First Evidence of Familial Transmission of Hereditary Gastrointestinal Polyposis Associated with Germline APC Variant in Jack Russell Terriers
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Information on Examined Families
2.2. Pedigree Analysis
2.3. Germline APC Variant Genotyping
2.4. Clinical Tests
2.5. Histopathological Analysis
3. Results
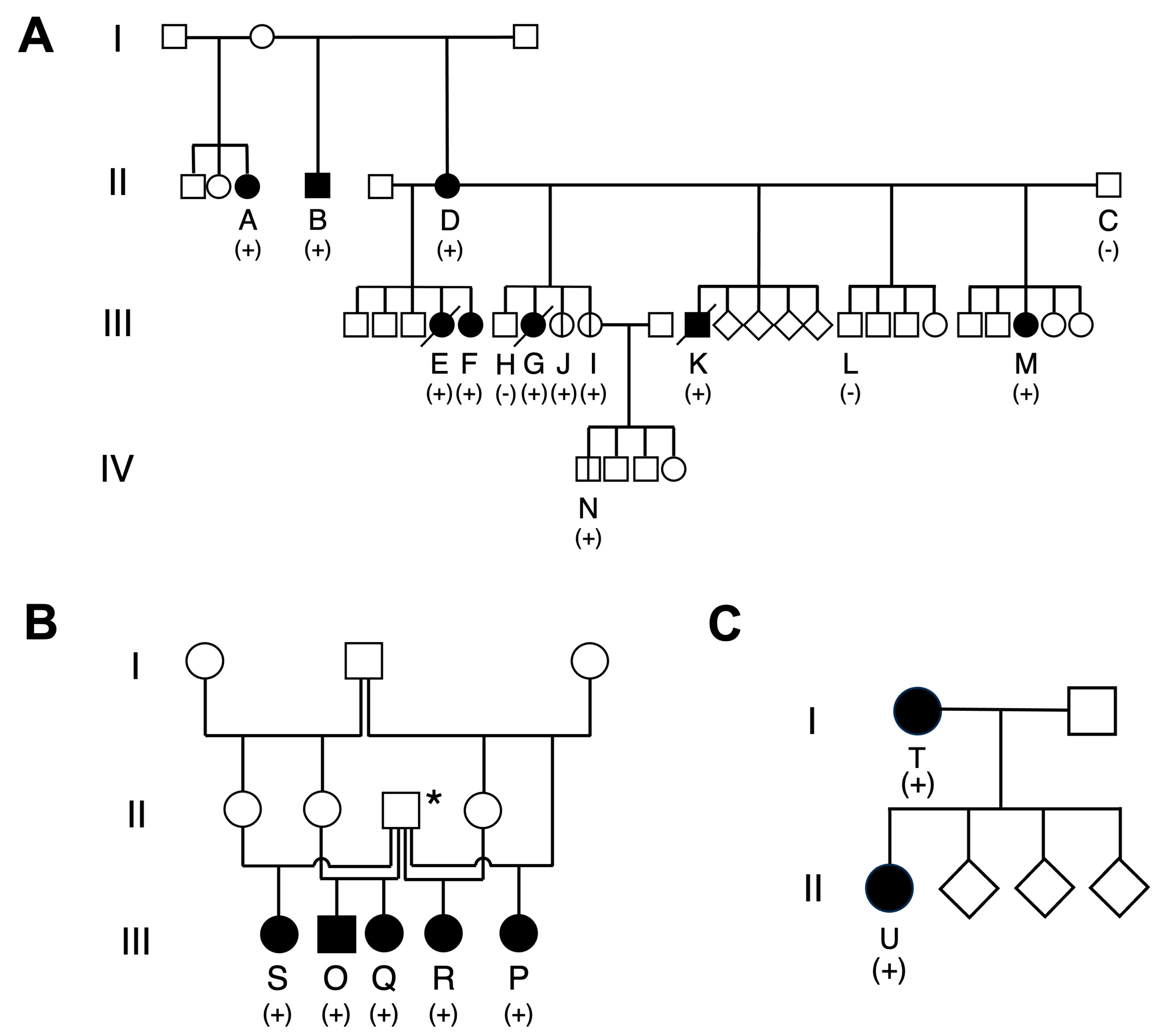
3.1. Family Structures, Germline APC Variant Status, and Medical History
3.1.1. Family 1
3.1.2. Family 2
3.1.3. Family 3
3.2. Disease Phenotype of the APC Variant Carriers
3.2.1. Brief Summary of the Medical Histories of the APC Variant Carriers
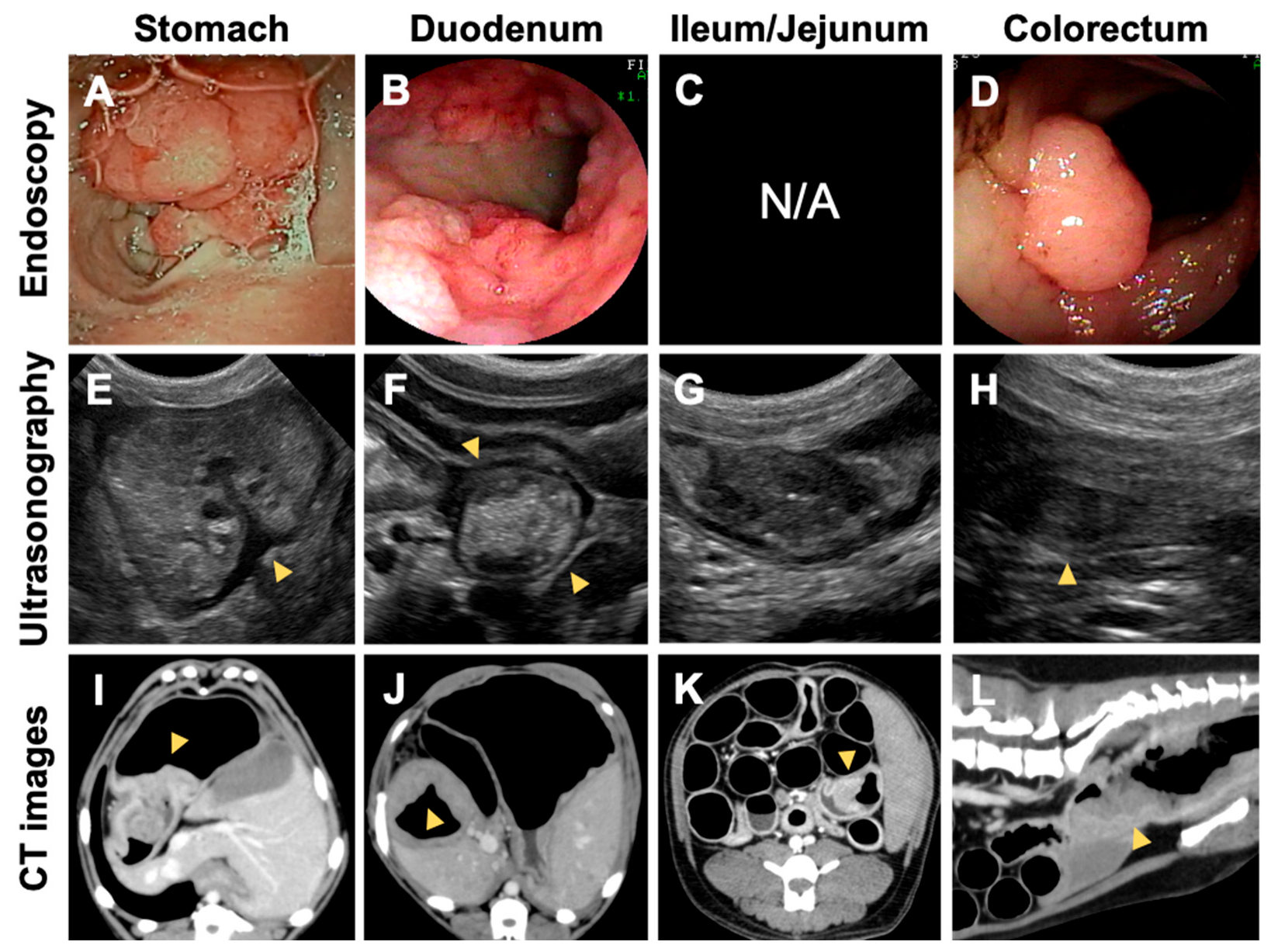
3.2.2. Number and Distribution of the GI Lesions
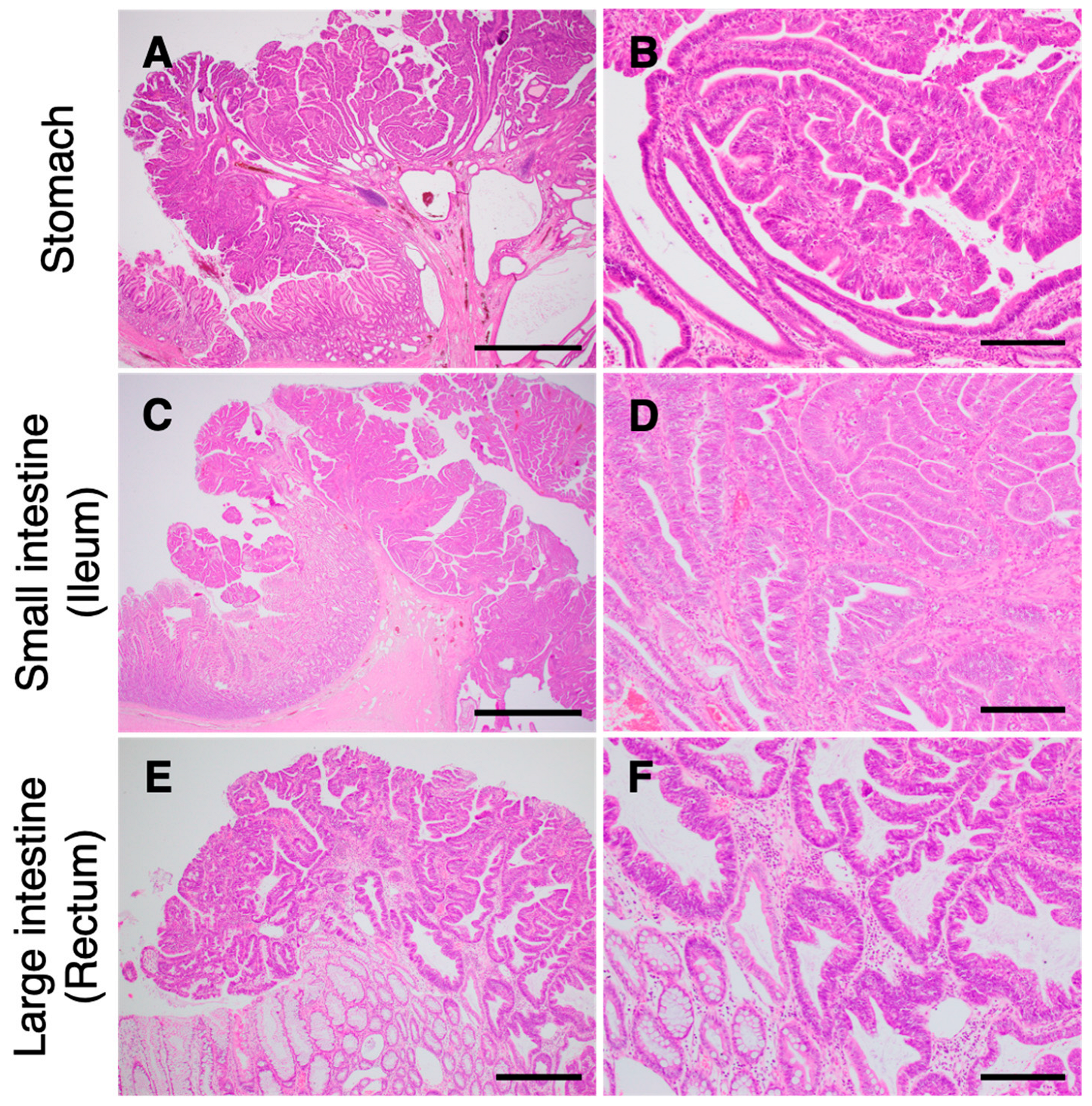
3.2.3. Pathological Findings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giger, U.; Sargan, D.R.; McNiel, E.A. Breed-specific Hereditary Diseases and Genetic Screening. In The Dog and Its Genome; Ostrander, E.A., Giger, U., Lindblad-Toh, K., Eds.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2006; pp. 249–289. [Google Scholar]
- Nicholas, F.W. Online Mendelian Inheritance in Animals (OMIA): A record of advances in animal genetics, freely available on the Internet for 25 years. Anim. Genet. 2021, 52, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Rokhsar, J.L.; Canino, J.; Raj, K.; Yuhnke, S.; Slutsky, J.; Giger, U. Web resource on available DNA variant tests for hereditary diseases and genetic predispositions in dogs and cats: An Update. Hum. Genet. 2021, 140, 1505–1515. [Google Scholar] [CrossRef] [PubMed]
- Japan Kennel Club. Number of Registered Dogs by Breeds. Available online: https://www.jkc.or.jp/archives/enrollment (accessed on 18 January 2023).
