Effects of Anemoside B4 on Plasma Metabolites in Cows with Clinical Mastitis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care
2.2. Preparation of Anemoside B4
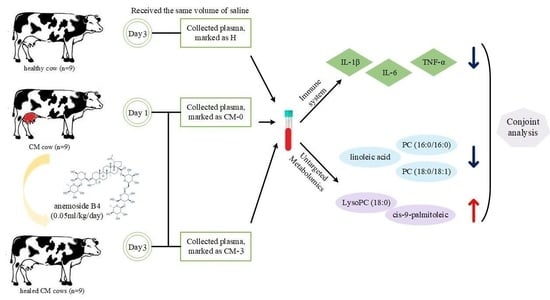
2.3. Experimental Design, Cows, and Management
2.4. Sample Collection
2.5. Biochemical Analysis
2.6. Metabolic Profiling Analysis
2.7. Data Processing and Statistical Analysis
3. Results
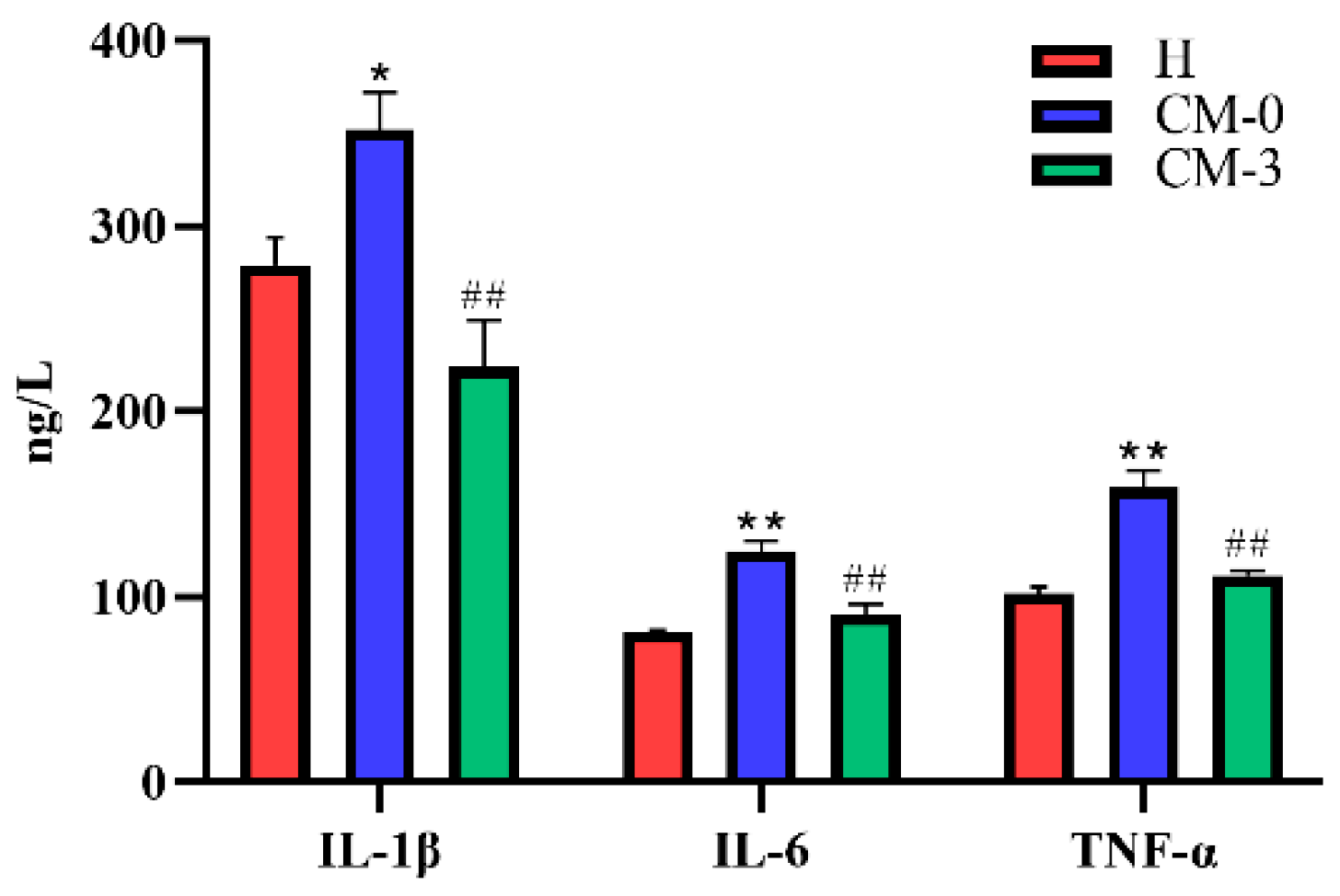
3.1. Biochemical Analysis
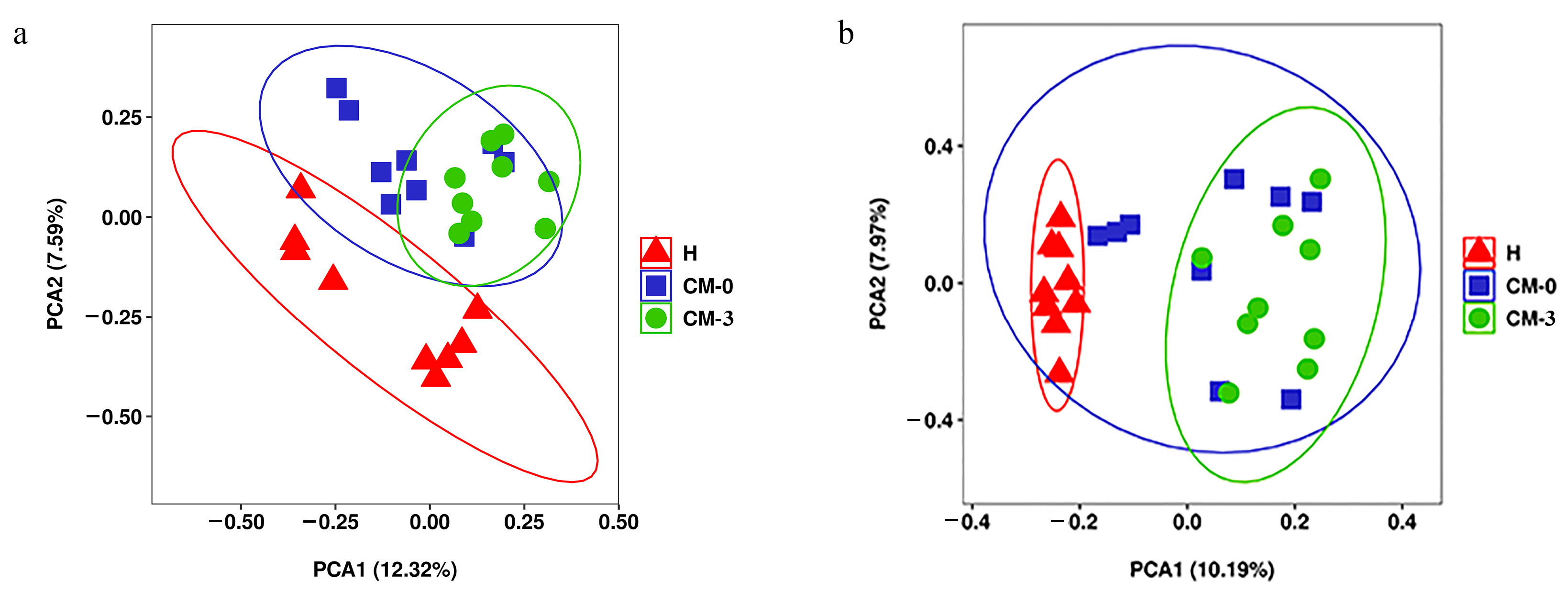
3.2. Metabolite Profiles and Data Analysis
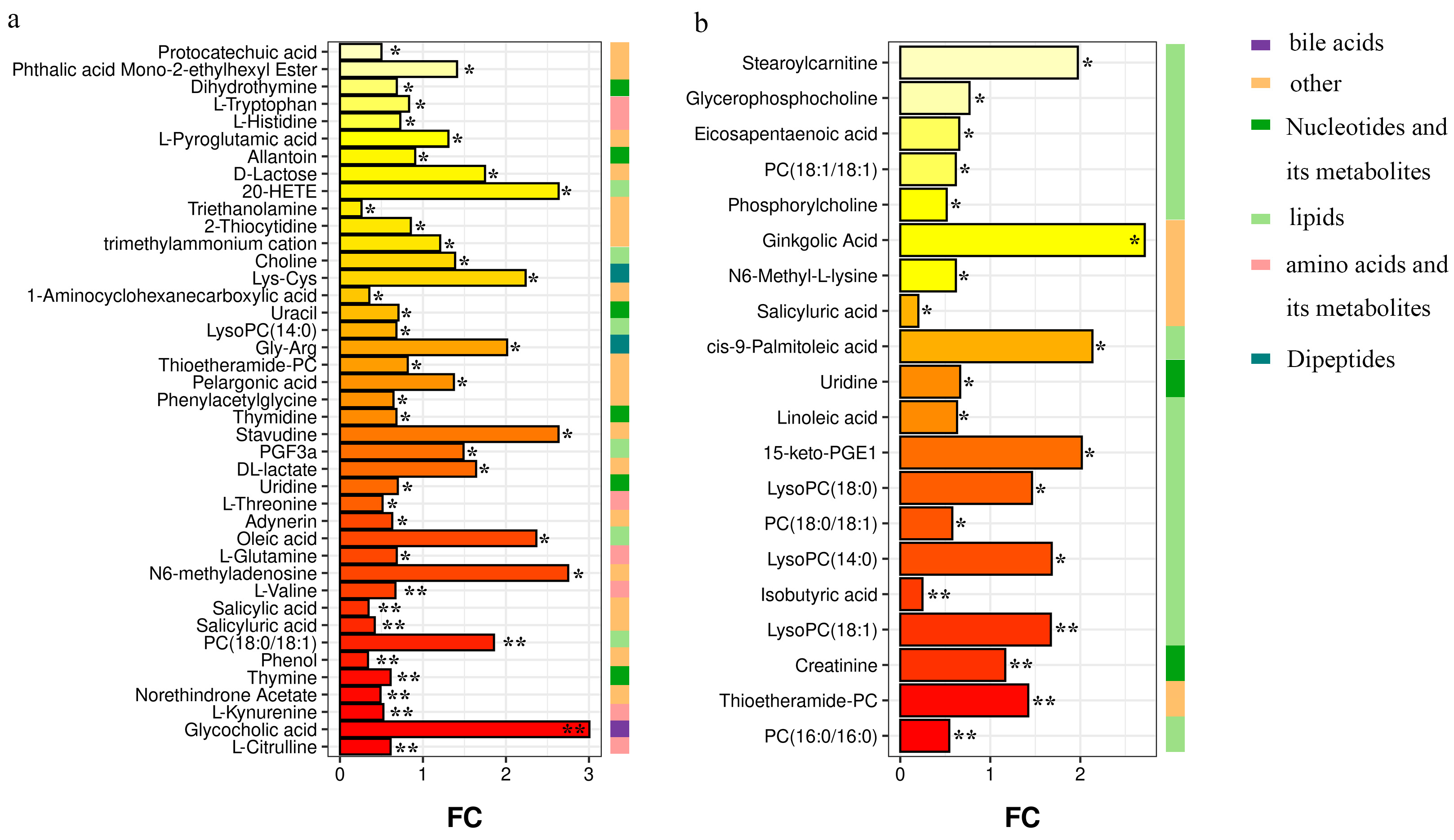
3.3. Differential Metabolites among Groups
3.4. Pathway Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rollin, E.; Dhuyvetter, K.C.; Overton, M.W. The cost of clinical mastitis in the first 30 days of lactation: An economic modeling tool. Prev. Vet. Med. 2015, 122, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Halasa, T.; Huijps, K.; Osteras, O.; Hogeveen, H. Economic effects of bovine mastitis and mastitis management: A review. Vet. Q. 2007, 29, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Gao, Y.; Xue, Y.; Liu, Y.; Zeng, X.; Cheng, Y.; Ma, J.; Wang, H.; Sun, J.; Wang, Z.; et al. Bacteriophage cocktails protect dairy cows against mastitis caused by drug resistant escherichia coli infection. Front. Cell. Infect. Microbiol. 2021, 11, 690377. [Google Scholar] [CrossRef] [PubMed]
- Abdelmegid, S.; Murugaiyan, J.; Abo-Ismail, M.; Caswell, J.L.; Kelton, D.; Kirby, G.M. Identification of Host Defense-Related Proteins Using Label-Free Quantitative Proteomic Analysis of Milk Whey from Cows with Staphylococcus aureus Subclinical Mastitis. Int. J. Mol. Sci. 2017, 19, 78. [Google Scholar] [CrossRef]
- Russell, C.D.; Widdison, S.; Leigh, J.A.; Coffey, T.J. Identification of single nucleotide polymorphisms in the bovine Toll-like receptor 1 gene and association with health traits in cattle. Vet. Res. 2012, 43, 17. [Google Scholar] [CrossRef]
