Biofilm-Producing Ability of Staphylococcus aureus Obtained from Surfaces and Milk of Mastitic Cows
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Dairy Cows and Udder Examination
2.2. Sanitation of Milking Equipment
2.3. Microbiological Examination of Milk Samples
2.4. Mastitis Forms
2.5. Samples from Surfaces and Microbiological Examination
2.6. Stainless Steel Coupons and Biofilm Preparation
2.7. Data Analysis
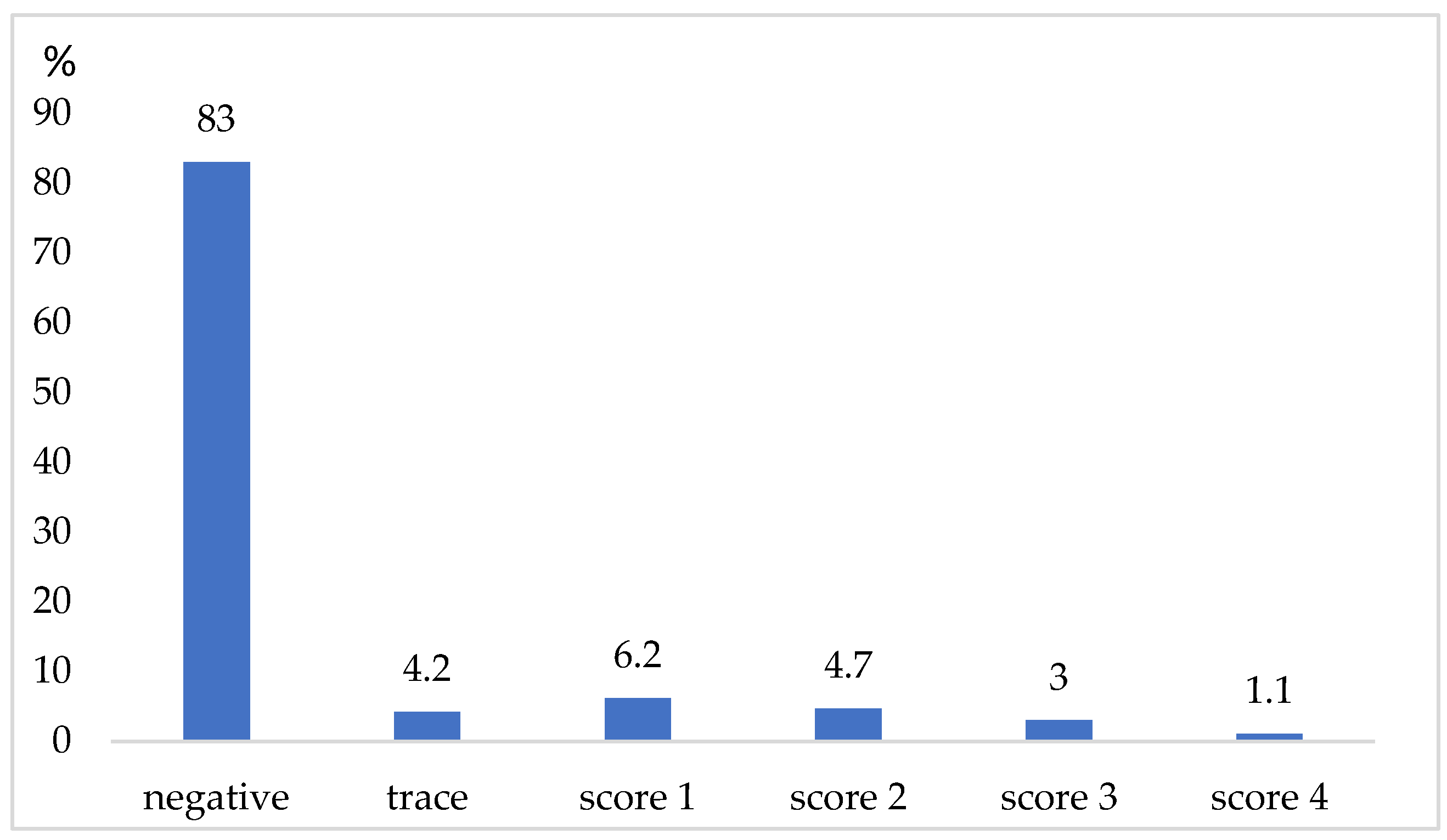
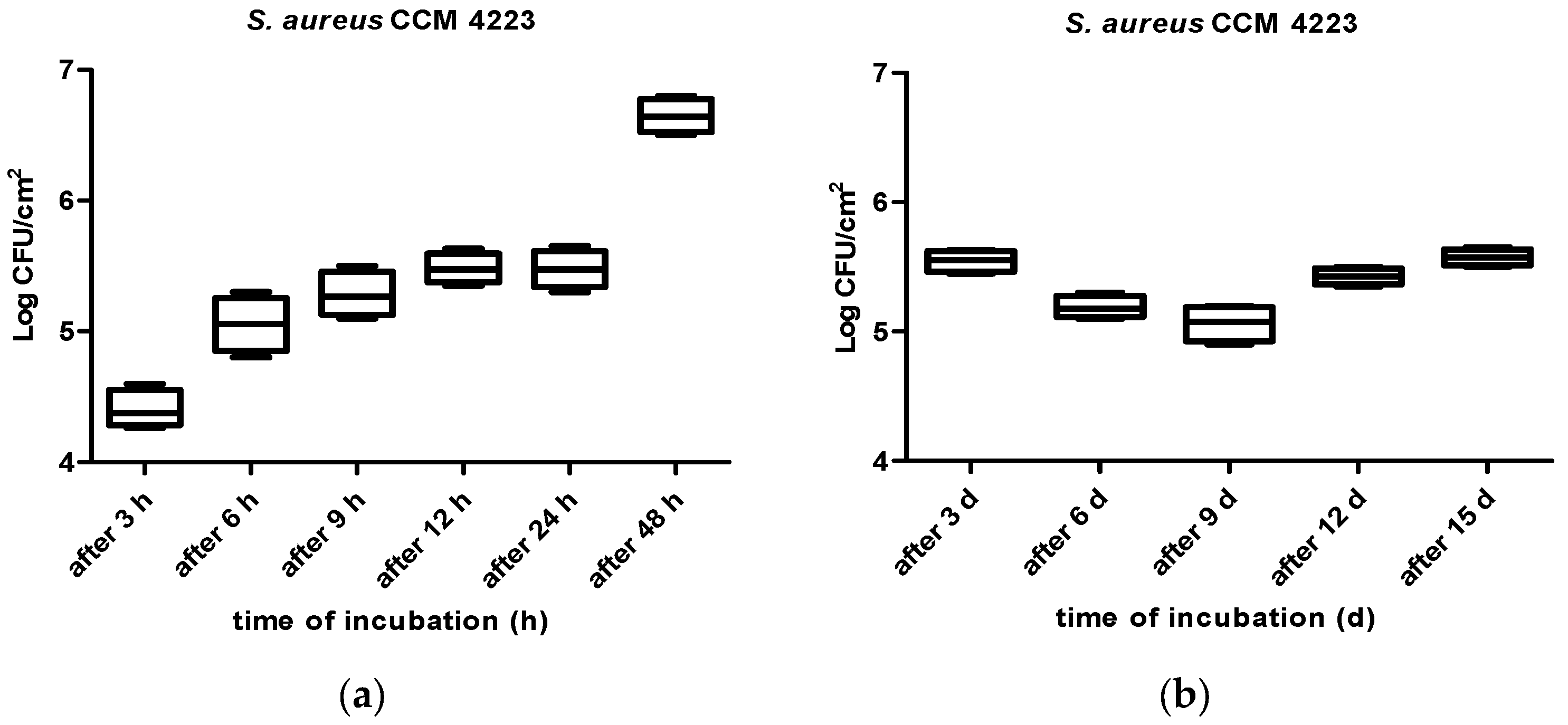
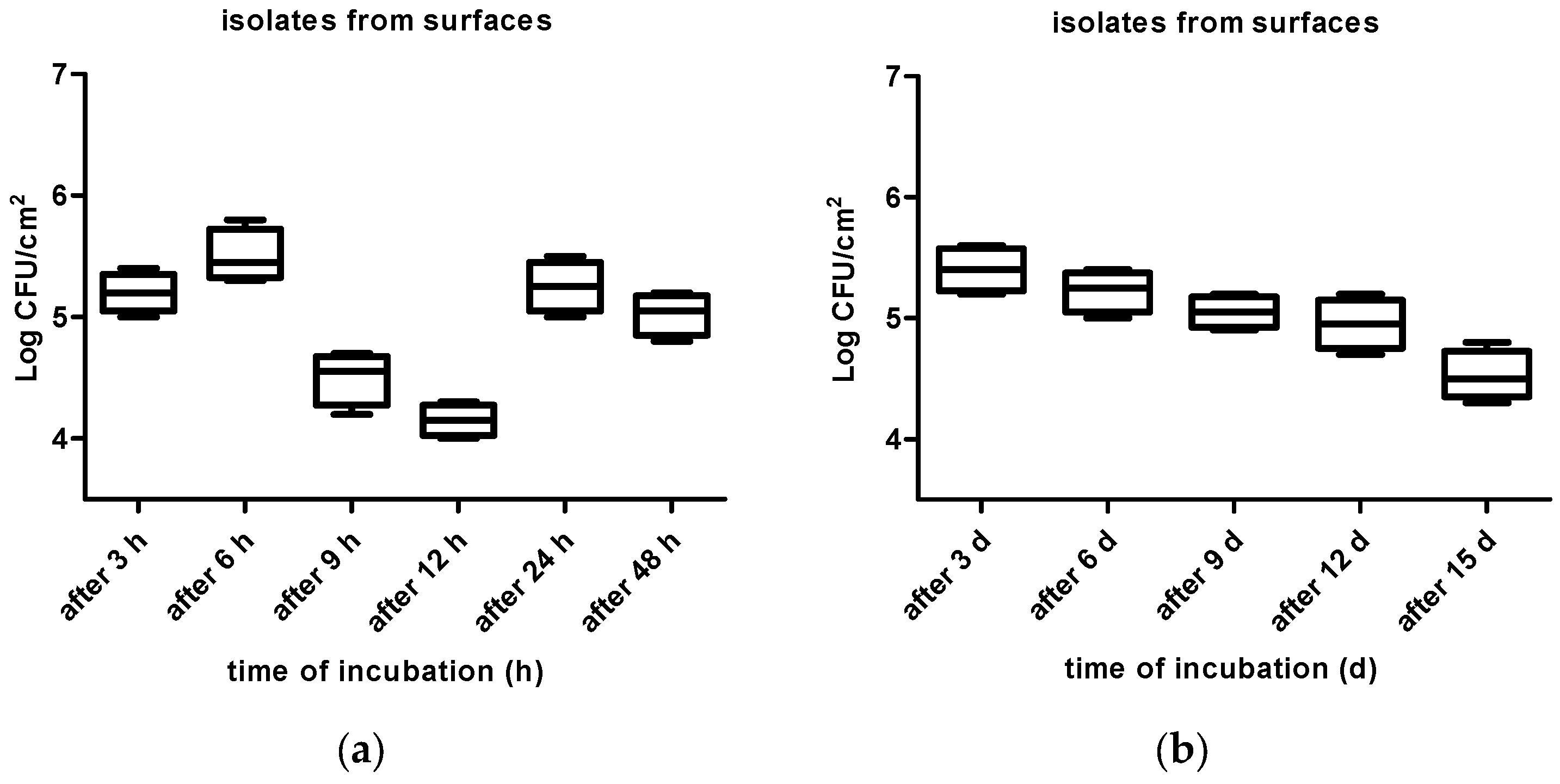
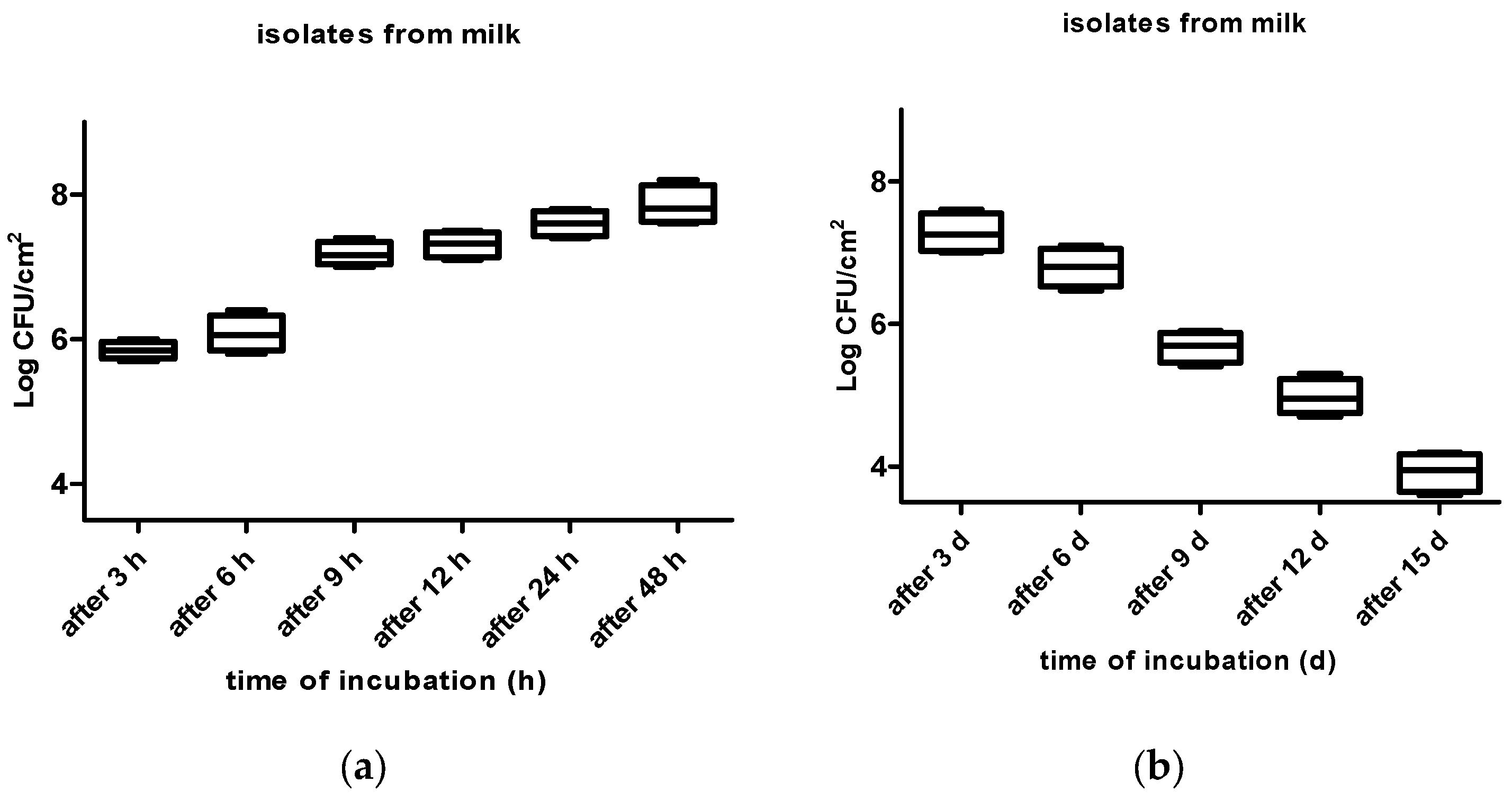
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hogeveen, H.; Huijps, K.; Lam, T.J. Economic aspects of mastitis: New developments. N. Z. Vet. J. 2021, 59, 16–23. [Google Scholar] [CrossRef]
- Woudstra, S.; Wente, N.; Zhang, Y.; Leimbach, S.; Kirkeby, C.; Gussmann, M.K.; Krömker, V. Reservoirs of Staphylococcus spp. and Streptococcus spp. Associated with Intramammary Infections of Dairy Cows. Pathogens 2023, 12, 699. [Google Scholar] [CrossRef] [PubMed]
- Tančin, V.; Mikláš, Š.; Čobirka, M.; Uhrinčať, M.; Mačuhová, L. Factors affecting raw milk quality of dairy cows under practical conditions. Potravin. Slovak J. Food Sci. 2020, 14, 744–749. [Google Scholar] [CrossRef]
- Zigo, F.; Farkašová, Z.; Výrostková, J.; Regecová, I.; Ondrašovičová, S.; Vargová, M.; Sasáková, N.; Pecka-Kielb, E.; Bursová, Š.; Kiss, D.S. Dairy Cows’ Udder Pathogens and Occurrence of Virulence Factors in Staphylococci. Animals 2022, 12, 470. [Google Scholar] [CrossRef] [PubMed]
- Cobirka, M.; Tančin, V.; Slama, P. Epidemiology and Classification of Mastitis. Animals 2020, 10, 2212. [Google Scholar] [CrossRef] [PubMed]
- Coelho, S.M.; Pereira, I.A.; Soares, L.C.; Pribul, B.R.; Souza, M.M. Profile of virulence factors of Staphylococcus aureus isolated from subclinical bovine mastitis in the state of Rio de Janeiro. Braz. J. Dairy Sci. 2011, 94, 3305–3310. [Google Scholar] [CrossRef] [PubMed]