- Ohmi, A.; Ohno, K.; Chambers, J.K.; Uchida, K.; Nakagawa, T.; Tomiyasu, H.; Tsujimoto, H. Clinical and histopathological features and prognosis of gastrointestinal adenocarcinomas in Jack Russell Terriers. J. Vet. Med. Sci. 2021, 83, 167–173. [Google Scholar] [CrossRef]
- Saito, T.; Nibe, K.; Chambers, J.K.; Uneyama, M.; Nakashima, K.; Ohno, K.; Tsujimoto, H.; Uchida, K.; Nakayama, H. A histopathological study on spontaneous gastrointestinal epithelial tumors in dogs. J. Toxicol. Pathol. 2020, 33, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, K.; Hirata, A.; Nishii, N.; Kawabe, M.; Goto, M.; Mori, T.; Sakai, H. Familial adenomatous polyposis in dogs: Hereditary gastrointestinal polyposis in Jack Russell Terriers with germline APC mutations. Carcinogenesis 2021, 42, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Head, K.; Cullen, J.M.; Dubielzig, R.; Else, R.; Misdorp, W.; Patnaik, A.K.; Tateyama, S.; Gaag, I. Histological classification of tumors of the alimentary system of domestic animals. In WHO Histological Classification of Tumors of Domestic Animal; Armed Forces Institute of Pathology: Washington, DC, USA, 2003. [Google Scholar]
- Lingeman, C.H.; Garner, F.M. Comparative study of intestinal adenocarcinomas of animals and man. J. Natl. Cancer Inst. 1972, 48, 325–346. [Google Scholar]
- Munday, J.S.; Löhr, C.V.; Kiupel, M. Tumors of the Alimentary Tract. In Tumors in Domestic Animals, 5th ed.; Meuten, D.J., Ed.; Willey & Sons: Hoboken, NJ, USA, 2016; pp. 499–601. [Google Scholar]
- Patnaik, A.K.; Hurvitz, A.I.; Johnson, G.F. Canine gastrointestinal neoplasms. Vet. Pathol. 1977, 14, 547–555. [Google Scholar] [CrossRef]
