Off-Label Use of Crisdesalazine (GedaCure) in Meningoencephalitis in Two Dogs
Abstract
Simple Summary
Abstract
1. Introduction
2. Case Description
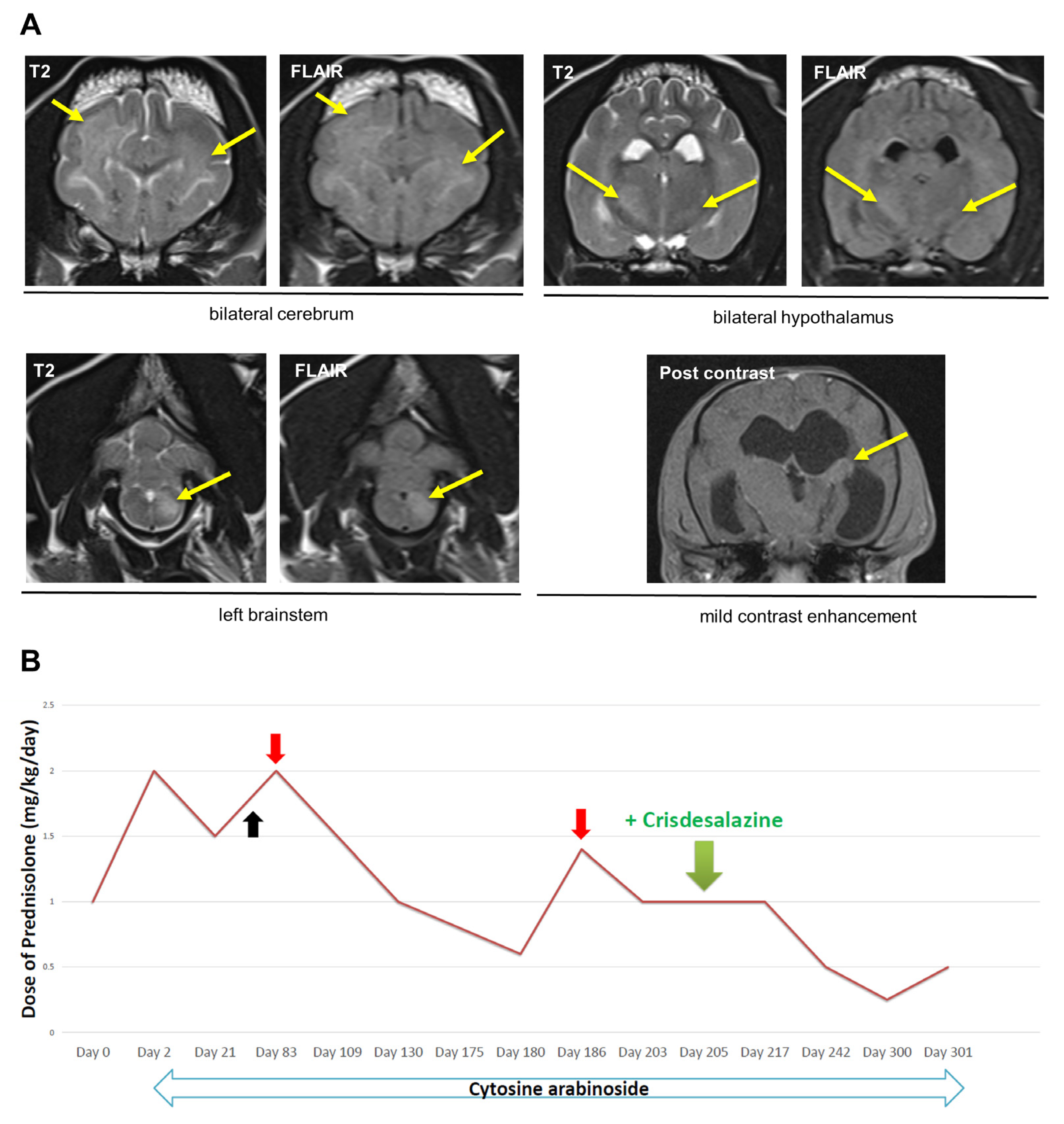
2.1. Dog 1
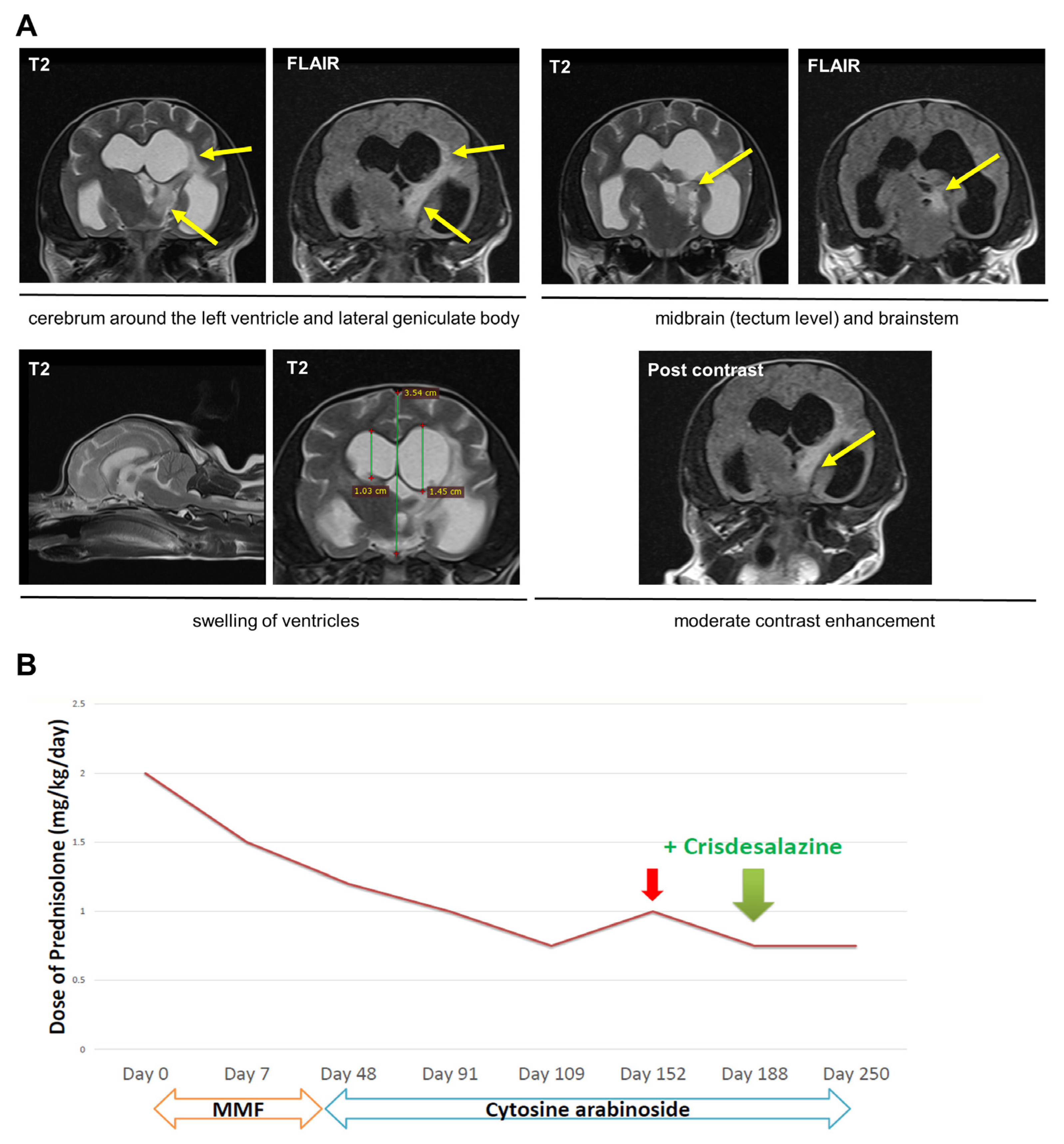
2.2. Dog 2
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Talarico, L.R.; Schatzberg, S.J. Idiopathic granulomatous and necrotising inflammatory disorders of the canine central nervous system: A review and future perspectives. J. Small Anim. Pract. 2010, 51, 138–149. [Google Scholar] [CrossRef]
- Cornelis, I.; Van Ham, L.; Gielen, I.; De Decker, S.; Bhatti, S.F.M. Clinical presentation, diagnostic findings, prognostic factors, treatment and outcome in dogs with meningoencephalomyelitis of unknown origin: A review. Vet. J. 2019, 244, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, N.; Granger, N. New insights into the treatment of meningoencephalomyelitis of unknown origin since 2009: A review of 671 cases. Front. Vet. Sci. 2023, 10, 1114798. [Google Scholar] [CrossRef] [PubMed]
- Brady, S.L.; Woodward, A.P.; le Chevoir, M. Survival time and relapse in dogs with meningoencephalomyelitis of unknown origin treated with prednisolone and ciclosporin: A retrospective study. Aust. Vet. J. 2020, 98, 491–498. [Google Scholar] [CrossRef]
- Barber, R.; Downey Koos, L. Treatment with Cytarabine at Initiation of Therapy with Cyclosporine and Glucocorticoids for Dogs with Meningoencephalomyelitis of Unknown Origin Is not Associated with Improved Outcomes. Front. Vet. Sci. 2022, 9, 925774. [Google Scholar] [CrossRef] [PubMed]