- Bogdanovičová, K.; Skočková, A.; Šťástková, Z.; Karpíšková, R. Occurrence and antimicrobial resistance of Staphylococcus aureus in bulk tank milk and milk filters. Potravin. Slovak J. Food Sci. 2014, 8, 97–101. [Google Scholar] [CrossRef]
- Rainard, P.; Foucras, G.; Fitzgerald, J.R.; Watts, J.L.; Koop, G.; Middleton, J.R. Knowledge gaps and research priorities in Staphylococcus aureus mastitis control. Transbound Emerg Dis. 2018, 65, 149–165. [Google Scholar] [CrossRef]
- Holko, I.; Tančin, V.; Vršková, M.; Tvarožková, K. Prevalence and antimicrobial susceptibility of udder pathogens isolated from dairy cows in Slovakia. J. Dairy Res. 2019, 86, 436–439. [Google Scholar] [CrossRef]
- Vasil, M.; Farkašová, Z.; Elečko, J.; Illek, J.; Zigo, F. Comparison of biofilm formation by Staphylococcus aureus and Staphylococcus epidermidis strains isolated from sheep milk using three diagnostic methods. Pol. J. Vet. Sci. 2017, 20, 795–801. [Google Scholar]
- Lee, S.H.; Mangolin, B.L.; Goncalves, J.L.; Neeff, D.V.; Silva, M.P.; Cruz, A.G.; Oliveira, C.A. Biofilm-producing ability of Staphylococcus aureus isolates from Brazilian dairy farms. J. Dairy Sci. 2014, 97, 1812–1816. [Google Scholar] [CrossRef]
- Gajewska, J.; Chajęcka-Wierzchowska, W. Biofilm Formation Ability and Presence of Adhesion Genes among Coagulase-Negative and Coagulase-Positive Staphylococci Isolates from Raw Cow’s Milk. Pathogens 2020, 9, 654. [Google Scholar] [CrossRef]
- Otto, M. Staphylococcal biofilms. Curr. Top. Microbiol. Immun. 2020, 322, 207–228. [Google Scholar]
- Costerton, J.W.; Cheng, K.J.; Geesey, G.G.; Ladd, T.I.; Nickel, J.C.; Dasgupta, M.; Marrie, T.J. Bacterial biofilms in nature and disease. Annu. Rev. Microbiol. 1987, 41, 435–464. [Google Scholar] [CrossRef]
