Green Turtle Fibropapillomatosis: Tumor Morphology and Growth Rate in a Rehabilitation Setting
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Individuals Selection
2.2. Tumor Categorization by Morphology and Tumor Area Measurement
2.3. Average Biweekly Growth Calculation and Statistical Analyses
3. Results
3.1. FP Tumor Progression and Regression Patterns per Turtle
3.2. Statistical Analysis Output
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, K.; Ariel, E.; Burgess, G.; Read, M. A Review of Fibropapillomatosis in Green Turtles (Chelonia Mydas). Vet. J. 2016, 212, 48–57. [Google Scholar] [CrossRef]
- Smith, G.M.; Coates, C.W. Fibro-epithelial growths of the skin in large marine turtles, Chelonia mydas (Linnaeus). Zoologica 1938, 23, 93–98. [Google Scholar] [CrossRef]
- Shaver, D.J.; Walker, J.S.; Backof, T.F. Fibropapillomatosis Prevalence and Distribution in Green Turtles Chelonia Mydas in Texas (USA). Dis. Aquat. Organ. 2019, 136, 175–182. [Google Scholar] [CrossRef]
- Herbst, L.; Ene, A.; Su, M.; Desalle, R.; Lenz, J. Tumor Outbreaks in Marine Turtles Are Not Due to Recent Herpesvirus Mutations. Curr. Biol. 2004, 14, 697–699. [Google Scholar] [CrossRef]
- Work, T.M.; Balazs, G.H.; Rameyer, R.A.; Morris, R.A. Retrospective Pathology Survey of Green Turtles Chelonia Mydas with Fibropapillomatosis in the Hawaiian Islands, 1993–2003. Dis. Aquat. Organ. 2004, 62, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Ene, A.; Su, M.; Lemaire, S.; Rose, C.; Schaff, S.; Moretti, R.; Lenz, J.; Herbst, L.H. Distribution of Chelonid Fibropapillomatosis-Associated Herpesvirus Variants in Florida: Molecular Genetic Evidence for Infection of Turtles Following Recruitment to Neritic Developmental Habitats. J. Wildl. Dis. 2005, 41, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Foley, A.M.; Schroeder, B.A.; Redlow, A.E.; Fick-Child, K.J.; Teas, W.G. Fibropapillomatosis in Stranded Green Turtles (Chelonia Mydas) from the Eastern United States (1980-98): Trends and Associations with Environmental Factors. J. Wildl. Dis. 2005, 41, 29–41. [Google Scholar] [CrossRef] [PubMed]
- van Houtan, K.S.; Hargrove, S.K.; Balazs, G.H. Land Use, Macroalgae, and a Tumor-Forming Disease in Marine Turtles. PLoS ONE 2010, 5, e12900. [Google Scholar] [CrossRef]
- Tagliolatto, A.B.; Guimarães, S.M.; Lobo-Hajdu, G.; Monteiro-Neto, C. Characterization of Fibropapillomatosis in Green Turtles Chelonia Mydas (Cheloniidae) Captured in a Foraging Area in Southeastern Brazil. Dis. Aquat. Organ. 2016, 121, 233–240. [Google Scholar] [CrossRef]
- Spring, J. A Histological Comparison of Tumor Characteristics in Adult Nesting Female and Foraging Juvenile Hawaiian Green Sea Turtles Is Regression Related to Age? Ph.D. Thesis, Columbia University, New York, NT, USA, 2006. [Google Scholar]
- Guimarães, S.M.; Gitirana, H.M.; Wanderley, A.V.; Monteiro-Neto, C.; Lobo-Hajdu, G. Evidence of Regression of Fibropapillomas in Juvenile Green Turtles Chelonia Mydas Caught in Niterói, Southeast Brazil. Dis. Aquat. Organ. 2013, 102, 243–247. [Google Scholar] [CrossRef]
- Kelley, J.R.; Kelley, K.L.; Savage, A.E.; Mansfield, K.L. Novel Disease State Model Finds Most Juvenile Green Turtles Develop and Recover from Fibropapillomatosis. Ecosphere 2022, 13, e4000. [Google Scholar] [CrossRef]
- Farrell, J.A.; Yetsko, K.; Whitmore, L.; Whilde, J.; Eastman, C.B.; Ramia, D.R.; Thomas, R.; Linser, P.; Creer, S.; Burkhalter, B.; et al. Environmental DNA Monitoring of Oncogenic Viral Shedding and Genomic pro Fi Ling of Sea Turtle Fi Bropapillomatosis Reveals Unusual Viral Dynamics. Commun. Biol. 2021, 4, 565. [Google Scholar] [CrossRef]
