Virulence of Shigatoxigenic and Enteropathogenic Escherichia coli O80:H2 in Galleria mellonella Larvae: Comparison of the Roles of the pS88 Plasmids and STX2d Phage
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Genome Sequencing
2.3. Construction of DH10B Transconjugant and Transductant
2.4. In Vivo Assay: The Galleria Mellonella Model
2.5. Statistical Analysis
3. Results
3.1. Virulotypes and Serotypes of the Four Genome Sequenced E. coli Strains
3.2. Identification of Transconjugant and Transductant
3.3. Virulence of E. coli Strains in G. mellonella Larvae
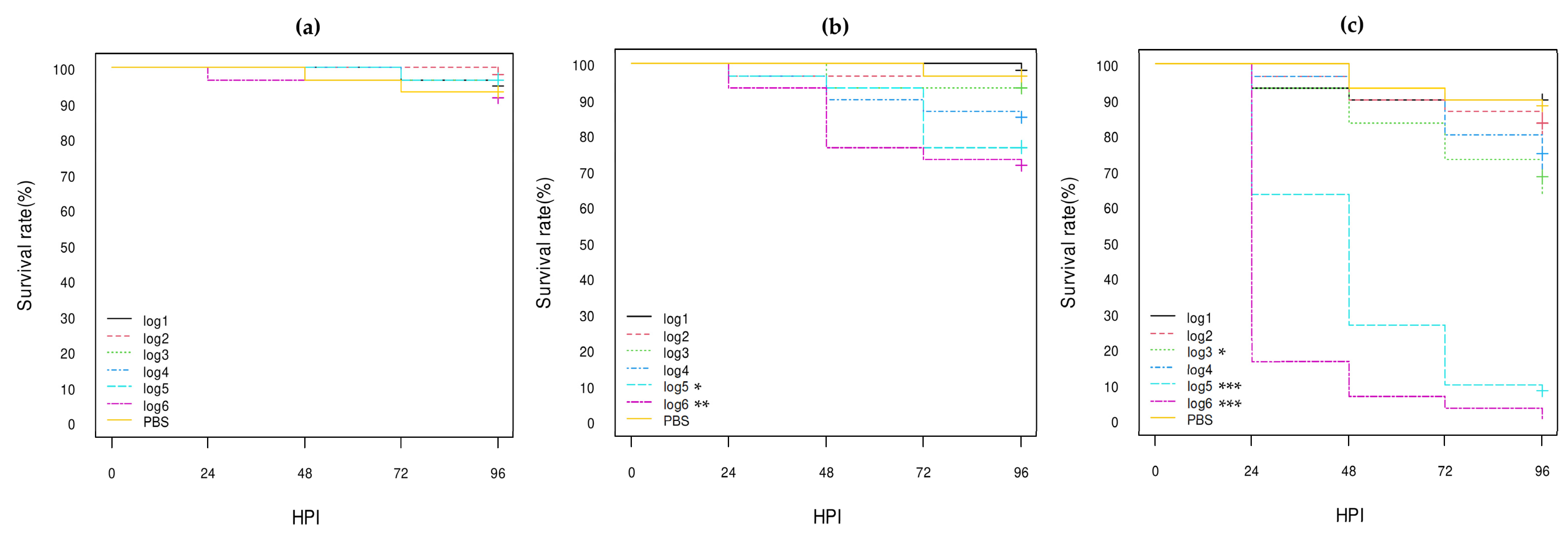
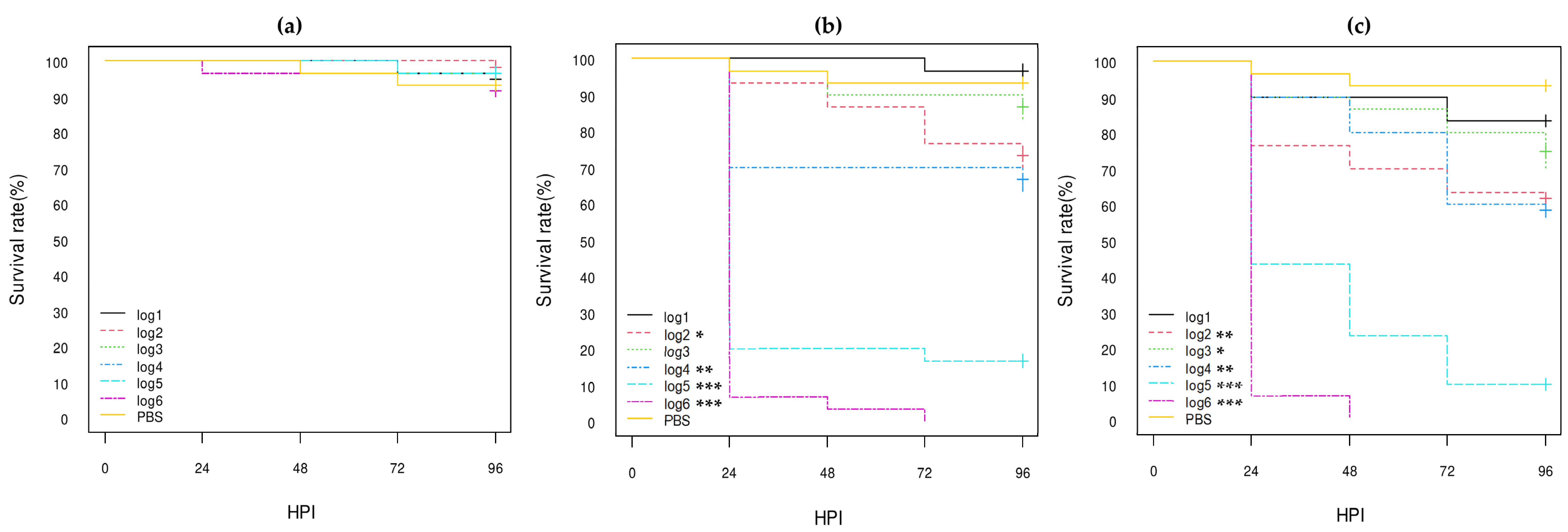
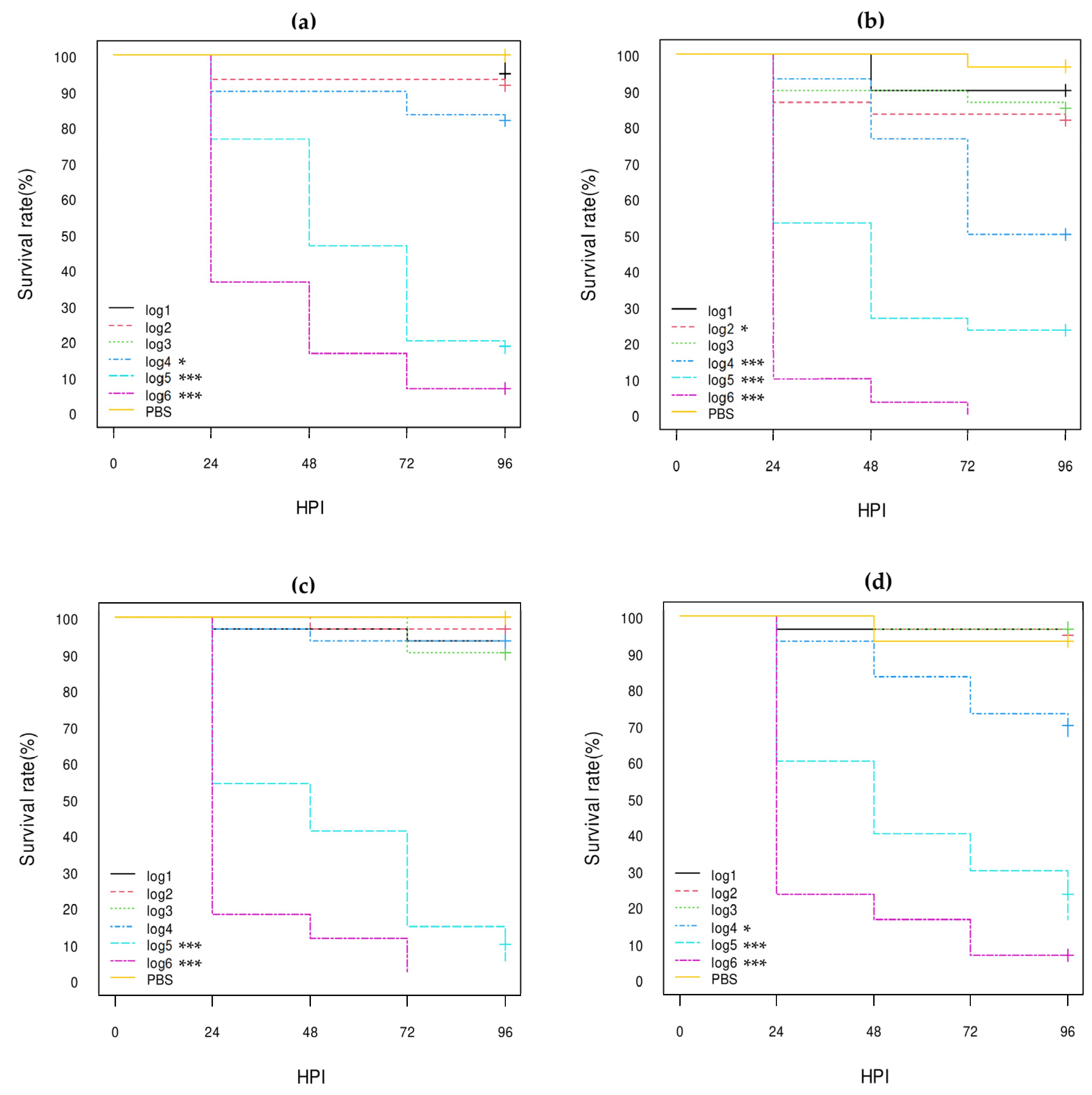
3.3.1. E. coli Non-O80:H2 Control Strains
3.3.2. Comparison of the E. coli O80:H2 Strains Belonging to Different Virulotypes
3.3.3. Comparison of the Role of the pS88 Plasmid and of the STX2d Phage
3.3.4. Comparison with Other E. coli O80 Serotypes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Russo, T.A.; Johnson, J.R. Proposal for a New Inclusive Designation for Extraintestinal Pathogenic Isolates of Escherichia coli: ExPEC. J. Infect. Dis. 2000, 181, 1753–1754. [Google Scholar] [CrossRef] [PubMed]
- Tozzoli, R.; Scheutz, F. Diarrheagenic Escherichia coli Infections in Humans. In Pathogenic Escherichia coli: Molecular and Cellular Microbiology; Morabito, S., Ed.; Caister Academic Press: Norfolk, UK, 2014; pp. 1–18. ISBN 978-1-908230-37-9. [Google Scholar]
- Mainil, J.G.; Fairbrother, M.J. Pathogenic Escherichia coli in Domestic Mammals and Birds. In Pahogenic Escherichia coli: Molecular and Cellular Microbiology; Morabito, S., Ed.; Caister Academic Press: Norfolk, UK, 2014; pp. 19–43. ISBN 978-1-908230-37-9. [Google Scholar]
- Borgatta, B.; Kmet-Lunaček, N.; Rello, J.E. coli O104:H4 Outbreak and Haemolytic-Uraemic Syndrome. Med. Intensiv. 2012, 36, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Piérard, D.; De Greve, H.; Haesebrouck, F.; Mainil, J. O157:H7 and O104:H4 Vero/Shiga Toxin-Producing Escherichia coli Outbreaks: Respective Role of Cattle and Humans. Vet. Res. 2012, 43, 13. [Google Scholar] [CrossRef]
- EFSA BIOHAZ Panel; Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Herman, L.; Hilbert, F.; et al. Pathogenicity Assessment of Shiga Toxin-Producing Escherichia coli (STEC) and the Public Health Risk Posed by Contamination of Food with STEC. EFSA J. 2020, 18, 5967. [Google Scholar] [CrossRef]