- Czechowski, M.H. Bacterial attachment to Buna-N gaskets in milk processing equipment. Aust. J. Dairy Technol. 1990, 45, 113–114. [Google Scholar]
- Fox, L.K.; Zadoks, R.N.; Gaskins, C.T. Biofilm production by Staphylococcus aureus associated with intramammary infection. Vet. Microbiol. 2005, 107, 295–299. [Google Scholar] [CrossRef] [PubMed]
- DeVita, M.D.; Wadhera, R.K.; Theis, M.L.; Ingham, S.C. Assessing the potential of Streptococcus pyogenes and Staphylococcus aureus transfer to foods and customers via a survey of hands, hand-contact surfaces and food-contact surfaces at foodservice facilities. J. Food Serv. 2007, 18, 76–79. [Google Scholar] [CrossRef]
- Herrera, F.C.; Santos, J.A.; Otero, A.; García-López, M.L. Occurrence of foodborne pathogenic bacteria in retail prepackaged portions of marine fish in Spain. J. Appl. Microbiol. 2006, 100, 527–536. [Google Scholar] [CrossRef]
- Sattar, S.A.; Springthorpe, S.; Mani, S.; Gallant, M.; Nair, R.C.; Scott, E.; Kain, J. Transfer of bacteria from fabrics to hands and other fabrics: Development and application of a quantitative method using Staphylococcus aureus as a model. J. Appl. Microbiol. 2001, 90, 962–970. [Google Scholar] [CrossRef]
- Simon, S.S.; Sanjeev, S. Prevalence of enterotoxigenic Staphylococcus aureus in fishery products and fish-processing factory workers. Food Control. 2007, 18, 1565–1568. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef] [PubMed]
- McDougald, D.; Rice, S.A.; Barraud, N.; Steinberg, P.D.; Kjelleberg, S. Should we stay or should we go: Mechanisms and ecological consequences for biofilm dispersal. Nat. Rev. Microbiol. 2012, 10, 39–50. [Google Scholar] [CrossRef]