- Tristan, T.; Shaver, D.J.; Kimbro, J.; DeMaar, T.; Metz, T.; George, J.; Amos, A. Identification of Fibropapillomatosis in Green Sea Turtles (Chelonia Mydas) on the Texas Coast. J. Herpetol. Med. Surg. 2012, 20, 109. [Google Scholar] [CrossRef]
- Garefino, V.E.; Milton, S.L. Influence of Sunlight on Vitamin D and Health Status in Green (Chelonia Mydas) Sea Turtles with Fibropapillomatosis. Animals 2022, 12, 488. [Google Scholar] [CrossRef]
- Norton, M.T.; Jacobson, R.E.; Sundberg, J.P. Cutaneous Fibropapillomas and Renal Myxofibroma in a Green Turtle, Chelonia Mydas. J. Wildl. Dis. 1990, 26, 265–270. [Google Scholar] [CrossRef] [PubMed]
- da Cunha Brito, F.L.; Maia, F.C.L.; de França, L.M.O.; de Albuquerque, A.R.; Cavalcanti, M.A.M.; Guimarães, E.S.G. Fibropapillomatosis and Multiple Fibromas in a Green Turtle from the South Coast of Pernambuco State, Brazil. Mar. Turt. Newsl. 2004, 106, 12. [Google Scholar]
- Page-Karjian, A.; Perrault, J.R.; Zirkelbach, B.; Pescatore, J.; Riley, R.; Stadler, M.; Zachariah, T.T.; Marks, W.; Norton, T.M. Tumor Re-Growth, Case Outcome, and Tumor Scoring Systems in Rehabilitated Green Turtles with Fibropapillomatosis. Dis. Aquat. Organ. 2019, 137, 101–108. [Google Scholar] [CrossRef]
- Perrault, J.R.; Levin, M.; Mott, C.R.; Bovery, C.M.; Bresette, M.J.; Chabot, R.M.; Gregory, C.R.; Guertin, J.R.; Hirsch, S.E.; Ritchie, B.W.; et al. Insights on Immune Function in Free-Ranging Green Sea Turtles (Chelonia Mydas) with and without Fibropapillomatosis. Animals 2021, 11, 861. [Google Scholar] [CrossRef]
- Hirama, S.; Ehrhart, L.M. Description, Prevalence and Severity of Green Turtle Fibropapillomatosis in Three Developmental Habitats on the East Coast of Florida. Florida Sci. 2007, 70, 435–448. [Google Scholar]
