Simple Summary
Sea turtles, Caretta caretta, are hosts for several animal and algal organisms which live on their body surface and develop a symbiotic association with them which can range from mutualistic to parasitic, depending primarily on the species. The copepod Balaenophilus manatorum has developed a true parasitic association in that, while exploiting turtle’s skin keratin as a food resource, it may be responsible for cutaneous lesions whenever the equilibrium host-parasite is lost. Studies reporting B. manatorum as a component of epibiotic communities of turtles are still sparse in the literature, and little is known about its distribution in the Mediterranean Sea. This study aimed to investigate its presence in C. caretta ranging in the Northwestern Adriatic Sea and to investigate the effects of Debilitated Turtle Syndrome (DTS) on the turtle–parasite equilibrium. The results of this study indicate that the copepod is a common epibiont of turtles feeding in this region and that individuals suffering from DTS are more frequently parasitized and have higher copepod burdens. Appropriate attention should be given, in debilitated animals, to proper treatment against external epibionts, taking into account the eventual co-presence of true parasites.
Abstract
Balenophilus manatorum (Copepoda: Harpaticoida) is one of the few components of the epibiontic fauna of Caretta caretta that show a “true” parasitic association with their host. From rrosive to ulcerative cutaneous lesions may seldom appear as a consequence of the copepod feeding on keratin on turtles’ skin. Debilitating Turtle Syndrome (DTS) is the final outcome of a chronic insufficient assumption of nutrients, generally occurring with the impairment of immune functions and high epibiota burdens. In this survey, the presence of B. manatorum in C. caretta from the Northwestern Adriatic Sea was investigated and the relation between infection indices and the co-occurrence of DTS was studied. Clinical examination was performed at the time of rescue, including routine hematological assessment; external parasites were isolated mechanically from turtles’ skin and morphologically identified through observation with an optic microscope and SEM. Ten turtles were classified as affected by DTS, all of them being small juveniles with typical clinical and clinicopathological presentation. A higher prevalence, abundance, and density of infection were found in turtles affected by the syndrome. The presence of massive skin coverage by the burrowing barnacle Pletylepas hexastylos prevented a proper evaluation of the pathology associated with B. manatorum in turtles affected by DTS. In any event, eventual skin damages caused by the parasite may represent a port of entry for secondary infections in such immunocompromised animals. Therefore, infection by B. manatorum should not go overlooked in debilitated turtles and should be opportunely treated.
1. Introduction
The Northern Adriatic Sea is a well-known neritic feeding ground for loggerhead sea turtles, Caretta caretta, in the Mediterranean Sea. Due to the co-existence of a relevant fishing effort in the same area, the region is also known as a hotspot for sea turtle bycatch, especially by trawlers [1]. Although anthropogenic activities represent the greatest threat to the conservation of the sea turtle subpopulation in the region, other health issues, unrelated to human activities, also affect the health status of these animals and are less frequently reported. Debilitated Turtle Syndrome (DTS) is a condition observed in stranded sea turtles all over the world, the most striking aspect of which is the massive epibiont burden which generally covers the skin and carapace of the affected turtles [2]. This clinical presentation is thought to represent the end-stage of starvation, assuming a chronic inadequate intake or absorption of nutrients at the origin [2,3]. In the absence of visible external lesions, the identification of primary causes is somewhat challenging, considering that consequences of the debilitation also occur simultaneously, overlapping and worsening the precedent condition. Insufficient food intake may be the consequence of simple food unavailability, or the impossibility to ingest food (e.g., obstruction of the digestive system, such as ingestion of foreign bodies, including hooks and lines). Any condition preventing normal swimming and predatory behaviour may virtually result in improper feeding, including traumatic lesions, entanglement, and cold stunning. Intestinal and metabolic diseases have also been reported as causes of the insufficient absorption of nutrients [3]. Though causes may widely differ, the consequences of debilitation are almost always consistent, resulting in common clinical signs and symptoms. High burdens of epibiota are an almost constant finding in debilitated sea turtles [2,4,5,6]. Excessive barnacle loads have been attributed to the inability of turtles to perform normal “self-grooming” behaviour, such as rubbing against objects, or accessing “cleaning stations,” in which specific omnivore fish species offer the mutual advantage of feeding on turtles’ epibionts while helping in the control of their burden [7,8]. Surface drag caused by the overgrowth of barnacles further decreases the ability of affected turtles to swim properly and feed, especially when their eyes and mouth are covered by sessile epibionts, and increases energy expenditure for performing normal movements [9]. Therapy generally focuses on rehydration, nutritional support, and the elimination of infectious diseases, along with the underlying causes, when identifiable.
Harpacticoid copepods are an important component of the meiofauna, including about 3000 known species that widespread in marine and freshwater ecosystems [10], and it is estimated that a great part of the diversity within this family is still overlooked [11]. Only five harpacticoid species live in association with vertebrate species, with the genus Balaenophilus, the most widespread, exploiting marine mammals and sea turtles as living substrates. The type of association developed with the vertebrate host has been a controversial topic for a long time. Evidence of a “true” parasitic association of Balaenophilus manatorum (previously Balaenophilus umigamecolus) with the sea turtle host has been provided by Badillo et al. (2007) [12] and Crespo-Picazo et al. (2017) [13], who demonstrated the ability of the species to feed on the keratin of the host skin, seldomly causing erosive to ulcerative lesions. Most severe disease has been described in captive hatchlings, with the underdeveloped immune system being cited as one of the predisposing factors for high parasitic loads [13].