- Johnzon, C.-F.; Dahlberg, J.; Gustafson, A.-M.; Waern, I.; Moazzami, A.A.; Östensson, K.; Pejler, G. The effect of Lipopolysaccharide-Induced experimental bovine mastitis on clinical parameters, inflammatory markers, and the metabolome: A kinetic approach. Front. Immunol. 2018, 9, 1487. [Google Scholar] [CrossRef]
- Sordillo, L.M.; Mavangira, V. The nexus between nutrient metabolism, oxidative stress and inflammation in transition cows. Anim. Prod. Sci. 2014, 54, 1204–1214. [Google Scholar] [CrossRef]
- Akers, R.M.; Nickerson, S.C. Mastitis and its impact on structure and function in the ruminant mammary gland. J. Mammary Gland. Biol. Neoplasia 2011, 16, 275–289. [Google Scholar] [CrossRef]
- Schabauer, A.; Pinior, B.; Gruber, C.-M.; Firth, C.L.; Käsbohrer, A.; Wagner, M.; Rychli, K.; Obritzhauser, W. The relationship between clinical signs and microbiological species, spa type, and antimicrobial resistance in bovine mastitis cases in Austria. Vet. Microbiol. 2018, 227, 52–60. [Google Scholar] [CrossRef]
- de Jong, A.; El Garch, F.; Simjee, S.; Moyaert, H.; Rose, M.; Youala, M.; Siegwart, E. Monitoring of antimicrobial susceptibility of udder pathogens recovered from cases of clinical mastitis in dairy cows across Europe: VetPath results. Vet. Microbiol. 2018, 213, 73–81. [Google Scholar] [CrossRef]
- Tang, X.; Liu, C.; Li, T.; Lin, C.; Hao, Z.; Zhang, H.; Zhao, G.; Chen, Y.; Guo, A.; Hu, C. Gambogic acid alleviates inflammation and apoptosis and protects the blood-milk barrier in mastitis induced by LPS. Int. Immunopharmacol. 2020, 86, 106697. [Google Scholar] [CrossRef]
- Li, Y.H.; Zou, M.; Han, Q.; Deng, L.R.; Weinshilboum, R.M. Therapeutic potential of triterpenoid saponin anemoside B4 from Pulsatilla chinensis. Pharmacol. Res. 2020, 160, 105079. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Tan, L.; Chen, M.; He, C. Pharmacological activities and molecular mechanisms of Pulsatilla saponins. Chin Med. 2022, 17, 59. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.-M.; Shu, Z.; He, W.-J.; Chen, L.-Y.; Yang, S.-L.; Yang, G.; Liu, Y.-L.; Li, X.-R. Antitumor activity of Pulsatilla chinensis (Bunge) Regel saponins in human liver tumor 7402 cells in vitro and in vivo. Phytomedicine 2012, 19, 293–300. [Google Scholar] [CrossRef]
- Pei, L.; He, L. Hepatoprotective effect of anemoside B4 against sepsis-induced acute liver injury through modulating the mTOR/p70S6K-mediated autophagy. Chem. Biol. Interact. 2021, 345, 109534. [Google Scholar] [CrossRef]
- Kang, N.; Shen, W.; Zhang, Y.; Su, Z.; Yang, S.; Liu, Y.; Xu, Q. Anti-inflammatory and immune-modulatory properties of anemoside B4 isolated from Pulsatilla chinensis in vivo. Phytomedicine 2019, 64, 152934. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.; You, L.; Zhang, Y.; Shen, Y.; Lyu, S.; Xiao, J.; Su, Z.; Dong, K.; Pei, M.; Zuo, C.; et al. Effects of pulsatilla saponin b4 on somatic cell count, enzymes, inflammtory and antioxidant factors in milk of dairy cows with clinical mastitis. Acta Agric. Univ. Jiangxiensis 2021, 43, 873–880. [Google Scholar] [CrossRef]
- Shen, L.H.; Qian, B.L.; You, L.C.; Zhang, Y.; Shen, Y.; Lyu, S.K.; Xiao, J.; Yu, S.; Su, Z.; Dong, K.; et al. Effect of Pulsatilla saponin B4 on treatment efficiency and serum inflammatory and immune factors of dairy cows with clinical mastitis. Acta Agric. Zhejiangensis 2021, 33, 1184–1191. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, X.; Lu, X.; Li, Y.; Zhan, C.; Akhtar, M.L.; Yang, L.; Bai, Y.; Zhao, J.; Wang, Y.; et al. Novel metabolomics serum biomarkers for pancreatic ductal adenocarcinoma by the comparison of pre-, postoperative and normal samples. J. Cancer 2020, 11, 4641–4651. [Google Scholar] [CrossRef]