- Moser, A.R.; Shoemaker, A.R.; Connelly, C.S.; Clipson, L.; Gould, K.A.; Luongo, C.; Dove, W.F.; Siggers, P.H.; Gardner, R.L. Homozygosity for the Minallele of Apc results in disruption of mouse development prior to gastrulation. Dev. Dyn. 1995, 203, 422–433. [Google Scholar] [CrossRef]
- Oshima, M.; Oshima, H.; Kitagawa, K.; Kobayashi, M.; Itakura, C.; Taketo, M. Loss of Apc heterozygosity and abnormal tissue building in nascent intestinal polyps in mice carrying a truncated Apc gene. Proc. Natl. Acad. Sci. USA 1995, 92, 4482–4486. [Google Scholar] [CrossRef]
- Yoneji, W.; Yoshizaki, K.; Hirata, A.; Yoneji, K.; Sakai, H. Clinical and Pathological Diagnosis of Hereditary Gastrointestinal Polyposis in Jack Russell Terriers. Vet. Sci. 2022, 9, 551. [Google Scholar] [CrossRef]
- Yoshizaki, K.; Hirata, A.; Matsushita, H.; Nishii, N.; Kawabe, M.; Mori, T.; Sakai, H. PCR-based genotyping assays to detect germline APC variant associated with hereditary gastrointestinal polyposis in Jack Russell terriers. BMC Vet. Res. 2021, 17, 32. [Google Scholar] [CrossRef] [PubMed]
- Giardiello, F.M.; Burt, R.W.; Järvinin, H.J.; Offerhaus, G.J. Familial adenomatous polyposis. In WHO Classification of Tumours of the Digestive System, 4th ed.; Bosman, F.T., Carneiro, F., Hruban, R.H., Theise, N.D., Eds.; World Health Organization Classification of Tumours; World Health Organization: Lyon, France, 2010; pp. 147–151. [Google Scholar]
- The UMD-APC Mutations Database. Available online: http://www.umd.be/APC/ (accessed on 18 January 2023).
- Cerasuolo, A.; Miele, E.; Russo, M.; Aversano, A.; Cammarota, F.; Duraturo, F.; Liccardo, R.; Izzo, P.; Rosa, M. Sporadic pediatric severe familial adenomatous polyposis: A case report. Mol. Clin. Oncol. 2020, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- D’Elia, G.; Caliendo, G.; Casamassimi, A.; Cioffi, M.; Molinari, A.M.; Vietri, M.T. APC and MUTYH Analysis in FAP Patients: A Novel Mutation in APC Gene and Genotype-Phenotype Correlation. Genes 2018, 9, 322. [Google Scholar] [CrossRef]