- Bai, X.; Fu, S.; Zhang, J.; Fan, R.; Xu, Y.; Sun, H.; He, X.; Xu, J.; Xiong, Y. Identification and Pathogenomic Analysis of an Escherichia coli Strain Producing a Novel Shiga Toxin 2 Subtype. Sci. Rep. 2018, 8, 6756. [Google Scholar] [CrossRef]
- Gill, A.; Dussault, F.; McMahon, T.; Petronella, N.; Wang, X.; Cebelinski, E.; Scheutz, F.; Weedmark, K.; Blais, B.; Carrillo, C. Characterization of Atypical Shiga Toxin Gene Sequences and Description of Stx2j, a New Subtype. J. Clin. Microbiol. 2022, 60, e0222921. [Google Scholar] [CrossRef]
- Martin, C.C.; Svanevik, C.S.; Lunestad, B.T.; Sekse, C.; Johannessen, G.S. Isolation and Characterisation of Shiga Toxin-Producing Escherichia coli from Norwegian Bivalves. Food Microbiol. 2019, 84, 103268. [Google Scholar] [CrossRef]
- Yang, X.; Bai, X.; Zhang, J.; Sun, H.; Fu, S.; Fan, R.; He, X.; Scheutz, F.; Matussek, A.; Xiong, Y. Escherichia coli Strains Producing a Novel Shiga Toxin 2 Subtype Circulate in China. Int. J. Med. Microbiol. 2020, 310, 151377. [Google Scholar] [CrossRef]
- Karmali, M.A.; Mascarenhas, M.; Shen, S.; Ziebell, K.; Johnson, S.; Reid-Smith, R.; Isaac-Renton, J.; Clark, C.; Rahn, K.; Kaper, J.B. Association of Genomic O Island 122 of Escherichia coli EDL 933 with Verocytotoxin-Producing Escherichia coli Seropathotypes That Are Linked to Epidemic and/or Serious Disease. J. Clin. Microbiol. 2003, 41, 4930–4940. [Google Scholar] [CrossRef]
- Soysal, N.; Mariani-Kurkdjian, P.; Smail, Y.; Liguori, S.; Gouali, M.; Loukiadis, E.; Fach, P.; Bruyand, M.; Blanco, J.; Bidet, P.; et al. Enterohemorrhagic Escherichia coli Hybrid Pathotype O80:H2 as a New Therapeutic Challenge. Emerg. Infect. Dis. 2016, 22, 1604–1612. [Google Scholar] [CrossRef] [PubMed]
- Nüesch-Inderbinen, M.; Morach, M.; Cernela, N.; Althaus, D.; Jost, M.; Mäusezahl, M.; Bloomberg, G.; Stephan, R. Serotypes and Virulence Profiles of Shiga Toxin-Producing Escherichia coli Strains Isolated during 2017 from Human Infections in Switzerland. Int. J. Med. Microbiol. 2018, 308, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Cointe, A.; Bizot, E.; Delannoy, S.; Fach, P.; Bidet, P.; Birgy, A.; Weill, F.-X.; Lefèvre, S.; Mariani-Kurkdjian, P.; Bonacorsi, S. Emergence of New ST301 Shiga Toxin-Producing Escherichia coli Clones Harboring Extra-Intestinal Virulence Traits in Europe. Toxins 2021, 13, 686. [Google Scholar] [CrossRef] [PubMed]