In the present paper, we aimed to (i) report the presence of B. manatorum in loggerhead sea turtles from the Northern Adriatic Sea and (ii) study the relationship between infection by B. manatorum and the co-presence of DTS, which is diagnosed in turtles by combining clinical and clinicopathological findings.
2. Materials and Methods
2.1. Clinical Data Collection
Twenty-four loggerhead turtles admitted to the rescue facility run by Centro Sperimentale per la Tutela degli Habitat (CESTHA) in Marina di Ravenna, between June and October 2021, were included in this study. Of these, 10 animals were found stranded along the shoreline or floating in the waters off the provinces of Ferrara and Ravenna (Figure 1). The remaining 14 turtles were rescued after being bycaught by trawlers in the corresponding offshore marine area. Water temperatures registered in the whole sampling period ranged from 20 °C to 27 °C.
Figure 1.
Sampling site in the Northern Adriatic Sea, embracing Ferrara and Ravenna provinces.
At admission, all turtles were submitted to clinical assessment, including physical examination and routine blood work (i.e., haematocrit and total protein estimate, and qualitative and quantitative white blood cell evaluation). Biometric data, including curved carapace length (CCL), notch-to-tip, and body weight were collected at the time of entry. Neurological examination was performed with the animals out of water and their ability to dive and surface was evaluated in water tanks after stabilization by a technician or a veterinarian. Nutritional condition was assessed always by the same veterinarian observing the axillary, inguinal, and neck regions, plastron concavity, and orbits, and assigning a Body Condition Score (BCS) from 1 to 5, ranging from cachectic to optimal condition [14]. External examination was conducted to exclude the presence of traumatic injuries and to evaluate the percentage of body surface covered by sessile epibiota. This was conducted by visual examination both immediately at the time of arrival by technicians on site and by taking pictures of the animals on dorsal and ventral recumbency which were later re-examined. A blood sample (at least 1 mL) was withdrawn from the cervical sinus of the jugular vein into lithium-heparin tubes after surgical preparation of the site. Hematocrit (HCT) and total protein (TP) concentration were obtained with standard centrifugation in microhematocrit capillary tubes; plasma was evaluated using a refractometer. In order to evaluate the immune status of the turtles, fresh blood smears were prepared, air-dried, and stained with Diff Quick for differential white blood cell count and observation of blood cell morphology [15,16]. Total white blood cell count was estimated with a Neubauer haemocytometer after dilution to 1:100 with Natt and Herrick’s staining solution (Bioanalytic GmbH, Umkirch, Germany). Ranges for the evaluation of HCT and TP, and for total WBC count, were compared with Casal and Orós (2009) [17]. All clinicopathological analyses were conducted by a trained veterinarian.
2.2. Parasitological Analyses
In order to detect B. manatorum, turtles were gently scrubbed with a nylon soft brush and washed with tap water soon after stabilization (i.e., within some hours from recovering normal surfacing behaviour), before entering rehabilitation tanks. Soft skin was also brushed to remove non-sessile external parasites. Washes were collected and filtered with a 0.5 mm sieve. All material kept on the filter was preserved in 70% ethanol flasks until examination at the Fish Pathology Unit of the Department of Veterinary Medical Sciences of Bologna University. For each sample, copepods were collected from washes at the stereomicroscope and counted. After isolation, morphometric characteristics of randomly selected specimens from each sample were studied under an optic microscope by NIS Elements D software (Nikon) (n = 21) and Scanning Electron Microscopy (SEM) (n = 5) to confirm species identification. Samples were examined by SEM in order to describe some morphological features of larval and adult stages. The specimens were postfixed in 1% osmium tetroxide in cacodylate buffer, dehydrated through a graded ethanol series, critical point dried, sputter-coated with platinum, and observed using a JEOL JSM 6700F SEM (Basiglio [MI], Italy) operating at 5.0 kV according to the process described in Tedesco et al. (2018) [18].
Morphometric characteristics of copepods were compared with the descriptions in the literature for species identification [19,20]. Similarly, specific key features were used for the identification of sessile epibionts [21].
2.3. Data Analysis
Prevalence (percentage of positive turtles), abundance (number of parasite individuals per turtle), and intensity values (number of parasite individuals per infected turtle) for B. manatorum were displayed using simple descriptive statistics. Since formulae for the calculation of body surface area in chelonians are lacking in the literature, weight was taken as a proxy of body surface area to calculate parasite density [22,23], assuming these two parameters to be positively correlated.
In order to explore any influence of debilitated turtle syndrome on the aforementioned epidemiological indices, turtles were classified as affected (DTSt) when presenting with the following findings: depressed sensorium associated with anamnesis of stranding or floating offshore, BCS < 3, extensive epibiota coverage (>50% percentage of body surface) or barnacles covering eyes or rhamphotheca, no evidence of traumatic injuries, anemia, and hypoproteinemia [2]. Animals deviating from this presentation, lacking two or more of the above-mentioned findings, were classified as non-affected (non-DTSt). Fisher’s exact test and Mann–Whitney test were used for the comparison of prevalence and abundance, intensity, and density between the two groups. Clopper–Pearson (exact) method was used for 95% CI definition. Student’s T test was used to compare HCT, TP, and total WBC values in DTSt and non--DTSt to assess the reliability of these values as markers for DTS in our sample.
Statistical tests were conducted on the online software Quantitative Parasitology QPweb [24]. Level of significance for all tests was set at p < 0.05.