- Djursby, M.; Wadt, K.; Frederiksen, J.H.; Madsen, M.B.; Berchtold, L.A.; Hasselby, J.P.; Willemoe, G.L.; Hansen, T.V.O.; Gerdes, A.M. A rare missense variant in APC interrupts splicing and causes AFAP in two Danish families. Hered. Cancer Clin. Pract. 2020, 18, 8. [Google Scholar] [CrossRef] [PubMed]
- Pang, M.; Liu, Y.; Hou, X.; Yang, J.; He, X.; Hou, N.; Liu, P.; Liang, L.; Fu, J.; Wang, K.; et al. A novel APC mutation identified in a large Chinese family with familial adenomatous polyposis and a brief literature review. Mol. Med. Rep. 2018, 18, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Christen, M.; de le Roi, M.; Jagannathan, V.; Becker, K.; Leeb, T. MYO5A Frameshift Variant in a Miniature Dachshund with Coat Color Dilution and Neurological Defects Resembling Human Griscelli Syndrome Type 1. Genes 2021, 12, 1479. [Google Scholar] [CrossRef] [PubMed]
- Thaiwong, T.; Corner, S.; Forge, S.; Kiupel, M. Dwarfism in Tibetan Terrier dogs with an LHX3 mutation. J. Vet. Diagn. Investig. 2021, 33, 740–743. [Google Scholar] [CrossRef]
- Tsuboi, M.; Watanabe, M.; Nibe, K.; Yoshimi, N.; Kato, A.; Sakaguchi, M.; Yamato, O.; Tanaka, M.; Kuwamura, M.; Kushida, K.; et al. Identification of the PLA2G6 c.1579G>A Missense Mutation in Papillon Dog Neuroaxonal Dystrophy Using Whole Exome Sequencing Analysis. PLoS ONE 2017, 12, e0169002. [Google Scholar] [CrossRef]
- Yamato, O.; Ochiai, K.; Masuoka, Y.; Hayashida, E.; Tajima, M.; Omae, S.; Iijima, M.; Umemura, T.; Maede, Y. GM1 gangliosidosis in shiba dogs. Vet. Rec. 2000, 146, 493–496. [Google Scholar] [CrossRef]