- Gigliucci, F.; van Hoek, A.H.A.M.; Chiani, P.; Knijn, A.; Minelli, F.; Scavia, G.; Franz, E.; Morabito, S.; Michelacci, V. Genomic Characterization of HlyF-Positive Shiga Toxin-Producing Escherichia coli, Italy and the Netherlands, 2000–2019. Emerg. Infect. Dis. 2021, 27, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Wijnsma, K.L.; Schijvens, A.M.; Rossen, J.W.A.; Kooistra-Smid, A.M.D.; Schreuder, M.F.; van de Kar, N.C.A.J. Unusual Severe Case of Hemolytic Uremic Syndrome Due to Shiga Toxin 2d-Producing E. coli O80:H2. Pediatr. Nephrol. 2017, 32, 1263–1268. [Google Scholar] [CrossRef]
- De Rauw, K.; Thiry, D.; Caljon, B.; Saulmont, M.; Mainil, J.; Piérard, D. Characteristics of Shiga toxin Producing- and Enteropathogenic Escherichia coli of the Emerging Serotype O80:H2 Isolated from Humans and Diarrheic Calves in Belgium. Clin. Microbiol. Infect. 2019, 25, 111.e5–111.e8. [Google Scholar] [CrossRef]
- Peigne, C.; Bidet, P.; Mahjoub-Messai, F.; Plainvert, C.; Barbe, V.; Médigue, C.; Frapy, E.; Nassif, X.; Denamur, E.; Bingen, E.; et al. The Plasmid of Escherichia coli Strain S88 (O45:K1:H7) That Causes Neonatal Meningitis Is Closely Related to Avian Pathogenic E. coli Plasmids and Is Associated with High-Level Bacteremia in a Neonatal Rat Meningitis Model. Infect. Immun. 2009, 77, 2272–2284. [Google Scholar] [CrossRef]
- Thiry, D.; Saulmont, M.; Takaki, S.; De Rauw, K.; Duprez, J.-N.; Iguchi, A.; Piérard, D.; Mainil, J.G. Enteropathogenic Escherichia coli O80:H2 in Young Calves with Diarrhea, Belgium. Emerg. Infect. Dis. 2017, 23, 2093–2095. [Google Scholar] [CrossRef]
- Habets, A.; Crombé, F.; Nakamura, K.; Guérin, V.; De Rauw, K.; Piérard, D.; Saulmont, M.; Hayashi, T.; Mainil, J.G.; Thiry, D. Genetic Characterization of Shigatoxigenic and Enteropathogenic Escherichia coli O80:H2 from Diarrhoeic and Septicaemic Calves and Relatedness to Human Shigatoxigenic E. coli O80:H2. J. Appl. Microbiol. 2021, 130, 258–264. [Google Scholar] [CrossRef]
- Cointe, A.; Birgy, A.; Mariani-Kurkdjian, P.; Liguori, S.; Courroux, C.; Blanco, J.; Delannoy, S.; Fach, P.; Loukiadis, E.; Bidet, P.; et al. Emerging Multidrug-Resistant Hybrid Pathotype Shiga Toxin-Producing Escherichia coli O80 and Related Strains of Clonal Complex 165, Europe. Emerg. Infect. Dis. 2018, 24, 2262–2269. [Google Scholar] [CrossRef]