- Hirama, S.; Ehrhart, L.M.; Rea, L.D.; Kiltie, R.A. Relating Fibropapilloma Tumor Severity to Blood Parameters in Green Turtles Chelonia Mydas. Dis. Aquat. Organ. 2014, 111, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Chabot, R.M.; Welsh, R.C.; Mott, C.R.; Guertin, J.R.; Shamblin, B.M.; Witherington, B.E. A Sea Turtle Population Assessment for Florida’s Big Bend, Northeastern Gulf of Mexico. Gulf Caribb. Res. 2021, 32, 19–33. [Google Scholar] [CrossRef]
- Lackovich, J.K.; Brown, D.R.; Homer, B.L.; Garber, R.L.; Mader, D.R.; Moretti, R.H.; Patterson, A.D.; Herbst, L.H.; Oros, J.; Jacobson, E.R.; et al. Association of Herpesvirus with Fibropapillomatosis of the Green Turtle Chelonia Mydas and the Loggerhead Turtle Caretta Caretta in Florida. Dis. Aquat. Organ. 1999, 37, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Rodenbusch, C.R.; Almeida, L.L.; Marks, F.S.; Ataíde, M.W.; Alievi, M.M.; Tavares, M.; Pereira, R.A.; Canal, C.W. Detection and Characterization of Fibropapilloma Associated Herpesvirus of Marine Turtles in Rio Grande Do Sul, Brazil. Pesqui. Vet. Bras. 2012, 32, 1179–1183. [Google Scholar] [CrossRef]
- Herbst, L.H.; Jacobson, E.R.; Kein, P.A.; Balazs, G.H.; Moretti, R.; Brown, T.; Sundberg, J.P. Comparative Pathology and Pathogenesis of Spontaneous and Experimentally Induced Fibropapillomas of Green Turtles (Chelonia Mydas). Vet. Pathol. 1999, 564, 551–564. [Google Scholar] [CrossRef]
- Herbst, L.H. Fibropapillomatosis of Marine Turtles. Annu. Rev. Fish Dis. 1994, 4, 389–425. [Google Scholar] [CrossRef]
- Greenblatt, R.J.; Work, T.M.; Dutton, P.; Sutton, C.A.; Spraker, T.R.; Casey, R.N.; Diez, C.E.; Parker, D.; St. Leger, J.; Balazs, G.H.; et al. Geographic Variation in Marine Turtle Fibropapillomatosis. J. Zoo Wildl. Med. 2005, 36, 527–530. [Google Scholar] [CrossRef]