- Yoshizaki, K.; Hirata, A.; Matsushita, H.; Sakaguchi, M.; Yoneji, W.; Owaki, K.; Sakai, H. Molecular epidemiological study of germline APC variant associated with hereditary gastrointestinal polyposis in dogs: Current frequency in Jack Russell Terriers in Japan and breed distribution. BMC Vet. Res. 2022, 18, 230. [Google Scholar] [CrossRef]
- Jasperson, K.W.; Tuohy, T.M.; Neklason, D.W.; Burt, R.W. Hereditary and familial colon cancer. Gastroenterology 2010, 138, 2044–2058. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, M.D.; Tomlinson, I.P.; Hodgson, S.V.; Neale, K.; Phillips, R.K.; Houlston, R.S. Explaining variation in familial adenomatous polyposis: Relationship between genotype and phenotype and evidence for modifier genes. Gut 2002, 51, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Connor, T.; McPhillips, M.; Hipwell, M.; Ziolkowski, A.; Oldmeadow, C.; Clapham, M.; Pockney, P.G.; Lis, E.; Banasiewicz, T.; Pławski, A.; et al. CD36 polymorphisms and the age of disease onset in patients with pathogenic variants within the mutation cluster region of APC. Hered. Cancer Clin. Pract. 2021, 19, 25. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, M.D.; Fletcher, C.; Churchman, M.; Hodgson, S.V.; Neale, K.; Phillips, R.K.; Tomlinson, I.P. Analysis of candidate modifier loci for the severity of colonic familial adenomatous polyposis, with evidence for the importance of the N-acetyl transferases. Gut 2004, 53, 271–276. [Google Scholar] [CrossRef]
| Dog No. | Birthday | Sex | APC Variant Status | Onset Age ‡ | Gastro Intestinal Sign | Age at Death | Cumulative Number and Location of Gastrointestinal Polyps | Treatment for Gastrointestinal Polyps | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stomach | Duodenum | Jejunum and Ileum | Large Intestine | Surgical Treatment | Medical Treatment | |||||||