- Cheng, S.; Shah, S.H.; Corwin, E.J.; Fiehn, O.; Fitzgerald, R.L.; Gerszten, R.E.; Illig, T.; Rhee, E.P.; Srinivas, P.R.; Wang, T.; et al. Potential impact and study considerations of metabolomics in cardiovascular health and disease: A scientific statement from the american heart association. Circ. Cardiovasc. Genet. 2017, 10, e000032. [Google Scholar] [CrossRef]
- Hailemariam, D.; Mandal, R.; Saleem, F.; Dunn, S.M.; Wishart, D.S.; Ametaj, B.N. Metabolomics approach reveals altered plasma amino acid and sphingolipid profiles associated with patholological state in transition dairy cows. Curr. Metab. 2015, 2, 184–195. [Google Scholar] [CrossRef]
- Sundekilde, U.K.; Poulsen, N.A.; Larsen, L.B.; Bertram, H.C. Nuclear magnetic resonance metabonomics reveals strong association between milk metabolites and somatic cell count in bovine milk. J. Dairy Sci. 2013, 96, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Shen, L.; Jiang, J.; Huang, Y.; Bai, L.; Yu, S.; Yao, X.; Ren, Z.; Yang, Y.; Cao, S. Plasma metabolite changes in dairy cows during parturition identified using untargeted metabolomics. J. Dairy Sci. 2019, 102, 4639–4650. [Google Scholar] [CrossRef]
- Huang, L.; Li, X.; Liu, T.; Wei, L.; Fan, C.; Tang, D.; Xiong, W.; Li, Y.; Wei, S.; Xiong, Z. Effect of intrahepatic cholestasis of pregnancy on infantile food allergy: A retrospective longitudinal study cohort in Southwest China. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 272, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, B. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis: A role for bifidobacteria and lactobacilli? Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 205. [Google Scholar] [CrossRef] [PubMed]
- Contreras, G.A.; Sordillo, L.M. Lipid mobilization and inflammatory responses during the transition period of dairy cows. Comp. Immunol. Microbiol. Infect. Dis. 2011, 34, 281–289. [Google Scholar] [CrossRef]
- Mavangira, V.; Gandy, J.C.; Zhang, C.; Ryman, V.E.; Daniel, J.A.; Sordillo, L.M. Polyunsaturated fatty acids influence differential biosynthesis of oxylipids and other lipid mediators during bovine coliform mastitis. J. Dairy Sci. 2015, 98, 6202–6215. [Google Scholar] [CrossRef]