- Manire, C.A.; Stacy, B.A.; Kinsel, M.J.; Daniel, H.T.; Anderson, E.T.; Wellehan, J.F.X. Proliferative Dermatitis in a Loggerhead Turtle, Caretta Caretta, and a Green Turtle, Chelonia Mydas, Associated with Novel Papillomaviruses. Vet. Microbiol. 2008, 130, 227–237. [Google Scholar] [CrossRef]
- Aguirre, A.A.; Spraker, T.R.; Balazs, G.H.; Zimmerman, B. Spirorchidiasis and Fibropapillomatosis in Green Turtles from the Hawaiian Islands. J. Wildl. Dis. 1998, 34, 91–98. [Google Scholar] [CrossRef]
- Formia, A.; Deem, S.; Billes, A.; Ngouessono, S.; Parnell, R.; Collinis, T.; Sounguet, G.P.; Gibudi, A.; Villarubia, A.; Balazs, G.H.; et al. Fibropapillomatosis Confirmed in Chelonia Mydas in the Gulf of Guinea, West Africa. Mar. Turt. Newsl. 2007, 116, 20–22. [Google Scholar]
- Rossi, S.; Sánchez-Sarmiento, A.M.; Vanstreels, R.E.T.; Dos Santos, R.G.; Prioste, F.E.S.; Gattamorta, M.A.; Grisi-Filho, J.H.H.; Matushima, E.R. Challenges in Evaluating the Severity of Fibropapillomatosis: A Proposal for Objective Index and Score System for Green Sea Turtles (Chelonia Mydas) in Brazil. PLoS ONE 2016, 11, e0167632. [Google Scholar] [CrossRef]
- Work, T.M.; Balazs, G.H. Relating Tumor Score to Hematology in Green Turtles with Fibropapillomatosis in Hawaii. J. Wildl. Dis. 1999, 35, 804–807. [Google Scholar] [CrossRef]
- Farrell, J.; Thomas, R.; Martindale, M.Q.; David, J. Characterisation of Fibropapillomatosis Tumour Growth Profiles in Green Sea Turtles (Chelonia Mydas). Testudo 2018, 8, 12–29. [Google Scholar]
- Lancaster, W.D.; Olson, C. Animal Papillomaviruses. Microbiol. Rev. 1982, 45, 191–207. [Google Scholar] [CrossRef] [PubMed]