- Melchior, M.B.; van Osch, M.H.J.; Lam, T.J.G.M.; Vernooij, J.C.M.; Gaastra, W.; Fink-Gremmels, J. Extended biofilm suscepti bility assay for Staphylococcus aureus bovine mastitis isolates: Evidence for association between genetic makeup and biofilm susceptibility. J. Dairy Sci. 2011, 94, 5926–5937. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, A.; Donatucci, C.F. Bacterial Biofilms and implantable prosthetic devices. Int. J. Impot. Res. 2003, 15, 150–154. [Google Scholar] [CrossRef]
- Fey, P.D.; Olson, M.E. Current concepts in biofilm formation of Staphylococcus epidermidis. Future Microbiol. 2010, 5, 917–933. [Google Scholar] [CrossRef]
- Nirwati, K.; Sinanjung, F.; Fahrunissa, F. Biofilm formation and antibiotic resistance of Klebsiella pneumoniae isolated from clinical samples in a tertiary care hospital. BMC Proc. 2019, 13, 20. [Google Scholar] [CrossRef]
- Wimpenny, J.; Manz, W.; Szewyk, U. Heterogeneity in biofilms. FEMS Microbiol. Rev. 2000, 24, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Swift, S.; Downie, J.A.; Whitehead, N.A. Quorum sensing as a population-density determinant of bacterial physiology. Adv. Microbiol. Physiol. 2001, 45, 199–270. [Google Scholar]
- Russell, A.D.; Chopra, I. Understanding Antibacterial Action and Resistance, 2nd ed.; Ellis Horwood: Chichester, UK, 1996. [Google Scholar]
- Gilbert, P.; McBain, A.J. Biocide use in the domestic setting and concern about aantibacterial and antibiotic resistance. J. Infect. 2001, 43, 85–91. [Google Scholar] [CrossRef]
- Batoni, G.; Maisetta, G.; Esin, S. Antimicrobial peptides and their interaction with biofilms of medically relevant bacteria. Biochim. Biophys. Acta (BBA)-Biomembr. 2016, 1858, 1044–1060. [Google Scholar] [CrossRef]
- Tewari, A.; Jain, B.; Dhamannapatil, P.S.; Saxena, M.K. Biofilm resistance to antimicrobial agents and novel approaches to combat biofilm mediated resistance in bacteria. EC Microbiol. 2018, 14, 71–77. [Google Scholar]