- Tanaka, N.; Yamaguchi, H.; Furugen, A.; Ogura, J.; Kobayashi, M.; Yamada, T.; Mano, N.; Iseki, K. Quantification of intracellular and extracellular eicosapentaenoic acid-derived 3-series prostanoids by liquid chromatography/electrospray ionization tandem mass spectrometry. Prostaglandins Leukot. Essent. Fat. Acids 2014, 91, 61–71. [Google Scholar] [CrossRef]
- Malik, V.S.; Chiuve, S.E.; Campos, H.; Rimm, E.B.; Mozaffarian, D.; Hu, F.B.; Sun, Q. Circulating Very-Long-Chain saturated fatty acids and incident coronary heart disease in US men and women. Circulation 2015, 132, 260–268. [Google Scholar] [CrossRef]
- Zhou, Z.; Garrow, T.A.; Dong, X.; Luchini, D.N.; Loor, J.J. Hepatic activity and transcription of Betaine-Homocysteine methyltransferase, methionine synthase, and cystathionine synthase in periparturient dairy cows are altered to different extents by supply of methionine and choline. J. Nutr. 2017, 147, 11–19. [Google Scholar] [CrossRef]
- Yan, J.; Jiang, X.; West, A.A.; Perry, C.A.; Malysheva, O.V.; Devapatla, S.; Pressman, E.; Vermeylen, F.; Stabler, S.P.; Allen, R.H.; et al. Maternal choline intake modulates maternal and fetal biomarkers of choline metabolism in humans. Am. J. Clin. Nutr. 2012, 95, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Hou, J.; Xiang, F.; Zhang, X.; Che, L.; Lin, Y.; Xu, S.; Wu, D.; Fang, Z. Reproductive stage associated changes in plasma fatty acid profile and proinflammatory cytokine expression in rat mammary glands. Anim. Nutr. 2016, 2, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Edward, A.D.; Paul, C.N. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015, 15, 511–523. [Google Scholar]
- Burke, J.E.; Dennis, E.A. Phospholipase A2 structure/function, mechanism, and signaling. J. Lipid Res. 2009, 50, S237–S242. [Google Scholar] [CrossRef] [PubMed]