3. Results
3.1. Clinical Data
Overall, 10 turtles out of the 24 were classified as affected by DTS, with all being small juveniles (mean CCL 18.6 cm, range 14.5–22.5 cm) and all but one being found stranded along the shore. One turtle was recovered while floating offshore, showing no voluntary attempts to escape at catching. At neurological examination, four turtles were severely depressed, with little or no response to lifting, five animals were classified as depressed, and one turtle as slightly depressed, showing a reduced but timely response to all stimuli. A BCS of 2/5 was attributed to all turtles of this group, which showed muscular atrophy, sunken eyes, and inguinal and axillary fossae from normal to slightly depressed. Barnacles of the species Platylepas hexastylos were found covering ≥50% of turtles’ skin, preferentially distributed on soft skin areas (axillary and inguinal regions and flippers), but were also abundant on the carapace, plastron, and head (Figure 2a). Occasionally, the eyelids were also affected. No other barnacle species were observed in this group. The detachment of P. hexastylos after freshwater baths revealed erosive to necrotic cutaneous lesions, and tissue loss along the margins of the extremities (Figure 2b,c). None of the turtles showed other external injuries (e.g., traumatic). Anaemia was classified as severe in eight animals (HCT range 8–12%) and moderate in two (14–16%). Hypoproteinaemia was registered in 5/10 animals (TP < 2.0 g/100 mL), leucocytosis in 1 individual (tot WBC > 19 × 103/µL) and heterophil toxicity of different degrees was observed in 3 individuals. The lymphocyte relative count ranged from 1% to 17% (Table 1 and Table S1).
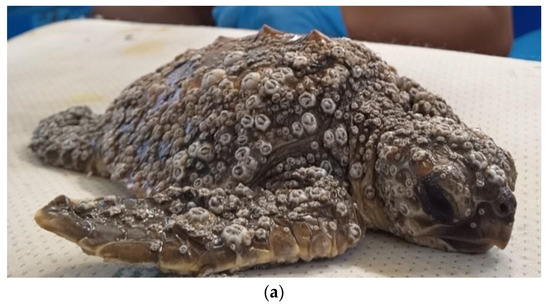
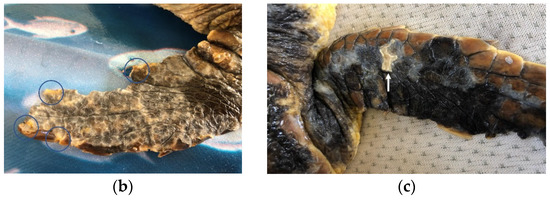
Figure 2.
Clinical presentation at arrival and cutaneous lesions on flippers of loggerhead sea turtles affected by DTS after barnacle removal. (a) Barnacle P. hexastylos covering most parts of body surface appearing as the almost exclusive barnacle species. (b) Diffuse erosive and multifocal ulcerative lesions (circles) on the fore-flipper surface; (c) deep ulcerative lesions (arrow) on the dorsal aspect of a fore-flipper.

Table 1.
Main clinicopathological values in loggerhead turtles in this study. Statistical comparison between DTSt and non-DTSt values is shown. Student’s T test results are given for the comparison between the two groups. HCT, hematocrit; TP, total protein; WBC, total white blood cells; lymph%, percentage of lymphocyte in white blood cell formula; het tox, toxic heterophils as evaluated from blood smear. * Statistically significant. All values are presented as mean ± standard deviation.
NonDTS turtles (n = 14) were all rescued from bycatch events in trawlers. The group included juvenile to adult animals, with CCLs ranging from 21 to 91 cm (mean 53.1 cm). Depressed mentation was observed in 6 out of 14 animals at admission, with the remnant being normally reactive to stimuli. The body condition scores ranged from two to four. The percentage of body surface covered by barnacles ranged from 0% to 30%, with Chelonibia sp. being the most frequently encountered barnacle species, preferentially fixed on the carapace. The HCT ranged within normality (26–46%), as did total protein (>2.2 g/100 mL). No heterophil toxicity was observed in the blood films. The total WBC count ranged within normality, except for one case of leucocytosis that probably attributable to stress leukogram and was not associated with clinical disease.
3.2. Parasitological Examination
Adult copepods were detected in 17/24 of the turtles. All of the specimens (n = 1803) were identified as Balaenophilus manatorum from morphology. The overall mean intensity of infection was 106.5, with a range from 3 to 377 individuals per turtle. The descriptive indices of infection in the overall sample and in DTSt and non-DTSt are reported in Table 2.

Table 2.
Epidemiological indices in the overall sample considered and in turtles affected and non-affected by DTS. p-values refer to the comparison between the latter two groups, obtained with Fisher’s exact test for prevalence values and Mann–Whitney test for intensity, abundance, and density. P%, prevalence (95% confidence interval calculated with Clopper Pearson exact method); medI, median intensity; medA, median abundance; medD, median density; IQR, interquartile range. * Statistically significant.
In detail, the morphological key features observed in our sample were the following: average body lenght ± sd (measured in µm) in females 1111.5 ± 61.6 (range 999.5–1189.46) (Figure 3a) and 1017 ± 79.8 (range 863.6–1133.5) in males; antennula made up of nine segments, with setae of variable numbers on segments 2–9; antenna with a short coxa, allobase carrying a coronula of spines on the inner side (Figure 3b,c), and a small exopod made up of two segments, having one and two setae, respectively; endopod of antenna carrying, overall, seven claws and three geniculate; maxilliped made up of a short syncoxa, having two setae at the distal end, a longer basis with two short setae at midlength, and a distal, hook-shaped endopod carrying two, very fine setae on the inner side; leg 1 having three small claws on both the endopod and exopod (Figure 3d,e); caudal rami being short (Figure 3f), with two elongated setae (average length 811.3 ± 56.7 in females, 831.1 ± 69.7 in males) (Figure 3g). No setae were observed on segment 2 of the exopode of leg 4.