- Prakash, B.; Veeregowda, B.; Krishnappa, G. Biofilms: A survival strategy of bacteria. Curr. Sci. 2003, 85, 1299–1307. [Google Scholar]
- Zhao, X.; Zhao, F.; Wang, J.; Zhong, N. Biofilm formation and control strategies of foodborne pathogens: Food safety perspectives, RSC Advances. Int. J. Microbiol. 2017, 7, 36670–36683. [Google Scholar] [CrossRef]
- Li, X.H.; Lee, J.H. Antibiofilm agents: A new perspective for antimicrobial strategy. J. Microbiol. 2017, 55, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Wang, Y.; Liu, L.; He, J. Biofilms: The microbial protective clothing” in extreme environments. Int. J. Mol. Sci. 2019, 20, 3423. [Google Scholar] [CrossRef]
- United States Department of Agriculture (USDA); Animal and Plant Health Inspection Service (APHIS). Determining U.S. Milk Quality Using Bulk-Tank Somatic Cell Counts; Veterinary Services Center for Epidemiology and Animal Health: Riverdale, ML, USA, 2018; pp. 1–8. [Google Scholar]
- Nutrient Requirements of Dairy Cattle (NRC), 7th ed.; The National Academies Press, National Research Council: Washington, DC, USA, 2001; pp. 381–390.
- Tančin, V. Somatic cell counts in milk of dairy cows under practical conditions. Slovak J. Anim. Sci. 2013, 46, 31–34. [Google Scholar]
- National Mastitis Council. National Mastitis Council Recommended Mastitis Control Program. In Proceedings of the National Mastitis Council Annual Meeting Proceedings, Reno, NV, USA, 11–14 February 2001; National Mastitis Council, Inc.: Verona, WI, USA, 2001; pp. 408–418. [Google Scholar]
- German Veterinary Association (GVA). Guidelines for Taking Milk Samples under Antiseptic Conditions and Isolation and Identification of Mastitis Pathogens, 2nd ed.; Verlag der Deutschen Veterinärmedizinischen Gesellschaft e.V.: Gießen, Germany, 2009. [Google Scholar]
