ± 0.709 b,1,2 |
| Glucose (mg/dL) | 105.0 ± 2.79 a,1 | 74.9 ± 7.15 b,1,2 | 63.4 ± 8.55 b,1 | 58.7 ± 4.92 b,1 |
| Ca++ (mmol/L) | 1.83 ± 0.0219 a,2 | 1.98 ± 0.0630 a,b,2 | 2.10 ± 0.0688 b,1 | 2.12 ± 0.0378 b,1 |
| Lactate (mmol/L) | 6.51 ± 0.258 a,2 | 9.46 ± 0.899 b,2,3 | 10.8 ± 0.832 b,1,2 | 11.4 ± 0.399 b,1 |
| Hematocrit (%) | 49.7 ± 0.445 a,2 | 51.2 ± 1.60 a,b,2,3 | 53.6 ± 1.89 a,b,1 | 54.2 ± 0.941 b,1 |
| HCO3− (mmol/L) | 19.5 ± 0.207 a,2 | 17.8 ± 0.712 a,2,3 | 14.2 ± 0.526 b,1 | 13.2 ± 0.273 b,1 |
| Q4 | ||||
| pH | 7.29 ± 0.0275 a,2 | 7.00 ± 0.0670 b,3 | 7.08 ± 0.0445 b,1 | 7.02 ± 0.0283 b,2 |
| pCO2 (mmHg) | 55.3 ± 2.13 a,2 | 83.5 ± 3.65 b,3 | 76.2 ± 3.05 b,1 | 83.1 ± 2.43 b,2 |
| pO2 (mmHg) | 12.7 ± 0.614 a,3 | 8.12 ± 1.13 b,3 | 10.0 ± 0.854 a,b,1 | 8.10 ± 0.731 b,2 |
| Glucose (mg/dL) | 103.0 ± 4.41 a,1 | 49.8 ± 4.14 b,2 | 61.2 ± 4.79 b,1 | 51.9 ± 3.05 b,1 |
| Ca++ (mmol/L) | 1.78 ± 0.0218 a,2 | 2.08 ± 0.0288 b,2 | 2.07 ± 0.0320 b,1 | 2.13 ± 0.0212 b,1 |
| Lactate (mmol/L) | 6.75 ± 0.443 a,2 | 11.9 ± 0.880 b,3 | 11.1 ± 0.630 b,2 | 12.0 ± 0.436 b,1 |
| Hematocrit (%) | 51.3 ± 0.689 a,2 | 56.7 ± 1.50 b,3 | 54.8 ± 0.970 a,b,1 | 55.0 ± 0.558 b,1 |
| HCO3− (mmol/L) | 19.1 ± 0.422 a,2 | 15.6 ± 0.970 b,3 | 12.8 ± 0.565 c,1 | 13.2 ± 0.514 b,c,1 |
| Staining Degree | Q1 n = 110 | Q2 n = 108 | Q3 n = 108 | Q4 n = 109 | ||||
|---|---|---|---|---|---|---|---|---|
| LB (%) | SB (%) | LB (%) | SB (%) | LB (%) | SB (%) | LB (%) | SB (%) | |
| Absent | 77 (70) a,1 | 0 a,1 | 58 (53) a,1 | 3 (2.7) a,1 | 51 (47.2) a,1 | 2 (1.8) a,1 | 26 (23.8) a,1 | 2 (1.8) a,1 |
| Mild | 12 (10.9) a,1,2 | 0 a,1,2 | 14 (12.9) a,1 | 0 a,1 | 11 (10.1) a,1 | 3 (2.7) a,1 | 6 (5.5) a,1 | 6 (5.5) a,1 |
| Moderate | 6 (5.4) a,2 | 3 (2.7) a,2 | 12 (11.1) a,1 | 2 (1.8) a,1 | 10 (9.2) a,1 | 5 (4.6) a,1 | 18 (16.5) a,1 | 10 (9.1) a,1 |
| Severe | 10 (9) a,1,2 | 2 (1.8) a,1,2 | 12 (11.1) a,1 | 7 (6.4) a,1 | 18 (16.6) a,1 | 8 (7.4) a,1 | 23 (21.1) a,1 | 18 (16.5) a,1 |
| Variables | Correlation Coefficient (rho) | p-Value |
|---|---|---|
| Q1 | ||
| pH | −0.125 | 0.193 |
| pCO2 (mmHg) | 0.273 | 0.004 * |
| pO2 (mmHg) | −0.137 | 0.154 |
| Glucose (mg/dL) | −0.165 | 0.085 |
| Ca++ (mmol/L) | 0.197 | 0.039 |
| Lactate (mmol/L) | 0.138 | 0.151 |
| Hematocrit (%) | 0.119 | 0.217 |
| HCO3− (mmol/L) | −0.109 | 0.257 |
| Q2 | ||
| pH | 0.178 | 0.065 |
| pCO2 (mmHg) | −0.205 | 0.034 * |
| PO2 (mmHg) | 0.168 | 0.082 |
| Glucose (mg/dL) | 0.324 | <0.001 * |
| Ca++ (mmol/L) | −0.038 | 0.695 |
| Lactate (mmol/L) | −0.214 | 0.026 * |
| Hematocrit (%) | −0.266 | 0.005 * |
| HCO3− (mmol/L) | 0.224 | 0.019 * |
| Q3 | ||
| pH | 0.167 | 0.084 |
| pCO2 (mmHg) | −0.006 | 0.954 |
| pO2 (mmHg) | 0.012 | 0.900 |
| Glucose (mg/dL) | 0.304 | 0.001 * |