- Lewis, D.I. Animal Experimentation: Implementation and Application of the 3Rs. Emerg. Top Life Sci. 2019, 3, 675–679. [Google Scholar] [CrossRef]
- Harnish, J.M.; Link, N.; Yamamoto, S. Drosophila as a Model for Infectious Diseases. Int. J. Mol. Sci. 2021, 22, 2724. [Google Scholar] [CrossRef] [PubMed]
- Ahlawat, S.; Sharma, K.K. Lepidopteran Insects: Emerging Model Organisms to Study Infection by Enteropathogens. Folia Microbiol. 2023, 68, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Cutuli, M.A.; Petronio Petronio, G.; Vergalito, F.; Magnifico, I.; Pietrangelo, L.; Venditti, N.; Di Marco, R. Galleria mellonella as a Consolidated in vivo Model Hosts: New Developments in Antibacterial Strategies and Novel Drug Testing. Virulence 2019, 10, 527–541. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.F.; Rossi, C.C.; da Silva, G.C.; Rosa, J.N.; Bazzolli, D.M.S. Galleria mellonella as an Infection Model: An In-Depth Look at why it Works and Practical Considerations for Successful Application. Pathog. Dis. 2020, 78, ftaa056. [Google Scholar] [CrossRef] [PubMed]
- Antoine, C.; Laforêt, F.; Blasdel, B.; Fall, A.; Duprez, J.-N.; Mainil, J.; Delcenserie, V.; Thiry, D. In vitro Characterization and in vivo Efficacy Assessment in Galleria mellonella Larvae of Newly Isolated Bacteriophages against Escherichia coli K1. Viruses 2021, 13, 2005. [Google Scholar] [CrossRef] [PubMed]
- Wojda, I.; Staniec, B.; Sułek, M.; Kordaczuk, J. The Greater Wax Moth Galleria mellonella: Biology and Use in Immune Studies. Pathog. Dis. 2020, 78, ftaa057. [Google Scholar] [CrossRef]
- Habets, A.; Antoine, C.; Wagemans, J.; Vermeersch, M.; Laforêt, F.; Diderich, J.; Lavigne, R.; Mainil, J.; Thiry, D. Impact of Shiga-Toxin Encoding Gene Transduction from O80:H2 Shiga Toxigenic Escherichia coli (STEC) on Non-STEC Strains. Sci. Rep. 2022, 12, 21587. [Google Scholar] [CrossRef]
- Chen, R.Y.; Keddie, B.A. The Galleria mellonella-Enteropathogenic Escherichia coli Model System: Characterization of Pathogen Virulence and Insect Immune Responses. J. Insect. Sci. 2021, 21, 7. [Google Scholar] [CrossRef]
- Morgan, J.K.; Ortiz, J.A.; Riordan, J.T. The Role for TolA in Enterohemorrhagic Escherichia coli Pathogenesis and Virulence Gene Transcription. Microb. Pathog. 2014, 77, 42–52. [Google Scholar] [CrossRef]