- Ozbey, G.; Cambay, Z.; Yilmaz, S.; Aytekin, O.; Zigo, F.; Ozçelik, M.; Otlu, B. Identification of bacterial species in milk by MALDI-TOF and assessment of some oxidant-antioxidant parameters in blood and milk from cows with different health status of the udder. Pol. J. Vet. Sci. 2022, 25, 269–277. [Google Scholar]
- El-Aziz, A.N.K.; Ammar, A.M.; El Damaty, H.M.; Abd Elkader, R.A.; Saad, H.A.; El-Kazzaz, W.; Khalifa, E. Environmental Streptococcus uberis Associated with Clinical Mastitis in Dairy Cows: Virulence Traits, Antimicrobial and Biocide Resistance, and Epidemiological Typing. Animals 2021, 11, 1849. [Google Scholar] [CrossRef]
- De Oliveira, C.E.V.; Stamford, T.L.M.; Gomes Neto, N.J.; de Souza, E.L. Inhibition of Staphylococcus aureus in broth and meat broth using synergies of phenolics and organic acids. Int. J. Food Microbiol. 2010, 137, 312–316. [Google Scholar] [CrossRef] [PubMed]
- ISO 6887-5:2010; Microbiology of Food and Animal Feeding Stuffs—Preparation of Test Samples, Initial Suspension and Decimal Dilutions for Microbiological Examination—Part 5: Specific Rules for the Preparation of Milk and Milk Products. Slovak Standards Institute: Bratislava, Slovakia, 2010.
- ISO 6888-1:1999; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Coagulase-Positive Staphylococci (Staphylococcus aureus and Other Species)—Part 1: Technique Using Baird-Parker Agar Medium. Slovak Standards Institute: Bratislava, Slovakia, 1999.
- Marques, S.C.; Rezende, J.G.O.S.; Alves, L.A.F.; Silva, B.C.; Alves, E.; Abreu, L.R.; Piccoli, R.H. Formation of biofilm by Staphylococcus aureus on stainless steel and glass surfaces and its resistance to some selected chemical sanitizers. Braz J Microbiol. 2007, 38, 538–543. [Google Scholar] [CrossRef]
- ISO 21528-1:2019; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Enterobacteriaceae—Part 1: Detection of Enterobacteriaceae. Slovak Standards Institute: Bratislava, Slovakia, 2019.