| Family 1 | ||||||||||||
| Generation II † | ||||||||||||
| A | 13 April 2010 | F | Carrier | 6 y 2 m | + | 5 | 0 | 0 | 0 | + | ||
| B | 18 March 2013 | M | Carrier | 4 y 10 m | + | 0 | 0 | 0 | 3 | + | ||
| C | 18 July 2013 | M | Non-carrier | − | − | |||||||
| D | 10 October 2013 | F | Carrier | 4 y 7 m | + | 2 | 0 | 2 | 2 | + | ||
| Generation III † | ||||||||||||
| E | 28 October 2014 | F | Carrier | 4 y 10 m | + | 7.6 y § | 10 | 0 | 2 | 15 | + | NSAID |
| F | 28 October 2014 | F | Carrier | 7 y 3 m | + | 1 | 0 | 0 | 8 | + | NSAID | |
| G | 29 May 2015 | F | Carrier | 3 y 2 m | + | 6.3 y §§ | 5 | 1 * | 9 | 3 | + | NSAID, Toceranib |
| H | 29 May 2015 | M | Non-carrier | − | − | |||||||
| I | 29 May 2015 | F | Carrier | − (at 4.4 years old) | NT | NT | NT | NT | − | |||
| J | 29 May 2015 | F | Carrier | − (at 5 years old) | NT | NT | NT | NT | − | |||
| K | 19 November 2015 | M | Carrier | 3 y 3 m | + | 4.6 y §§§ | 1 | 1 | 7 | 11 | + | NSAID, Toceranib |
| L | 30 June 2016 | M | Non-carrier | − | − | |||||||
| M | 11 December 2016 | F | Carrier | 3 y 10 m | + | 0 | 0 | 4 | 5 | + | ||
| Generation IV † | ||||||||||||
| N | 30 May 2017 | M | Carrier | NT | + | NT | NT | NT | NT | − | ||
| Family 2 | ||||||||||||
| O | 3 November 2011 | M | Carrier | 7 y 3 m | + | 5 | 0 | 0 | 0 | + | NSAID | |
| P | 12 July 2013 | F | Carrier | 8 y | + | 4 | 0 | 0 | 0 | + | NSAID | |
| Q | 30 September 2013 | F | Carrier | 6 y 8 m | + | 0 | 1 * | 1 | 1 | − | NSAID | |
| R | 15 October 2014 | F | Carrier | 6 y | + | 1 | 0 | NT | NT | − | ||
| S | 21 January 2017 | F | Carrier | 3 y 10 m | + | NT | NT | NT | 1 | + | ||