- Lawn, R.W.; Harcourt-Brown, T.R. Risk factors for early death or euthanasia within 100 days of diagnosis in dogs with meningoencephalitis of unknown origin. Vet. J. 2022, 287, 105884. [Google Scholar] [CrossRef]
- Beasley, M.J.; Shores, A. Perspectives on pharmacologic strategies in the management of meningoencephalomyelitis of unknown origin in dogs. Front. Vet. Sci. 2023, 10, 1167002. [Google Scholar] [CrossRef]
- Bhatti, S.F.; De Risio, L.; Muñana, K.; Penderis, J.; Stein, V.M.; Tipold, A.; Berendt, M.; Farquhar, R.G.; Fischer, A.; Long, S.; et al. International Veterinary Epilepsy Task Force consensus proposal: Medical treatment of canine epilepsy in Europe. BMC Vet. Res. 2015, 11, 176. [Google Scholar] [CrossRef]
- Podell, M.; Volk, H.A.; Berendt, M.; Löscher, W.; Muñana, K.; Patterson, E.E.; Platt, S.R. 2015 ACVIM Small Animal Consensus Statement on Seizure Management in Dogs. J. Vet. Intern. Med. 2016, 30, 477–490. [Google Scholar] [CrossRef]
- Shin, J.H.; Lee, Y.A.; Lee, J.K.; Lee, Y.B.; Cho, W.; Im, D.S.; Lee, J.H.; Yun, B.S.; Springer, J.E.; Gwag, B.J. Concurrent blockade of free radical and microsomal prostaglandin E synthase-1-mediated PGE2 production improves safety and efficacy in a mouse model of amyotrophic lateral sclerosis. J. Neurochem. 2012, 122, 952–961. [Google Scholar] [CrossRef]
- Ryu, B.R.; Lee, Y.A.; Won, S.J.; Noh, J.H.; Chang, S.Y.; Chung, J.M.; Choi, J.S.; Joo, C.K.; Yoon, S.H.; Gwag, B.J. The novel neuroprotective action of sulfasalazine through blockade of NMDA receptors. J. Pharmacol. Exp. Ther. 2003, 305, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Baek, I.S.; Kim, T.K.; Seo, J.S.; Lee, K.W.; Lee, Y.A.; Cho, J.; Gwag, B.J.; Han, P.L. AAD-2004 attenuates progressive neuronal loss in the brain of Tg-betaCTF99/B6 mouse model of Alzheimer disease. Exp. Neurobiol. 