- Babu, M.S.; Veena, P.; Kumar, R.S.; Amaravathi, P.; Vani, G. Clinical, Pathological and Immunohistochemical Bovine Papillomatosis. Pharma Innov. 2020, 9, 243–246. [Google Scholar] [CrossRef]
- Yetsko, K.; Farrell, J.A.; Blackburn, N.B.; Whitmore, L.; Stammnitz, M.R.; Whilde, J.; Eastman, C.B.; Ramia, D.R.; Thomas, R.; Krstic, A.; et al. Molecular Characterization of a Marine Turtle Tumor Epizootic, Profiling External, Internal and Postsurgical Regrowth Tumors. Commun. Biol. 2021, 4, 152. [Google Scholar] [CrossRef]
- Jacobson, E.R.; Mansell, J.L.; Sundberg, J.P.; Hajjar, L.; Reichmann, M.E.; Ehrhart, L.M.; Walsh, M.; Murru, F. Cutaneous Fibropapillomas of Green Turtles (Chelonia Mydas). J. Comp. Pathol. 1989, 101, 39–52. [Google Scholar] [CrossRef]
- Aguirre, A.A.; Balazs, G.H.; Zimmerman, B.; Spraker, T.R. Evaluation of Hawaiian Green Turtles (Chelonia Mydas) for Potential Pathogens Associated with Fibropapillomas. J. Wildl. Dis. 1994, 30, 8–15. [Google Scholar] [CrossRef] [PubMed]
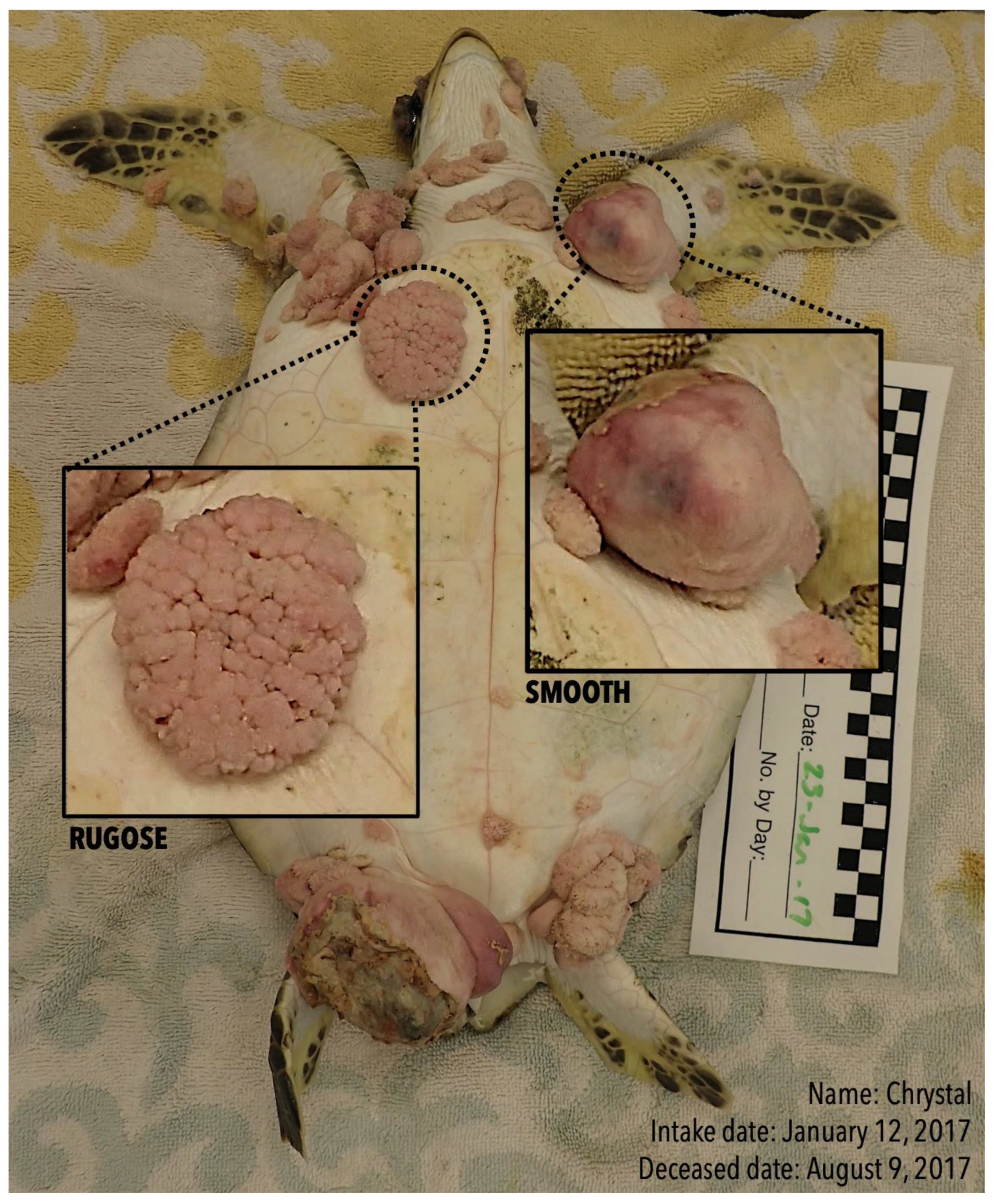
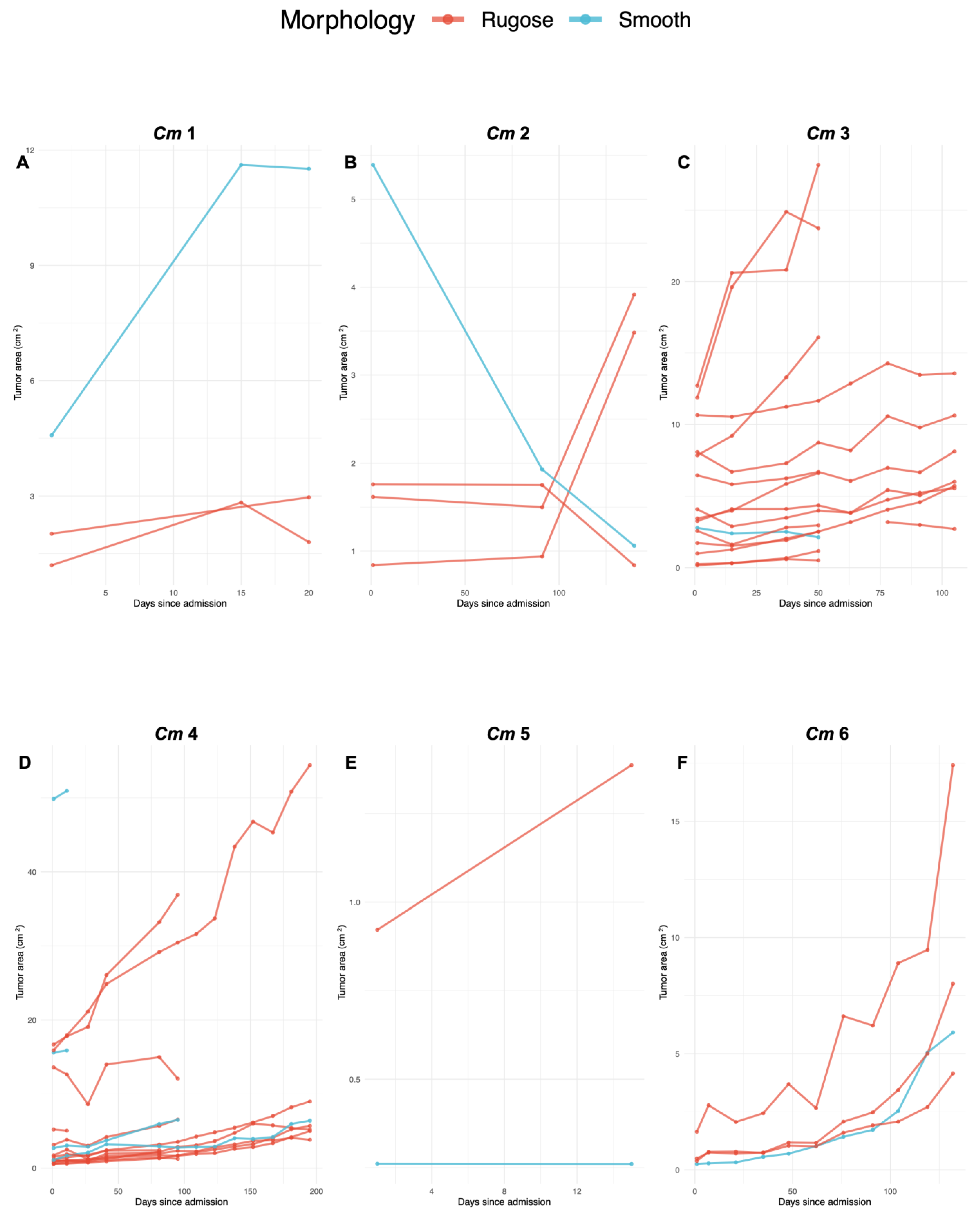
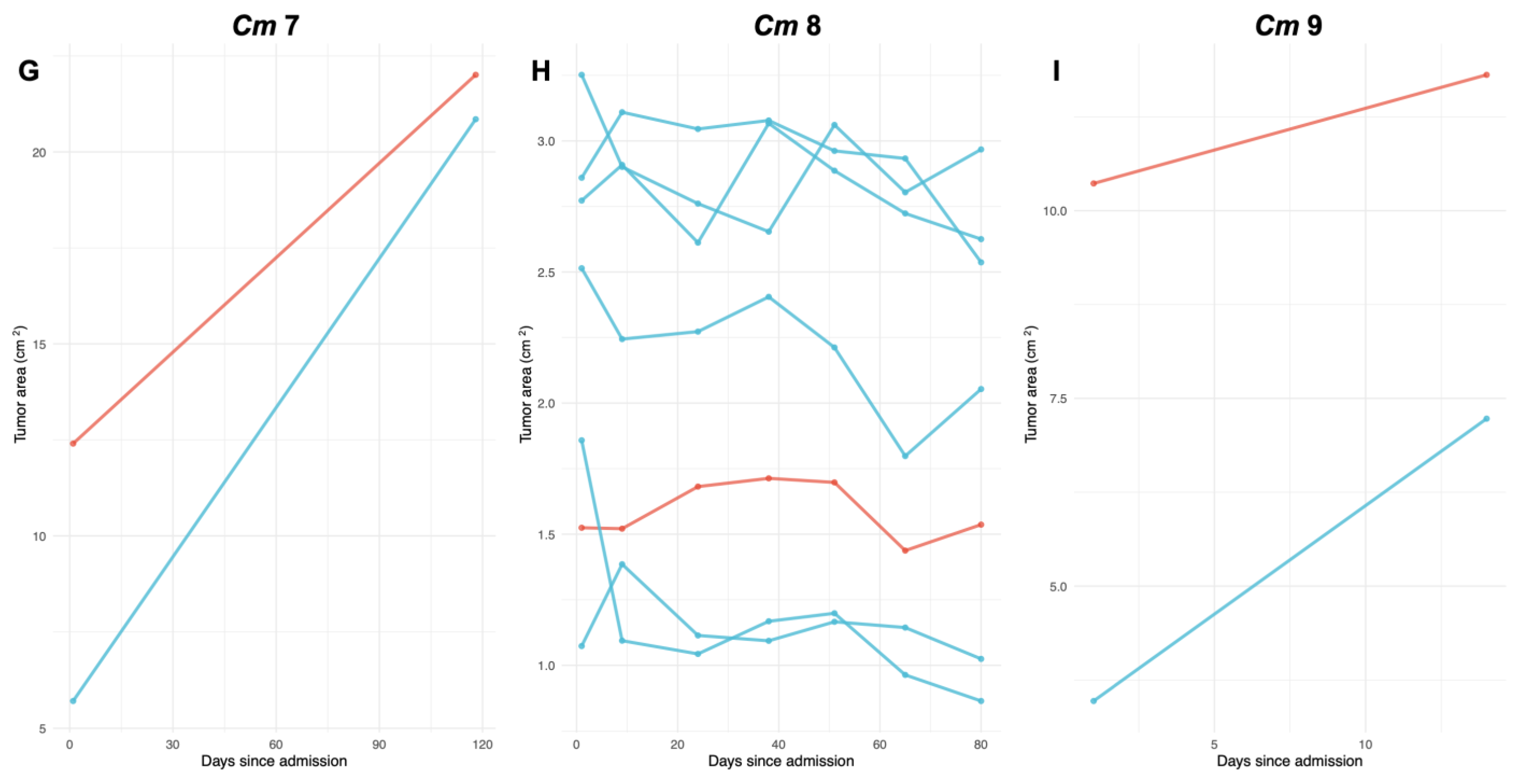

| Turtle ID | Straight Carapace Length (cm) | Weight (kg) | # Smooth Tumors | Change in Size of Smooth Tumors (%) | # Rugose Tumors | Change in Size of Rugose Tumors (%) | Total # FP Tumors |
|---|---|---|---|---|---|---|---|
| Cm 1 | NA | 4 | 1 | 76.5 | 2 | 48.4 ± 2.1 (46.9–50.0) | 3 |
| Cm 2 | 64.6 | 34 | 1 | −6.76 | 3 | 5.53 ± 14.5 (−10.8–17.0) | 4 |
| Cm 3 | 32.8 | 4 | 1 | −8.1 | 14 | 23.5 ± 20.4 (3.6–71.2) | 15 |
| Cm 4 | 29.2 | 2.8 | 4 | 10.7 ± 10 (1.7–21.1) | 14 | 16.1 ± 11.1 (−2.9–43.7) | 18 |
| Cm 5 | 35.1 | 4.4 | 1 | −0.1 | 1 | 50.4 | 2 |
| Cm 6 | 32.5 | 4.1 | 1 | 39.3 | 3 | 33.9 ± 2.9 (30.5–36.1) | 4 |
| Cm 7 | 38.4 | 5.0 | 1 | 17.5 | 1 | 7.3 | 2 |