- De la Cruz, M.A.; Morgan, J.K.; Ares, M.A.; Yanez-Santos, J.A.; Riordan, J.T.; Giron, J.A. The Two-Component Sytem CpxRA Negatively Regulates the Locus of Enterocyte Effacement of Enterohemorrhagic Escherichia coli Involving σ32 and Lon Protease. Front. Cell. Infect. Microbiol. 2016, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, R.; Nakamura, K.; Saulmont, M.; Habets, A.; Duprez, J.-N.; Korsak, N.; Hayashi, T.; Thiry, D.; Mainil, J.G. Escherichia coli O80 in Healthy Cattle: Absence of Shigatoxigenic and Enteropathogenic E. coli O80:H2 and (Phylo) Genomics of Non-Clonal Complex 165 E. coli O80. Microorganisms 2023, 11, 230. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Reeves, P.R. Escherichia coli K12 Regains Its O Antigen. Microbiology 1994, 140 Pt 1, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Gilson, L.; Mahanty, H.K.; Kolter, R. Four Plasmid Genes Are Required for Colicin V Synthesis, Export, and Immunity. J. Bacteriol. 1987, 169, 2466–2470. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic Susceptibility Testing by a Standardized Single Disk Method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Pohl, P. Introduction à la Plasmidologie: Données actuelles sur sa Signification en Thérapeutique et en Pathologie. J. Pharm. Belg. 1981, 36, 165–174. [Google Scholar]
- Brosius, J.; Holy, A. Regulation of Ribosomal RNA Promoters with a Synthetic Lac Operator. Proc. Natl. Acad. Sci. USA 1984, 81, 6929–6933. [Google Scholar] [CrossRef]
- Iguchi, A.; Iyoda, S.; Seto, K.; Morita-Ishihara, T.; Scheutz, F.; Ohnishi, M. Pathogenic E. coli Working Group in Japan Escherichia coli O-Genotyping PCR: A Comprehensive and Practical Platform for Molecular O Serogrouping. J. Clin. Microbiol. 2015, 53, 2427–2432. [Google Scholar] [CrossRef]
- Banjo, M.; Iguchi, A.; Seto, K.; Kikuchi, T.; Harada, T.; Scheutz, F.; Iyoda, S. Pathogenic E. coli Working Group in Japan Escherichia coli H-Genotyping PCR: A Complete and Practical Platform for Molecular H Typing. J. Clin. Microbiol. 2018, 56, e00190-18. [Google Scholar] [CrossRef]
- Vandekerchove, D.; Vandemaele, F.; Adriaensen, C.; Zaleska, M.; Hernalsteens, J.-P.; De Baets, L.; Butaye, P.; Van Immerseel, F.; Wattiau, P.; Laevens, H.; et al. Virulence-Associated Traits in Avian Escherichia coli: Comparison between Isolates from Colibacillosis-Affected and Clinically Healthy Layer Flocks. Vet. Microbiol. 2005, 108, 75–87. [Google Scholar] [CrossRef]