| Family 3 | ||||||||||||
| T | 25 November 2008 | F | Carrier | 5 y 1 m | + | 8.9 y §§§§ | 2 | 0 | 0 | 1 | ||
| U | 24 January 2012 | F | Carrier | 8 y 9 m | + | 3 | 0 | 0 | 1 | |||
| Onset Age of Gastrointestinal Polyps | |||
|---|---|---|---|
| Aged < 5 Years | Aged ≥ 5 Years | p-Value * | |
| Family 1 † | 6 | 3 | 0.01 |
| Previous cases (Ref. [7]) ‡ | 3 | 17 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoneji, W.; Yoshizaki, K.; Hirota, T.; Yoneji, K.; Yoshikawa, R.; Mori, T.; Sakai, H.; Hirata, A. First Evidence of Familial Transmission of Hereditary Gastrointestinal Polyposis Associated with Germline APC Variant in Jack Russell Terriers. Vet. Sci. 2023, 10, 439. https://doi.org/10.3390/vetsci10070439
Yoneji W, Yoshizaki K, Hirota T, Yoneji K, Yoshikawa R, Mori T, Sakai H, Hirata A. First Evidence of Familial Transmission of Hereditary Gastrointestinal Polyposis Associated with Germline APC Variant in Jack Russell Terriers. Veterinary Sciences. 2023; 10(7):439. https://doi.org/10.3390/vetsci10070439
Chicago/Turabian StyleYoneji, Wakana, Kyoko Yoshizaki, Teruaki Hirota, Kensuke Yoneji, Ryutaro Yoshikawa, Takashi Mori, Hiroki Sakai, and Akihiro Hirata. 2023. "First Evidence of Familial Transmission of Hereditary Gastrointestinal Polyposis Associated with Germline APC Variant in Jack Russell Terriers" Veterinary Sciences 10, no. 7: 439. https://doi.org/10.3390/vetsci10070439
APA StyleYoneji, W., Yoshizaki, K., Hirota, T., Yoneji, K., Yoshikawa, R., Mori, T., Sakai, H., & Hirata, A. (2023). First Evidence of Familial Transmission of Hereditary Gastrointestinal Polyposis Associated with Germline APC Variant in Jack Russell Terriers. Veterinary Sciences, 10(7), 439. https://doi.org/10.3390/vetsci10070439