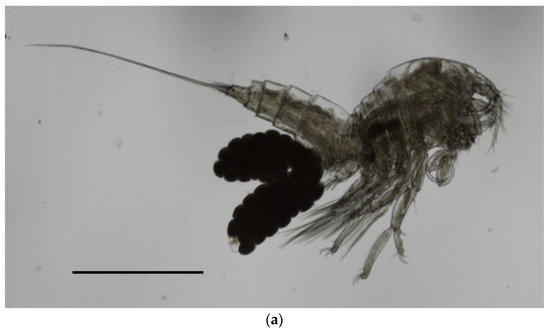
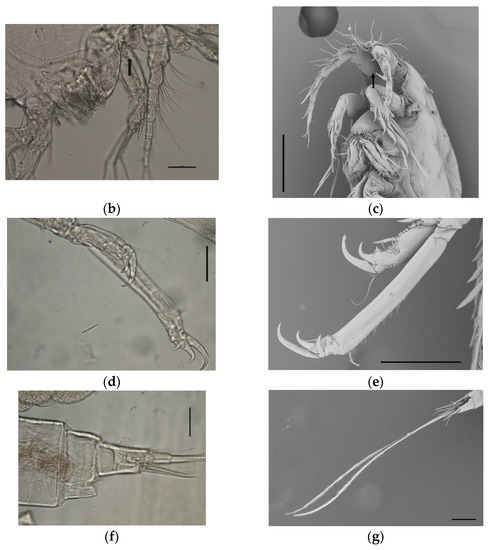
Figure 3.
Microphotographs of individuals of B. manatorum collected from Caretta caretta from the Northern Adriatic Sea using optic microscope (left side) and SEM (right side). (a) Full-body image of an adult female. (b,c) A coronula of spines is visible on the inner face of the antennula allobase (black arrow); (d,e) Leg 1 showing three hooks on the endopode and exopode; (f) caudal rami short; (g) terminal setae. Scale bars: (a)—500 µm; (b,d,f)—50 µm; (c,e,g)—100 µm.
4. Discussion
This study aimed to detect the copepod B. manatorum in sea turtles from the Adriatic Sea and relate infection indices with clinical and clinicopathological findings. Turtles affected by DTS that were included in the study were all juvenile specimens stranded along the north-western coast of the Adriatic Sea, which appeared to have a higher risk for hosting the copepod and have a higher burden with respect to turtles coming from bycatch. B. manatorum apparently has a worldwide distribution, as this species has been reported on the Eastern and Western Pacific coasts [20,25] as well in the Western Mediterranean Sea [12,13,26]. Previous surveys that were conducted on the epibiota of C. caretta in the Adriatic Sea [27,28], as well as in the Central [29,30] and Eastern Mediterranean [31], did not adopt specific methods for the detection of B. manatorum, and, consequently, no earlier reports of the species are present from these regions. As Domènech et al. (2014) [32] pointed out, the detection of this tiny ectoparasite is subordinated to the application of proper research methods, i.e., the use of a preliminary wash and filtering through a small mesh sieve, which was not included in the methodology followed by the aforementioned studies. Recently, Karaa et al. (2019) [33] reported the presence of one specimen of Harpacticoida on the carapace of one loggerhead turtle from the Tunisian coast, suggesting its identification as Balaenophilus sp., but the methods for epibiont collection were not disclosed in that study. This finding further suggests a pan-Mediterranean distribution of B. manatorum in loggerhead turtles. Data on the prevalence of infection in free-ranging loggerhead sea turtles have only been provided for the area of the Western Mediterranean (82.7%) [12]. Our data suggest a potentially comparable prevalence in loggerhead sea turtles from the Adriatic Sea (70.8%), but given the limited sample herein considered, caution is warranted when considering this value. The existence of different sibling species of B. manatorum in the Mediterranean should not be ruled out, considering separate spatial distribution [19], at least until a complete molecular study is conducted on the isolates.
A higher prevalence and intensity of infection by macro and microparasites are generally expected in animals suffering from depression of their immune functions, following alteration of the equilibrium between the host and its parasites [34]. Defects in the activity of lymphocytes have been reported in debilitated turtles, which finally results in the suppression of the humoral immunity in these individuals [2]. Suppression of the adapted immune response is a general consequence of extreme energy restrictions during starvation in mammals [35,36], and similar effects are expected in reptiles. Innate immunity apparently activates as compensation for this deficit in sea turtles to provide immediate defence against bacterial and parasitic infections [2]. Although no specific evaluation of immune functions was conducted in this study, and the lymphocyte relative counts were in some cases overlapped between individuals of the two groups, there was a significative trend in DTSt of have lower lymphocyte counts, which is correlated with lower immune defence, and the presence of toxic heterophils in this group supports the hypothesis of underlying inflammation and the compensatory activation of innate immunity [2,15]. Underdevelopment of the immune system was indeed suspected to be one of the co-factors inducing severe lesions by B. manatorum in hatchling loggerheads by Crespo-Picazo et al. (2017) [13], along with stress from captivity and high water temperatures. Similarly, high temperatures may also have increased the recruitment rate of B. manatorum in our study, as all animals were sampled in the warmer period of the year. Future studies should assess the impact of seasonal temperature variation and other abiotic factors on infection prevalence and intensity. The softer skin of hatchlings and small juveniles may also have facilitated the infection by B. manatorum in the study by Crespo-Picazo et al. (2017) [13] and in our study as well. Definite conclusions are not possible, given the overlap of young age and immune deficiency in both studies. Appropriate prophylactic and therapeutic measures, especially, should be adopted when dealing with small juveniles in captivity.