- Johnson, T.J.; Siek, K.E.; Johnson, S.J.; Nolan, L.K. DNA Sequence of a ColV Plasmid and Prevalence of Selected Plasmid-Encoded Virulence genes among Avian Escherichia coli Strains. J. Bacteriol. 2006, 188, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.; Pan, H.; Gao, Q.; Xiong, L.; Zhou, Y.; Zhang, D.; Gao, S.; Liu, X. Aerobactin Synthesis Genes iucA and iucC Contribute to the Pathogenicity of Avian Pathogenic Escherichia coli O2 Strain E058. PLoS ONE 2013, 8, e57794. [Google Scholar] [CrossRef] [PubMed]
- Braun, V.; Helbig, S.; Patzer, S.I.; Pramanik, A.; Römer, C. Import and Export of Bacterial Protein Toxins. Int. J. Med. Microbiol. 2015, 305, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Tarr, P.I.; Bilge, S.S.; Vary, J.C., Jr.; Jelacic, S.; Habeeb, R.L.; Ward, T.R.; Baylor, M.R.; Besser, T.E. Iha: A Novel Escherichia coli O157:H7 Adherence-conferring Molecule Encoded on a Recently Acquired Chromosomal Island of Conserved Structure. Infect. Immun. 2000, 68, 1400–1407. [Google Scholar] [CrossRef]
- Schmidt, H.; Hensel, M. Pathogenicity Islands in Bacterial Pathogenesis. Clin. Microbiol. Rev. 2004, 17, 14–56. [Google Scholar] [CrossRef]
- Mellata, M.; Dho-Moulin, M.; Dozois, C.M.; Curtiss, R.; Brown, P.K.; Arné, P.; Brée, A.; Desautels, C.; Fairbrother, J.M. Role of Virulence Factors in Resistance of Avian Pathogenic Escherichia coli to Serum and in Pathogenicity. Infect. Immun. 2003, 71, 536–540. [Google Scholar] [CrossRef]
- Caza, M.; Lépine, F.; Milot, S.; Dozois, C.M. Specific Roles of the iroBCDEN Genes in Virulence of an Avian Pathogenic Escherichia coli O78 Strain and in Production of Salmochelins. Infect. Immun. 2008, 76, 3539–3549. [Google Scholar] [CrossRef]
- Gyles, C.; Boerlin, P. Horizontally Transferred Genetic Elements and their Role in Pathogenesis of Bacterial Disease. Vet. Pathol. 2014, 51, 328–340. [Google Scholar] [CrossRef]
- Datsenko, K.A.; Wanner, B.L. One-Step Inactivation of Chromosomal Genes in Escherichia coli K-12 Using PCR Products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar] [CrossRef]
- Hutchens, M.; Luker, G.D. Applications of Bioluminescence Imaging to the Study of Infectious Diseases. Cell. Microbiol. 2007, 9, 2315–2322. [Google Scholar] [CrossRef]