2013, 22, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Ikeda-Matsuo, Y. The role of mPGES-1 in inflammatory brain diseases. Biol. Pharm. Bull. 2017, 40, 557–563. [Google Scholar] [CrossRef]
- Chaudhry, U.A.; Zhuang, H.; Crain, B.J.; Doré, S. Elevated microsomal prostaglandin-E synthase-1 in Alzheimer’s disease. Alzheimers Dement. 2008, 4, 6–13. [Google Scholar] [CrossRef]
- Lee, H.H.; Moon, Y.; Shin, J.S.; Lee, J.H.; Kim, T.W.; Jang, C.; Park, C.; Lee, J.; Kim, Y.; Kim, Y.; et al. A novel mPGES-1 inhibitor alleviates inflammatory responses by downregulating PGE2 in experimental models. Prostaglandins Other Lipid Mediat. 2019, 144, 106347. [Google Scholar] [CrossRef]
- Herzig, R.; Beckmann, K.; Körner, M.; Steffen, F.; Rohrer Bley, C. A shortened whole brain radiation therapy protocol for meningoencephalitis of unknown origin in dogs. Front. Vet. Sci. 2023, 10, 1132736. [Google Scholar] [CrossRef] [PubMed]
- Nessler, J.N.; Oevermann, A.; Schawacht, M.; Gerhauser, I.; Spitzbarth, I.; Bittermann, S.; Steffen, F.; Schmidt, M.J.; Tipold, A. Concomitant necrotizing encephalitis and granulomatous meningoencephalitis in four toy breed dogs. Front. Vet. Sci. 2022, 9, 957285. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef]
- Ranguelova, K.; Mason, R.P. The fidelity of spin trapping with DMPO in biological systems. Magn. Reson. Chem. 2011, 49, 152–158. [Google Scholar] [CrossRef]