The facilitation of B. manatorum colonization in DTSt group may have also been offered by the previous overgrowth of P. hexastylos on the turtles’ body surface. Although the temporal succession of epibiont communities on sea turtles hosts is still poorly understood, it is thought that hard, sessile species, such as barnacles, are the first to colonize turtle carapaces; these “pioneer species” [37] generate new niches for colonization by motile epibionts, facilitating their settlement to turtle carapace [38,39,40]. Lazo-Wasem et al. (1997) [25] also found the co-occurrence of B. manatorum with the sessile barnacle Stomatolepas praegustator to not be coincidental and hypothesized that B. manatorum may feed on irritated skin close to the embedded barnacles. Specialized hooks and claws likely allow adult and immature B. manatorum to grasp tightly to turtles’ skin, independently from the presence of other epibiotic species, as the motile amphipod Caprella spp. does; both species, indeed, can be found as an exclusive component of the epibiotic community of turtles [13,38]. High burdens of B. manatorum were isolated from one turtle of the non-DTSt group in which the overgrowth of algae was also observed, covering the caudal midpart of the carapace. Further studies should test whether colonization by animals and/or algal epibionts facilitates the recruitment of B. manatorum.
The higher prevalence of P. hexastylos in DTSt vs. C. testudinaria is difficult to interpret. Both species are described in C. caretta in the Adriatic Sea [28]. P. hexastylos was thought to be a useful indicator of habitat use, being more typical of turtles floating in pelagic areas [30]. Ten et al. (2019) [41] recently reported this species to have a limited value as a habitat indicator, as it has been detected in both oceanic and neritic turtles with similar abundance, but has been negatively correlated with the abundance of C. testudinaria. The soft carapace skin of juveniles may have facilitated the recruitment all over the body surface of the burrowing barnacle P. hexastylos, whose presence is generally limited to the skin of throat, axillary areas, and flanks, finally competing with C. testudinaria for its niche. A comparison with subadult, DTS-affected loggerhead sea turtles should be conducted to further explore this issue. Meanwhile, conclusions regarding this aspect sill remain speculative.
The high loads of the barnacle P. hexastylos in the DTSt group prevented a proper evaluation of the pathological effect of the copepods on turtles’ skin. Erosions and ulcers were evident on all body surfaces of the turtles at the time of barnacle removal, with most severe lesions being represented by areas of necrotizing ulcerative dermatitis, mostly on soft skin areas (neck and flippers). Barnacles of the species P. hexastylos are listed among the “burrowing epibionts,” here indicating a varied group of organisms whose bodies can become embedded within both the hard and soft tissues of host turtles; the opposite, non-burrowing barnacles (e.g., Chelonibia spp.) simply fix their bodies on the epidermis surface with no tissue penetration [38]. Although P. hexastylos is found technically external to the skin, as a thin epidermal layer remains intact under their bodies, at the time of their detachment, erosions and ulcerative lesions can become evident, as well as the loss of the external beta-keratin layer [42,43]. Similar erosive lesions were extensively present in the DTSt group, but their appearance strikingly overlaps the lesions caused by B. manatorum, as reported in the literature [12,13,20]. As an exception among marine symbionts, indeed, B. manatorum has been demonstrated to be able to feed on keratin, exploiting the skin of manatees and sea turtles as optimal substrates [12]. Discoloured or yellowish areas are described on the skin of turtles as the result of keratin consumption by the copepods, mostly on soft skin areas, and occasionally on the carapace and plastron [12,20]. At histology, ulcerative necrotizing dermatitis involving the superficial dermal layer is reported in severe cases, as well as dyskeratosis and inflammation of varying degrees [12,13]. In this study, no biopsies were conducted to detect the presence of copepods within the lesions. Therefore, it still remains to be ascertained to what extent the copepods may have contributed to altering the skin barrier in the DTS-affected turtles, potentially favouring secondary bacterial infections in immunodeficient animals. The mean intensity of infection in turtles with lesions is variable in the literature but higher than ours in the study by Crespo-Picazo et al. (2017) [13] (337.4 copepods per turtle). Badillo et al. 2007 [12] reported neither gross nor microscopic tissue alterations in turtles infected with 113 copepods, and only inflammatory reactions were evident at histopathology in turtles with from 585 to 1193 specimens. Nevertheless, the size of the turtles was not known in this latter study, making it difficult to compare with our study.
A mass stranding event of juvenile loggerhead turtles was reported along the same North-Western Adriatic coast in 2009 [44]; comparably to the present case, the stranding of such juvenile turtles happened in the same season—from late July to September—and involved only small individuals, with CCL ranging from 17 to 25 cm. Massive infestation by P. hexastylos was reported in those specimens as well, covering the skin and mucosae; similar clinicopathological findings were reported [45], but sampling for B. manatorum was not performed. The reasons for the unusual stranding of small juvenile C. caretta in the area remain to be ascertained; specimens of this size represent an unusual finding in the region, which is mainly populated by large juveniles, and subadult and adult C. caretta at their neritic phase. The shallow waters of the Northern Adriatic Sea probably represent an unsuitable feeding ground for juveniles in their pelagic life-stage, and may have led to the development of DTS, which would also be confirmed by the almost exclusive presence of algae in their feces for days after rescue (Segati, pers. Comm.), as also reported ain 2009 [45], which are an uncommon food item for loggerheads at this ontogenic stage. Prompt supporting therapy is mandatory for the recovery of debilitated turtles; repeated freshwater baths are administered for progressive barnacle removal and may be resolutive for infection by B. manatorum as well [13].