| Cm 8 | 42.8 | 7.5 | 6 | −2.6 ± 3.7 (−10.1–0.3) | 1 | 0.4 | 7 |
| Cm 9 | 33.6 | 4.6 | 1 | 108.2 | 1 | 13.9 | 2 |
| Tumor Anatomic Region | Rugose | Smooth |
|---|---|---|
| Along flipper | 4 | 1 |
| Base of flipper | 26 | 14 |
| Ventral neck | 1 | 0 |
| Plastron | 10 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s) but do represent the views of the U.S. Geological Survey. MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manes, C.; Herren, R.M.; Page, A.; Dunlap, F.D.; Skibicki, C.A.; Rollinson Ramia, D.R.; Farrell, J.A.; Capua, I.; Carthy, R.R.; Duffy, D.J. Green Turtle Fibropapillomatosis: Tumor Morphology and Growth Rate in a Rehabilitation Setting. Vet. Sci. 2023, 10, 421. https://doi.org/10.3390/vetsci10070421
Manes C, Herren RM, Page A, Dunlap FD, Skibicki CA, Rollinson Ramia DR, Farrell JA, Capua I, Carthy RR, Duffy DJ. Green Turtle Fibropapillomatosis: Tumor Morphology and Growth Rate in a Rehabilitation Setting. Veterinary Sciences. 2023; 10(7):421. https://doi.org/10.3390/vetsci10070421
Chicago/Turabian StyleManes, Costanza, Richard M. Herren, Annie Page, Faith D. Dunlap, Christopher A. Skibicki, Devon R. Rollinson Ramia, Jessica A. Farrell, Ilaria Capua, Raymond R. Carthy, and David J. Duffy. 2023. "Green Turtle Fibropapillomatosis: Tumor Morphology and Growth Rate in a Rehabilitation Setting" Veterinary Sciences 10, no. 7: 421. https://doi.org/10.3390/vetsci10070421
APA StyleManes, C., Herren, R. M., Page, A., Dunlap, F. D., Skibicki, C. A., Rollinson Ramia, D. R., Farrell, J. A., Capua, I., Carthy, R. R., & Duffy, D. J. (2023). Green Turtle Fibropapillomatosis: Tumor Morphology and Growth Rate in a Rehabilitation Setting. Veterinary Sciences, 10(7), 421. https://doi.org/10.3390/vetsci10070421