- Witcomb, L.A.; Czupryna, J.; Francis, K.P.; Frankel, G.; Taylor, P.W. Non-Invasive Three-Dimensional Imaging of Escherichia coli K1 Infection Using Diffuse Light Imaging Tomography Combined with Micro-Computed Tomography. Methods 2017, 127, 62–68. [Google Scholar] [CrossRef] [PubMed]
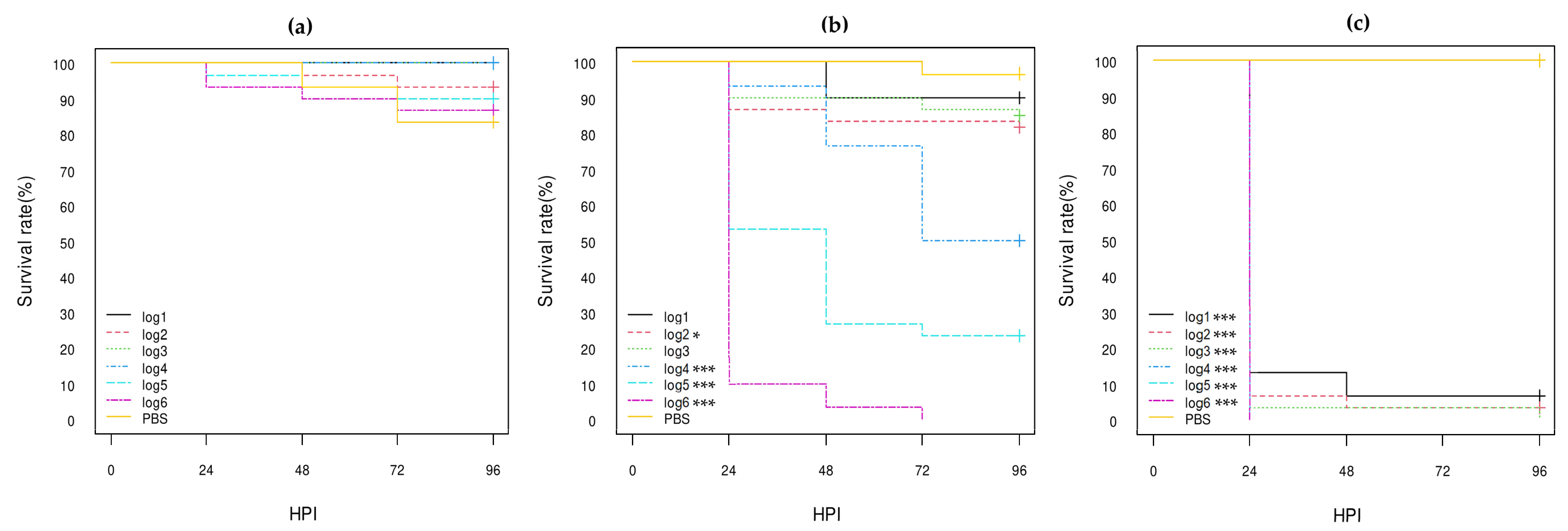
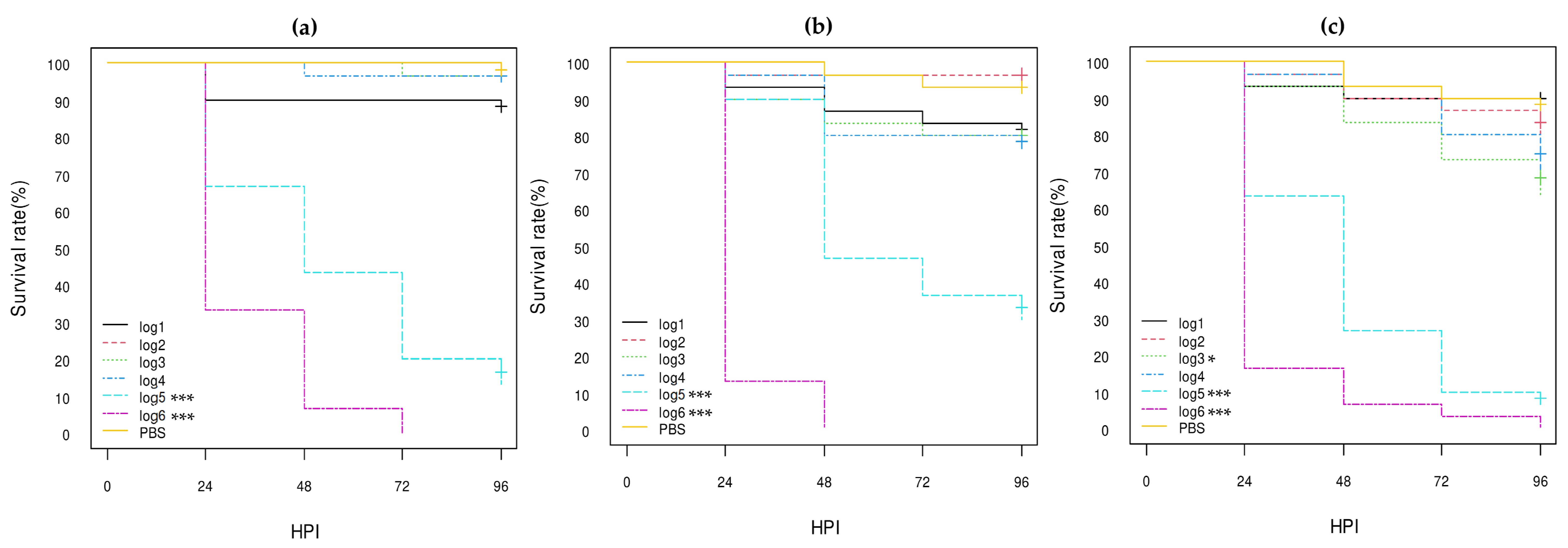
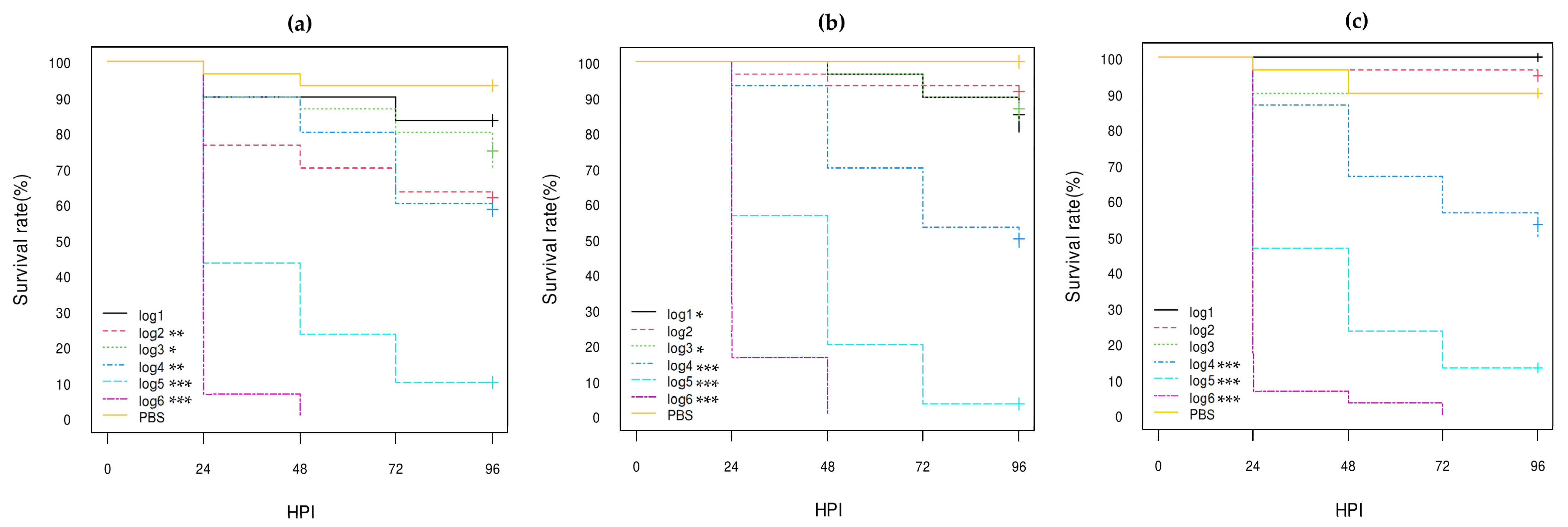
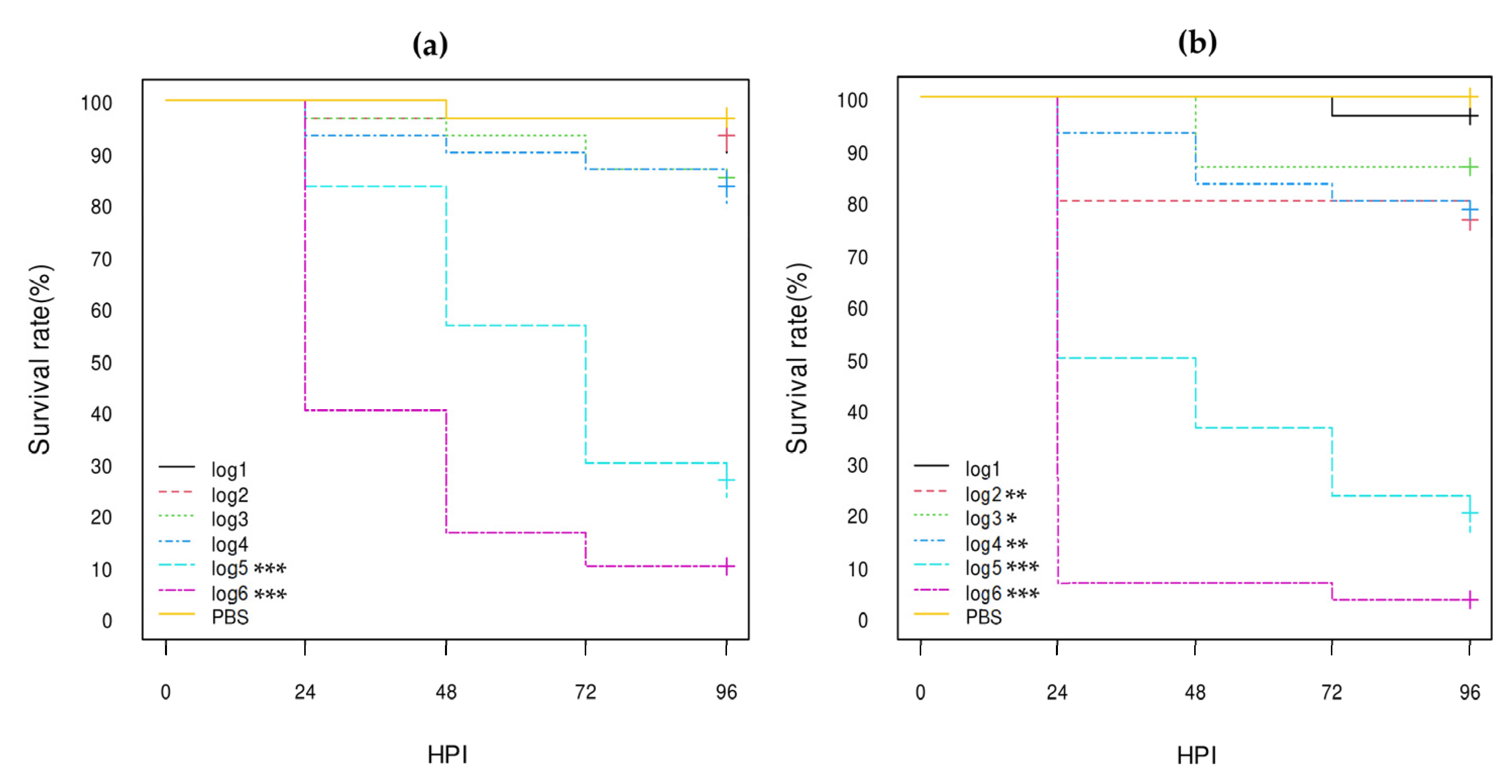
| Isolate References 1 | Serotype | stx Genes | eae Gene | pS88 Plasmid Virulotype (iucC/etsC Genes) | Reference of Genome Sequencing |
|---|---|---|---|---|---|
| SES5320 | O80:H2 | 1a | ξ | nd/nd 2 | This study |
| SES5363 | 1a | ξ | d/d | ||
| EH2282 3,4 | 1a | ξ | d/d | [21] | |
| EH3160 4 | 2d | ξ | d/d | ||
| EH3307/SES2959 | 2d | ξ | nd/nd | ||
| EH3320/SES3090 5 | 2d | ξ | d/d | ||
| EH3308/SES2973 | nd | ξ | nd/nd | ||
| EH3322/SES3122 | nd | ξ | d/d | ||
| SES6039 | O80:H6 | nd | nd | --- 6 | [34] |
| SES5725 | O80:H45 | nd | nd | --- | |
| SES6156 | nd | nd | --- | ||
| Serotype collection | O80:H26 5 | nd | nd | --- | This study |
| O78:H4 | nd | nd | d/d |
| PCR | Target Genes | Primers | Amplified Fragment Length | Reference |
|---|---|---|---|---|
| Serotype O80 | wzyO80 | Og80-F: 5′-TGGTGTTGATTCCACTAGCGT-3′ Og80-R: 5′-CGAGAGTACCTGGTTCCCAAA-3′ | 285 bp | [40] |
| Serotype H2 | fliCH2 | Hg2-F: 5′-TGATCCGACACTTCCTGATG-3′ Hg2-R: 5′-CCGTCATCACCAATCAACGC-3′ | 228 bp | [41] |
| Serotype O16 | wzxO16 | Og16-F: 5′-GGTTTCAATCTCACAGCAACTCAG-3′ Og16-R: 5′-GTTAGAGGGATAATAGCCAAGCGG-3′ | 302 bp | [40] |
| Colicin V_wt | cvaC_wt | CvaC-F: 5′-TATGAGAACTCTGACTCTAAAT-3′ CvaC-R: 5′-ATTTATAAACAAACATCACTAA-3′ | 314 bp | [42] |
| Colicin V_M 1 | cvaC_M | 1555 bp 1 | ||
| Avian hemolysin | hlyF | HlyF-F: 5′-GGCGATTTAGGCATTCCGATACTC-3′ HlyF-R: 5′-ACGGGGTCGCTAGTTAAGGAG-3′ | 599 bp | [19] |
| E. coli Serotypes and Strains | O80:H2 1,2 | O80:H6 3 | O80:H26 4 | O80:H45 3 | O78:H4 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genes Detected after Genome Analysis | SES5320 | SES5363 | EH2282 5 | EH3307/ SES2959 | EH3320/ SES3090 | EH3160 | EH3308/ SES2973 | EH3322/ SES3122 | SES6039 | EH3161 | SES5725 | SES6156 | O78C | |
| Phage-located genes | stx1a | + 6 | + | + | ||||||||||
| stx2d | + | + | + | |||||||||||