- Drobnik, W.; Liebisch, G.; Audebert, F.-X.; Fröhlich, D.; Glück, T.; Vogel, P.; Rothe, G.; Schmitz, G. Plasma ceramide and lysophosphatidylcholine inversely correlate with mortality in sepsis patients. J. Lipid Res. 2003, 44, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Zandkarimi, F.; Vanegas, J.; Fern, X.; Maier, C.S.; Bobe, G. Metabotypes with elevated protein and lipid catabolism and inflammation precede clinical mastitis in prepartal transition dairy cows. J. Dairy Sci. 2018, 101, 5531–5548. [Google Scholar] [CrossRef]
- Turk, R.; Rošić, N.; Kuleš, J.; Horvatić, A.; Gelemanovic, A.; Galen, A.; Ljubić, B.B.; Benić, M.; Stevanović, V.; Mrljak, V.; et al. Milk and serum proteomes in subclinical and clinical mastitis in Simmental cows. J. Proteom. 2021, 244, 104277. [Google Scholar] [CrossRef]
- Yamamoto, T.; Koyama, H.; Kurajoh, M.; Shoji, T.; Tsutsumi, Z.; Moriwaki, Y. Biochemistry of uridine in plasma. Clin. Chim. Acta 2011, 412, 1712–1724. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, L.; Shen, Y.; Zhang, Y.; Cao, S.; Yu, S.; Zong, X.; Su, Z. Effects of Anemoside B4 on Plasma Metabolites in Cows with Clinical Mastitis. Vet. Sci. 2023, 10, 437. https://doi.org/10.3390/vetsci10070437
Shen L, Shen Y, Zhang Y, Cao S, Yu S, Zong X, Su Z. Effects of Anemoside B4 on Plasma Metabolites in Cows with Clinical Mastitis. Veterinary Sciences. 2023; 10(7):437. https://doi.org/10.3390/vetsci10070437
Chicago/Turabian StyleShen, Liuhong, Yu Shen, Yue Zhang, Suizhong Cao, Shumin Yu, Xiaolan Zong, and Zhetong Su. 2023. "Effects of Anemoside B4 on Plasma Metabolites in Cows with Clinical Mastitis" Veterinary Sciences 10, no. 7: 437. https://doi.org/10.3390/vetsci10070437
APA StyleShen, L., Shen, Y., Zhang, Y., Cao, S., Yu, S., Zong, X., & Su, Z. (2023). Effects of Anemoside B4 on Plasma Metabolites in Cows with Clinical Mastitis. Veterinary Sciences, 10(7), 437. https://doi.org/10.3390/vetsci10070437