5. Conclusions
B. manatorum is a component of the epibiotic communities of loggerhead sea turtles in the Northern Adriatic Sea. Studies on the real prevalence in free ranging turtles are encouraged in the area in order to increase our knowledge on epibiotic communities’ composition in C. caretta, and especially for that part of the epibiota which is considered, per definition, parasitic. Turtles suffering from DTS and, more generally, those carrying high burdens of burrowing barnacles, should be carefully evaluated for the presence of B. manatorum and opportunely treated.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vetsci10070427/s1, Table S1: Individual clinical and parasitological data of loggerhead sea turtles included in the study.
Author Contributions
Conceptualization, E.M.; methodology, A.G., E.M., F.M. and J.L.C.-P.; writing—original draft preparation, E.M.; writing—review and editing, F.M., A.G. and J.L.C.-P.; sample collection and parasitological analyses: V.V., S.S., S.D., E.M., F.M. and A.G.; Clinical data collection and analyses E.M. and S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the non-applicability of the European Parliament and Council normative 201063/UE on the protection of animals used for scientific purposes, in that the “non-experimental clinical veterinary practice” for the collection of data, applied in this study, is excluded from the scope of the legislation (Article 2, Section 5, letter b and f).
Informed Consent Statement
Not applicable.
Data Availability Statement
Dataset providing information supporting results of this study is reported in Supplementary materials.
Acknowledgments
We thank Perla Tedesco and Valentina Luci for preparation and technical support for SEM analysis at Bologna University and Gianfranco Medri (Clinica Veterinaria San Marco, Ravenna) for providing clinical and diagnostic support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lucchetti, A.; Pulcinella, J.; Angelini, V.; Pari, S.; Russo, T.; Cataudella, S. An interaction index to predict turtle bycatch in a Mediterranean bottom trawl fishery. Ecol. Indic. 2016, 60, 557–564. [Google Scholar] [CrossRef]
- Stacy, N.I.; Lynch, J.M.; Arendt, M.D.; Avens, L.; Braun McNeill, J.; Cray, C.; Day, R.D.; Harms, C.A.; Lee, A.M.; Peden-Adams, M.M.; et al. Chronic debilitation in stranded loggerhead sea turtles (Caretta caretta) in the southeastern United States: Morphometrics and clinicopathological findings. PLoS ONE 2018, 113, e0200355. [Google Scholar] [CrossRef] [PubMed]
- Manire, C.A.; Stacy, N.I.; Norton, T.M. Chronic debilitation. In Sea Turtle Medicine and Rehabilitation; Manire, C.A., Norton, T.M., Stacy, B.A., Innis, C.J., Harms, C.A., Eds.; J. Ross Publishing: Plantation, FL, USA, 2017; pp. 707–724. [Google Scholar]
- Deem, S.L.; Norton, T.M.; Mitchell, M.; Segars, A.; Alleman, A.R.; Cray, C.; Poppenga, R.H.; Dodd, M.; Karesh, W.B. Comparison of blood values in foraging, nesting, and stranded loggerhead turtles (Caretta caretta) along the coast of Georgia, USA. J. Wildl. Dis. 2009, 45, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Flint, M.; Patterson-Kane, J.C.; Limpus, C.J.; Work, T.M.; Blair, D.; Mills, P.C. Postmortem Diagnostic Investigation of Disease in Free-Ranging Marine Turtle Populations: A Review of Common Pathologic Findings and Protocols. J. Vet. Diagn. Investig. 2009, 21, 733–759. [Google Scholar] [CrossRef] [PubMed]
- Nolte, C.R.; Nel, R.; Pfaff, M.C. Determining body condition of nesting loggerhead sea turtles (Caretta caretta) in the South-west Indian Ocean. J. Mar. Biol. Assoc. U. K. 2020, 100, 291–299. [Google Scholar] [CrossRef]
- Schofield, G.; Katselidis, K.A.; Dimopoulos, P.; Pantis, J.D.; Hays, G.C. Behavioural analysis of loggerhead sea turtle Caretta caretta from direct in-water observation. Endang. Species Res. 2006, 2, 71–79. [Google Scholar] [CrossRef]
- Losey, G.S.; Balazs, G.H.; Privitera, L.A. Cleaning Symbiosis between the Wrasse, Thalassoma duperry, and the Green Turtle, Chelonia mydas. Copeia 1994, 3, 684–690. [Google Scholar] [CrossRef]
- Seigel, R.A. Occurrence and Effects of Barnacle Infestations on Diamondback Terrapins (Malaclemys terrapin). Am. Mid. Nat. 1983, 109, 34–39. [Google Scholar] [CrossRef]
- Wells, J.B.J. An Annotated Checklist and Keys to the Species of Copepod a Harpacticoida (Crustacea); Zootaxa Series, 1568; Magnolia Press: Auckland, New Zealand, 2007; pp. 1–872. [Google Scholar]
- Rossel, S.; Martínez Arbizu, P. Revealing higher than expected diversity of Harpacticoida (Crustacea:Copepoda) in the North Sea using MALDI-TOF MS and molecular barcoding. Sci. Rep. 2019, 9, 9182. [Google Scholar] [CrossRef]
- Badillo, F.J.; Puig, L.; Montero, F.E.; Raga, J.A.; Aznar, F.J. Diet of Balaenophilus spp. (Copepoda: Harpacticoida): Feeding on keratin at sea? Mar. Biol. 2007, 151, 751–758. [Google Scholar] [CrossRef]
- Crespo-Picazo, J.L.; García-Parraga, D.; Domènech, F.; Tomás, J.; Aznar, F.J.; Ortega, J.; Corpa, J.M. Parasitic outbreak of the copepod Balaenophilus manatorum in neonate loggerhead sea turtles (Caretta caretta) from a head-starting program. BMC Vet. Res. 2017, 13, 154. [Google Scholar] [CrossRef] [PubMed]
- LafeberVet Web Site, Body Condition Scoring the Sea Turtle. Available online: https://lafeber.com/vet/body-condition-scoring-the-sea-turtle/ (accessed on 8 March 2023).