- Rode, T.M.; Langsrud, S.; Holck, A.; Moretto, T. Different patterns of biofilm formation in Staphylococcus aureus under food-related stress conditions. Int. J. Food Microbiol. 2007, 116, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Turk, R.; Rošic, N.; Kuleš, J.; Horvatic, A.; Gelemanovic, A.; Galen, A.; Ljubic, B.B.; Benic, M.; Stevanovic, V.; Mrljak, V.; et al. Milk and serum proteomes in subclinical and clinical mastitis in Simmental cows. J. Proteomics 2021, 244, 104277. [Google Scholar] [CrossRef]
- Monistero, V.; Graber, H.U.; Pollera, C.; Cremonesi, P. Staphylococcus aureus Isolates from Bovine Mastitis in Eight Countries: Genotypes, Detection of Genes Encoding Different Toxins and Other Virulence Genes. Toxins 2018, 10, 247. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Godden, S.M.; Royster, E.; Crooker, B.A.; Timmerman, J.; Fox, L. Relationships among bedding materials, bedding bacteria counts, udder hygiene, milk quality, and udder health in US dairy herds. J. Dairy Sci. 2019, 102, 10213–10234. [Google Scholar] [CrossRef]
- Idriss, S.E.; Foltys, V.; Tančin, V.; Kirchnerová, K.; Zaujec, K. Mastitis pathogens in milk of dairy cows in Slovakia. Slovak J. Anim. Sci. 2013, 46, 115–119. [Google Scholar]
- Thiran, E.; Di Ciccio, P.A.; Graber, H.U.; Zanardi, E.; Ianeri, A.; Hummerjohann, J. Biofilm formation of Staphylococcus aureus dairy isolates representing different genotypes. J. Dairy Sci. 2018, 101, 1000–1012. [Google Scholar] [CrossRef]
- Unlu, A.; Sar, T.; Seker, G.; Erman, A.G.; Kalpar, E.; Akbas, M.Y. Biofilm formation by Staphylococcus aureus strains and their control by selected phytochemicals. Int. J. Dairy Tech. 2018, 71, 637–646. [Google Scholar] [CrossRef]
- Latorre, A.A.; Munoz, M.A. On-farm evaluation of surfaces in contact with milk during milking as a factor affecting the microbiological quality of bulk tank milk on Chilean dairy farms: A Preliminary Report. In Proceedings of the Annual Meeting of the National Mastitis Council, Memphis, TN, USA, 1–3 February 2015; pp. 231–232. [Google Scholar]
- Latorre, A.A.; Pacha, P.; Munoz, M.A. Staphylococcus aureus containing biofilms on surfaces in contact with milk: Implications for the microbiological quality of milk and udder health. In Proceedings of the 6th International Dairy Federation Conference, Nantes, France, 7–9 September 2016; pp. 71–77. [Google Scholar]
- Mosteller, T.M.; Bishop, J.R. Sanitizer efficacy againstattached bacteria in a milk biofilm. J. Food Prot. 1993, 56, 34–41. [Google Scholar] [CrossRef]
- Morton, L.H.G.; Greenway, D.L.A.; Gaylarde, C.C.; Surman, S.B. Consideration of some implications of the resistance of biofilms to biocides. Int. Biodeter. Biodegr. 1998, 41, 247–259. [Google Scholar] [CrossRef]
- Pagedar, A.; Singhr, J.; Batish, V.K. Surface hydrophobicity, nutritional contents affect Staphylococcus aureus biofilms and temperature influences its survival in preformed biofilms. J. Basic Microbiol. 2010, 50, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Sánchez, D.; Habimana, O.; Holck, A. Impact of Food-Related Environmental Factors on the Adherence and Biofilm Formation of Natural Staphylococcus aureus Isolates. Curr Microbiol. 2013, 66, 110–121. [Google Scholar] [CrossRef]
- Wirtanen, G.; Ahola, W.; Mattila-Sandholm, T. Evaluation of cleaning procedures in elimination of biofilm from stainless steel surface in process equipment. Food Bioprod. Process. 1995, 73, 9–16. [Google Scholar]
- Zottola, E.A.; Sasahara, K.C. Microbial biofilms in the food processing industry—Should they be a concern? Int. J. Food Microbiol. 1994, 23, 125–148. [Google Scholar] [CrossRef]