| Ca++ (mmol/L) | −0.213 | 0.027 * |
| Lactate (mmol/L) | 0.063 | 0.516 |
| Hematocrit (%) | 0.017 | 0.861 |
| HCO3− (mmol/L) | 0.039 | 0.686 |
| Q4 | ||
| pH | −0.587 | <0.001 * |
| pCO2 (mmHg) | 0.621 | <0.001 * |
| PO2 (mmHg) | −0.512 | <0.001 * |
| Glucose (mg/dL) | −0.579 | <0.001 * |
| Ca++ (mmol/L) | 0.412 | <0.001 * |
| Lactate (mmol/L) | 0.603 | <0.001 * |
| Hematocrit (%) | 0.532 | <0.001 * |
| HCO3− (mmol/L) | −0.541 | <0.001 * |
| Variables | Correlation Coefficient (r) | p-Value |
|---|---|---|
| All puppies (quartiles) | 0.3319875 | p < 0.001 |
| Q1 | 0.111 | 0.248 |
| Q2 | −0.200 | 0.038 * |
| Q3 | 0.023 | 0.811 |
| Q4 | 0.214 | 0.025 * |
| Variables | Correlation Coefficient (r) | p-Value |
|---|---|---|
| Q1 | ||
| Vitality Score min 1 | 0.0644 | 0.514 |
| Vitality Score min 5 | 0.116 | 0.238 |
| Vitality Score min 60 | 0.0325 | 0.742 |
| Q2 | ||
| Vitality Score min 1 | 0.0587 | 0.568 |
| Vitality Score min 5 | 0.148 | 0.148 |
| Vitality Score min 60 | 0.174 | 0.0882 |
| Q3 | ||
| Vitality Score min 1 | −0.0323 | 0.762 |
| Vitality Score min 5 | 0.0430 | 0.687 |
| Vitality Score min 60 | 0.143 | 0.179 |
| Q4 | ||
| Vitality Score min 1 | 0.0912 | 0.443 |
| Vitality Score min 5 | 0.0822 | 0.489 |
| Vitality Score min 60 | 0.0887 | 0.456 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lezama-García, K.; Martínez-Burnes, J.; Baqueiro-Espinosa, U.; Olmos-Hernández, A.; Hernández-Ávalos, I.; Domínguez-Oliva, A.; Mota-Rojas, D. Assessment of Vitality, Blood Profile, and Degree of Meconium Staining on the Skin in Newborn Dogs According to Its Birth Weight. Vet. Sci. 2023, 10, 453. https://doi.org/10.3390/vetsci10070453
Lezama-García K, Martínez-Burnes J, Baqueiro-Espinosa U, Olmos-Hernández A, Hernández-Ávalos I, Domínguez-Oliva A, Mota-Rojas D. Assessment of Vitality, Blood Profile, and Degree of Meconium Staining on the Skin in Newborn Dogs According to Its Birth Weight. Veterinary Sciences. 2023; 10(7):453. https://doi.org/10.3390/vetsci10070453
Chicago/Turabian StyleLezama-García, Karina, Julio Martínez-Burnes, Uri Baqueiro-Espinosa, Adriana Olmos-Hernández, Ismael Hernández-Ávalos, Adriana Domínguez-Oliva, and Daniel Mota-Rojas. 2023. "Assessment of Vitality, Blood Profile, and Degree of Meconium Staining on the Skin in Newborn Dogs According to Its Birth Weight" Veterinary Sciences 10, no. 7: 453. https://doi.org/10.3390/vetsci10070453
APA StyleLezama-García, K., Martínez-Burnes, J., Baqueiro-Espinosa, U., Olmos-Hernández, A., Hernández-Ávalos, I., Domínguez-Oliva, A., & Mota-Rojas, D. (2023). Assessment of Vitality, Blood Profile, and Degree of Meconium Staining on the Skin in Newborn Dogs According to Its Birth Weight. Veterinary Sciences, 10(7), 453. https://doi.org/10.3390/vetsci10070453