- Stacy, N.I.; Alleman, A.R.; Sayler, K.A. Diagnostic Hematology of Reptiles. Clin. Lab. Med. 2011, 31, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Stacy, N.I.; Innis, C.J. Clinical Pathology. In Sea Turtle Medicine and Rehabilitation; Manire, C.A., Norton, T.M., Stacy, B.A., Innis, C.J., Harms, C.A., Eds.; J. Ross Publishing: Plantation, FL, USA, 2017; pp. 147–207. [Google Scholar]
- Casal, A.B.; Orós, J. Plasma biochemistry and haematology values in juvenile loggerhead sea turtles undergoing rehabilitation. Vet. Rec. 2009, 164, 663–665. [Google Scholar] [CrossRef]
- Tedesco, P.; Gustinelli, A.; Caffara, M.; Patarnello, P.; Terlizzi, A.; Fioravanti, M.L. Hysterothylacium fabri (Nematoda: Raphidascarididae) in Mullus surmuletus (Perciformes: Mullidae) and Uranoscopus scaber (Perciformes: Uranoscopidae) from the Mediterranean. J. Parasitol. 2018, 104, 262–274. [Google Scholar] [CrossRef]
- Aznar, F.J.; Badillo, F.J.; Mateu, P.; Raga, J.A. Balaenophilus manatorum (Ortíz, Lalana and Torres, 1992) (Copepoda: Harpacticoida) from loggerhead sea turtles, Caretta caretta, from Japan and the western Mediterranean: Amended description and geographical comparison. J. Parasitol. 2010, 96, 299–307. [Google Scholar] [CrossRef]
- Ogawa, K.; Matsuzaki, K.; Misaki, H. A new species of Balaenophilus (Copepoda: Harpacticoida), an ectoparasite of a sea turtle in Japan. Zool. Sci. 1997, 14, 691–699. [Google Scholar] [CrossRef]
- Relini, G. Cirripedi Toracici. Guide per il Risonoscimento delle Specie Animali Acque Lagunari e Costiere Italiane; Consiglio Nazionale delle Recherche: Genova, Italy, 1980; p. 112. [Google Scholar]
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 1997, 83, 75–583. [Google Scholar] [CrossRef]
- Margolis, L.; Esch, G.W.; Holmes, J.C.; Kuris, A.M.; Schad, G.A. The Use of Ecological Terms in Parasitology (Report of an Ad Hoc Committee of the American Society of Parasitologists). J. Parasitol. 1982, 68, 131–133. [Google Scholar] [CrossRef]
- Reiczigel, J.; Marozzi, M.; Fábián, I.; Rózsa, L. Biostatistics for parasitologists—A primer to Quantitative Parasitology. Trends Parasitol. 2019, 35, 277–281. [Google Scholar] [CrossRef]
- Lazo-Wasem, E.A.; Pinou, T.; Peña De Niz, A.; Salgado, M.A.; Schenker, E. New Records of the Marine Turtle Epibiont Balaenophilus umigamecolus (Copepoda: Harpacticoida: Balaenophilidae): New Host Records and Possible Implications for Marine Turtle Health. Bull. Am. Mus. Nat. Hist. 2007, 48, 153–156. [Google Scholar] [CrossRef]
- Badillo, F.J.; Aznar, F.J.; Tomas, J.; Raga, J.A. Epibiont fauna of Caretta caretta in the Spanish Mediterranean. In Proceedings of the First Mediterranean Conference on Marine Turtles; Margaritoulis, D., Demetropoulos, A., Eds.; Barcelona Convention–Bern Convention–Bonn Convention (CMS): Nicosia, Cyprus, 2003; pp. 62–66. [Google Scholar]
- Galluzzo, G.; Cirelli, G.; Ottone, E.; Pisto, A.; Salvemini, P.; Colucci, A.; Costantini, F.; Colangelo, M.A. Analysis of the epibiont communities of the loggerhead sea turtle Caretta caretta recovered along the southern Italian coasts. In Proceedings of the International Workshop on Metrology for the Sea; Learning to Measure Sea Health Parameters (MetroSea), Reggio Calabria, Italy, 4–6 October 2021. [Google Scholar] [CrossRef]
- Scaravelli, D.; Affronte, M.; Costa, F. Analysis of epibiont presence on Caretta caretta from Adriatic sea. In Proceedings of the First Mediterranean Conference on Marine Turtles; Margaritoulis, D., Demetropoulos, A., Eds.; Barcelona Convention–Bern Convention–Bonn Convention (CMS): Nicosia, Cyprus, 2003; pp. 221–225. [Google Scholar]
- Blasi, M.F.; Rotini, A.; Bacci, T.; Targusi, M.; Ferraro, G.B.; Vecchioni, L.; Alduina, R.; Migliore, L. On Caretta caretta’s shell: First spatial analysis of micro- and macro-epibionts on the Mediterranean loggerhead sea turtle carapace. Mar. Biol. Res. 2021, 17, 762–774. [Google Scholar] [CrossRef]