- Meira, Q.G.S.; Barbosa, I.M.; Athayde, A.J.A.A.; Siqueira, J.P., Jr.; Souza, E.L. Influence of temperature and surface kind on 386 biofilm formation by Staphylococcus aureus from food contact surfaces and sensitivity to sanitizers. Food Cont. 2012, 25, 469–475. [Google Scholar] [CrossRef]
- Souza, E.L.; Siqueira-Junior, J.P. Biofilm formation by Staphylococcus aureus from food contact surfaces in a meat-based broth and sensitivity to sanitizers. Braz. J. Microbiol. 2014, 45, 67–75. [Google Scholar] [CrossRef] [PubMed]
| Isolated Bacteria | Subclinical 2 | Clinical 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CM1 | CM2 | CM3 | ||||||||
| n | % | n | % | n | % | n | % | n | % | |
| Staphylococcus spp. | ||||||||||
| S. aureus | 11 | 25.6 | 2 | 4.7 | 4 | 9.3 | 4 | 9.3 | 1 | 2.3 |
| S. intermedius | 3 | 7.0 | -- | -- | 3 | 7.0 | -- | -- | -- | -- |
| NAS 1 | ||||||||||
| S. chromogenes | 5 | 11.6 | 3 | 7.0 | -- | -- | 2 | 4.7 | -- | -- |
| S. haemolyticus | 3 | 7.0 | -- | -- | 2 | 4.7 | 1 | 2.3 | -- | -- |
| S. warneri | 2 | 4.7 | 1 | 2.3 | 1 | 2.3 | -- | -- | -- | -- |
| S. xylosus | 2 | 4.7 | 2 | 4.7 | -- | -- | -- | -- | -- | -- |
| Streptococcus spp. | ||||||||||
| Str. uberis | 4 | 9.3 | 1 | 2.3 | 2 | 4.7 | 1 | 2.3 | -- | -- |
| Str. faecalis | 2 | 4.7 | 2 | 4.7 | -- | -- | -- | -- | -- | -- |
| Other bacteria | ||||||||||
| E. coli | 4 | 9.3 | 2 | 4.7 | -- | -- | 2 | 4.7 | -- | -- |
| Pseudomonas spp. | 3 | 7.0 | 3 | 7.0 | -- | -- | -- | -- | -- | -- |
| Mixed infection * | 4 | 9.3 | 1 | 2.3 | 2 | 4.7 | -- | -- | -- | -- |
| Total | 43 | 100 | 17 | 39.5 | 14 | 32.5 | 10 | 23.2 | 1 | 2.3 |
| Sample Source | n | Isolated Bacteria | Positive for S. aureus n (%) |
|---|---|---|---|
| Floor | 9 | Streptococcus spp. S. aureus E. coli Enterococcus faecalis | (15%) |
| Teat cup | 9 | S. aureus E. coli Campylobacter spp. | 7 (26%) |
| Dairy cow restraints | 9 | S. aureus Hafnia alvei Ralstonia insidiosa E. coli | 3 (11%) |
| Total | 27 | 14/(52%) |
| Isolated Bacteria | Sample Source | Testing Value | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Floor (n) | % | Teat Cup (n) | % | Cow Restraints (n) | % | Milk (n) | % | χ2 | p | |
| S. aureus | 4 | 15 | 7 | 26.0 | 3 | 11.1 | 11 | 25.6 | 4.99 * | 0.025 * |
| NAS | 0 | 0 | 0 | 0 | 0 | 0 | 12 | 27.9 | 0.078 | 0.780 |
| Streptococcus spp. | 1 | 3.7 | 0 | 0 | 0 | 0 | 6 | 14.0 | 1.93 | 0.164 |
| E. coli | 2 | 7.4 | 1 | 3.7 | 4 | 14.8 | 7 | 16.2 | 4.13 * | 0.042 * |
| Other bacteria | 2 | 7.4 | 1 | 3.7 | 2 | 7.4 | 7 | 16.2 | 0.95 | 0.329 |
| Total | 9 | 33.5 | 9 | 33.2 | 9 | 33.3 | 43 | 100 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargová, M.; Zigo, F.; Výrostková, J.; Farkašová, Z.; Rehan, I.F. Biofilm-Producing Ability of Staphylococcus aureus Obtained from Surfaces and Milk of Mastitic Cows. Vet. Sci. 2023, 10, 386. https://doi.org/10.3390/vetsci10060386
Vargová M, Zigo F, Výrostková J, Farkašová Z, Rehan IF. Biofilm-Producing Ability of Staphylococcus aureus Obtained from Surfaces and Milk of Mastitic Cows. Veterinary Sciences. 2023; 10(6):386. https://doi.org/10.3390/vetsci10060386
Chicago/Turabian StyleVargová, Mária, František Zigo, Jana Výrostková, Zuzana Farkašová, and Ibrahim F. Rehan. 2023. "Biofilm-Producing Ability of Staphylococcus aureus Obtained from Surfaces and Milk of Mastitic Cows" Veterinary Sciences 10, no. 6: 386. https://doi.org/10.3390/vetsci10060386
APA StyleVargová, M., Zigo, F., Výrostková, J., Farkašová, Z., & Rehan, I. F. (2023). Biofilm-Producing Ability of Staphylococcus aureus Obtained from Surfaces and Milk of Mastitic Cows. Veterinary Sciences, 10(6), 386. https://doi.org/10.3390/vetsci10060386