- Buettner, G.R. Spin trapping: ESR parameters of spin adducts. Free Radic. Biol. Med. 1987, 3, 259–303. [Google Scholar] [CrossRef]
- Günther, C.; Steffen, F.; Alder, D.S.; Beatrice, L.; Geigy, C.; Beckmann, K. Evaluating the use of cytosine arabinoside for treatment for recurrent canine steroid-responsive meningitis-arteritis. Vet. Rec. 2020, 187, e7. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, K.; Lappin, M.; Legare, M.; Veir, J. A retrospective study of adverse effects of mycophenolate mofetil administration to dogs with immune-mediated disease. J. Vet. Intern. Med. 2021, 35, 2215–2221. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Song, W.-J.; Park, J.; Kim, M.; Choen, S.; Kim, M.-C.; Jeong, H.; Yun, Y. Off-Label Use of Crisdesalazine (GedaCure) in Meningoencephalitis in Two Dogs. Vet. Sci. 2023, 10, 438. https://doi.org/10.3390/vetsci10070438
Lee S, Song W-J, Park J, Kim M, Choen S, Kim M-C, Jeong H, Yun Y. Off-Label Use of Crisdesalazine (GedaCure) in Meningoencephalitis in Two Dogs. Veterinary Sciences. 2023; 10(7):438. https://doi.org/10.3390/vetsci10070438
Chicago/Turabian StyleLee, Saeyoung, Woo-Jin Song, Jongjin Park, Minkun Kim, Sangkyung Choen, Myung-Chul Kim, Hyohoon Jeong, and Youngmin Yun. 2023. "Off-Label Use of Crisdesalazine (GedaCure) in Meningoencephalitis in Two Dogs" Veterinary Sciences 10, no. 7: 438. https://doi.org/10.3390/vetsci10070438
APA StyleLee, S., Song, W.-J., Park, J., Kim, M., Choen, S., Kim, M.-C., Jeong, H., & Yun, Y. (2023). Off-Label Use of Crisdesalazine (GedaCure) in Meningoencephalitis in Two Dogs. Veterinary Sciences, 10(7), 438. https://doi.org/10.3390/vetsci10070438