- Casale, P.; D’Addario, M.; Freggi, D.; Argano, R. Barnacles (Cirripedia, Thoracica) and associated epibionts from sea turtles in the central Mediterranean. Crustaceana 2012, 85, 533–549. [Google Scholar] [CrossRef]
- Fuller, W.J.; Broderick, A.C.; Enever, R.; Thorne, P.; Godley, B.J. Motile homes: A comparison of the spatial distribution of epibiont communities on Mediterranean sea turtles. J. Nat. Hist. 2010, 44, 1743–1753. [Google Scholar] [CrossRef]
- Domènech, F.; Badillo, F.J.; Tomás, J.; Raga, J.A.; Aznar, F.J. Epibiont communities of loggerhead marine turtles (Caretta caretta) in the western Mediterranean: Influence of geographic and ecological factors. J. Mar. Biol. Assoc. U. K. 2014, 95, 851–861. [Google Scholar] [CrossRef]
- Karaa, S.; Jribi, I.; Marouani, S.; Jrijer, J.; Bradai, M.N. Preliminary study on parasites in loggerhead turtles (Caretta caretta) from the Southern Tunisian waters. Am. J. Biomed. Sci. Res. 2019, 5, 373–376. [Google Scholar]
- Borgsteede, F.H.M. The effect of parasites on wildlife. Vet. Quart. 1996, 18, 138–140. [Google Scholar] [CrossRef]
- Demas, G.E.; Drazen, D.L.; Nelson, R.J. Reductions in total body fat decrease humoral immunity. Proc. Biol. Sci. 2003, 270, 905–911. [Google Scholar] [CrossRef]
- Chandra, R.K. Nutrition and the immune system from birth to old age. Eur. J. Clin. Nutr. 2002, 56, S73–S76. [Google Scholar] [CrossRef]
- Frick, M.G.; Williams, K.L.; Veljacic, D.; Jackson, J.A.; Knight, S.E. Epibiont community succession on nesting loggerhead sea turtles, Caretta caretta, from Georgia, USA. In Proceedings of the 20th Annual Symposium on Sea Turtle Biology and Conservation, Miami, FL, USA, 29 February–4 March 2000; NOAA Technical Memorandum NMFS-SEFSC 2002. pp. 280–282. [Google Scholar]
- Frick, M.G.; Pfaller, J.B. Sea turtle epibiosis. In The Biology of Sea Turtles; Wyneken, J., Lohmann, K.J., Musick, J.A., Eds.; CRC Press: Boca Raton, FL, USA, 2013; Volume 3, pp. 399–426. [Google Scholar]
- Frick, M.G.; Zardus, J.D.; Lazo-Wasem, E.A. A New Coronuloid Barnacle Subfamily, Genus and Species from Cheloniid Sea Turtles. Bull. Peabody Mus. Nat. Hist. 2010, 51, 169–177. [Google Scholar] [CrossRef]
- Pfaller, J.; Bjorndal, K.; Reich, K.; Williams, K.; Frick, M. Distribution patterns of epibionts on the carapace of loggerhead turtles, Caretta caretta. Mar. Biodiv. Rec. 2008, 1, E36. [Google Scholar] [CrossRef]
- Ten, S.; Pascual, L.; Pérez-Gabaldón, M.I.; Tomás, J.; Domènech, F.; Aznar, F.J. Epibiotic barnacles of sea turtles as indicators of habitat use and fishery interactions: An analysis of juvenile loggerhead sea turtles, Caretta caretta, in the western Mediterranean. Ecol. Indic. 2019, 107, 105672. [Google Scholar] [CrossRef]
- Stacy, B.A.; Werneck, M.R.; Walden, H.S.; Harms, C.A. Parasitology. In Sea Turtle Medicine and Rehabilitation; Manire, C.A., Norton, T.M., Stacy, B.A., Innis, C.J., Harms, C.A., Eds.; J. Ross Publishing: Plantation, FL, USA, 2017; pp. 727–750. [Google Scholar]
- Bugoni, L.; Krause, L.; Almeida, A.O.; Bueno-A, A.P. Commensal barnacles of sea turtles in Brazil. MTN 2001, 94, 7–9. [Google Scholar]
- Novarini, N.; Mizzan, L.; Basso, R.; Perlasca, P.; Richard, J.; Gelli, D.; Poppi, L.; Verza, E.; Boschetti, E.; Vianello, C. Segnalazioni di tartarughe marine in laguna di venezia e lungo le coste venete—Anno 2009, (Reptilia, testudines). Boll. Mus. St. Nat. Venezia 2010, 61, 59–81. [Google Scholar]
- Vallini, C.; Rubini, S.; Tarricone, L.; Mazziotti, C.; Gaspari, S. Unusual stranding of live, small, debilitated loggerhead turtles along the northwestern Adriatic coast. MTN 2011, 131, 25–28. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).