| LEE-located genes | eaeξ | + | + | + | + | + | + | + | + | |||||
| espA/B/F/P | + | + | + | + | + | + | + | + | ||||||
| tir | + | + | + | + | + | + | + | + | ||||||
| pS88 plasmid-located genes | cia | + | + | + | + | + | + | + | + | + | ||||
| cvaA | + | + | + | + | + | + | + | + | + | |||||
| hlyF | + | + | + | + | + | + | + | + | + | |||||
| iroN | + | + | + | + | + | + | + | + | + | |||||
| iss | + | + | + | + | + | + | + | + | + | + | + | + | ||
| ompT | + | + | + | + | + | + | + | + | + | + | + | |||
| sitA | + | + | + | + | + | + | + | + | + | + | + | |||
| etsC | + | + | + | + | + | + | ||||||||
| iucC | + | + | + | + | + | + | ||||||||
| Chromosome-located genes | cma | + | + | + | + | |||||||||
| iha | + | + | + | + | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikeda, R.; Laforêt, F.; Antoine, C.; Adachi, M.; Nakamura, K.; Habets, A.; Kler, C.; De Rauw, K.; Hayashi, T.; Mainil, J.G.; et al. Virulence of Shigatoxigenic and Enteropathogenic Escherichia coli O80:H2 in Galleria mellonella Larvae: Comparison of the Roles of the pS88 Plasmids and STX2d Phage. Vet. Sci. 2023, 10, 420. https://doi.org/10.3390/vetsci10070420
Ikeda R, Laforêt F, Antoine C, Adachi M, Nakamura K, Habets A, Kler C, De Rauw K, Hayashi T, Mainil JG, et al. Virulence of Shigatoxigenic and Enteropathogenic Escherichia coli O80:H2 in Galleria mellonella Larvae: Comparison of the Roles of the pS88 Plasmids and STX2d Phage. Veterinary Sciences. 2023; 10(7):420. https://doi.org/10.3390/vetsci10070420
Chicago/Turabian StyleIkeda, Rie, Fanny Laforêt, Céline Antoine, Mare Adachi, Keiji Nakamura, Audrey Habets, Cassandra Kler, Klara De Rauw, Tetsuya Hayashi, Jacques G. Mainil, and et al. 2023. "Virulence of Shigatoxigenic and Enteropathogenic Escherichia coli O80:H2 in Galleria mellonella Larvae: Comparison of the Roles of the pS88 Plasmids and STX2d Phage" Veterinary Sciences 10, no. 7: 420. https://doi.org/10.3390/vetsci10070420
APA StyleIkeda, R., Laforêt, F., Antoine, C., Adachi, M., Nakamura, K., Habets, A., Kler, C., De Rauw, K., Hayashi, T., Mainil, J. G., & Thiry, D. (2023). Virulence of Shigatoxigenic and Enteropathogenic Escherichia coli O80:H2 in Galleria mellonella Larvae: Comparison of the Roles of the pS88 Plasmids and STX2d Phage. Veterinary Sciences, 10(7), 420. https://doi.org/10.3390/vetsci10070420





