Effects of Black Soldier Fly Larvae (Hermetia illucens Larvae) Meal on the Production Performance and Cecal Microbiota of Hens
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. HILM
2.2. Laying Hens, Diets, and Experimental Design
2.3. Production Performance
2.4. Cecal Microbiome
2.4.1. Total Genomic DNA Extraction
2.4.2. Quantify DNA Concentration
2.4.3. PCR Amplification
2.4.4. Illumina MiSeq Sequencing
2.4.5. Processing of Sequencing Data
2.5. Statistical Analysis
3. Results
3.1. Production Performance
3.2. Cecal Microbiota
3.2.1. Rarefaction Curves
3.2.2. Alpha Diversity Analysis
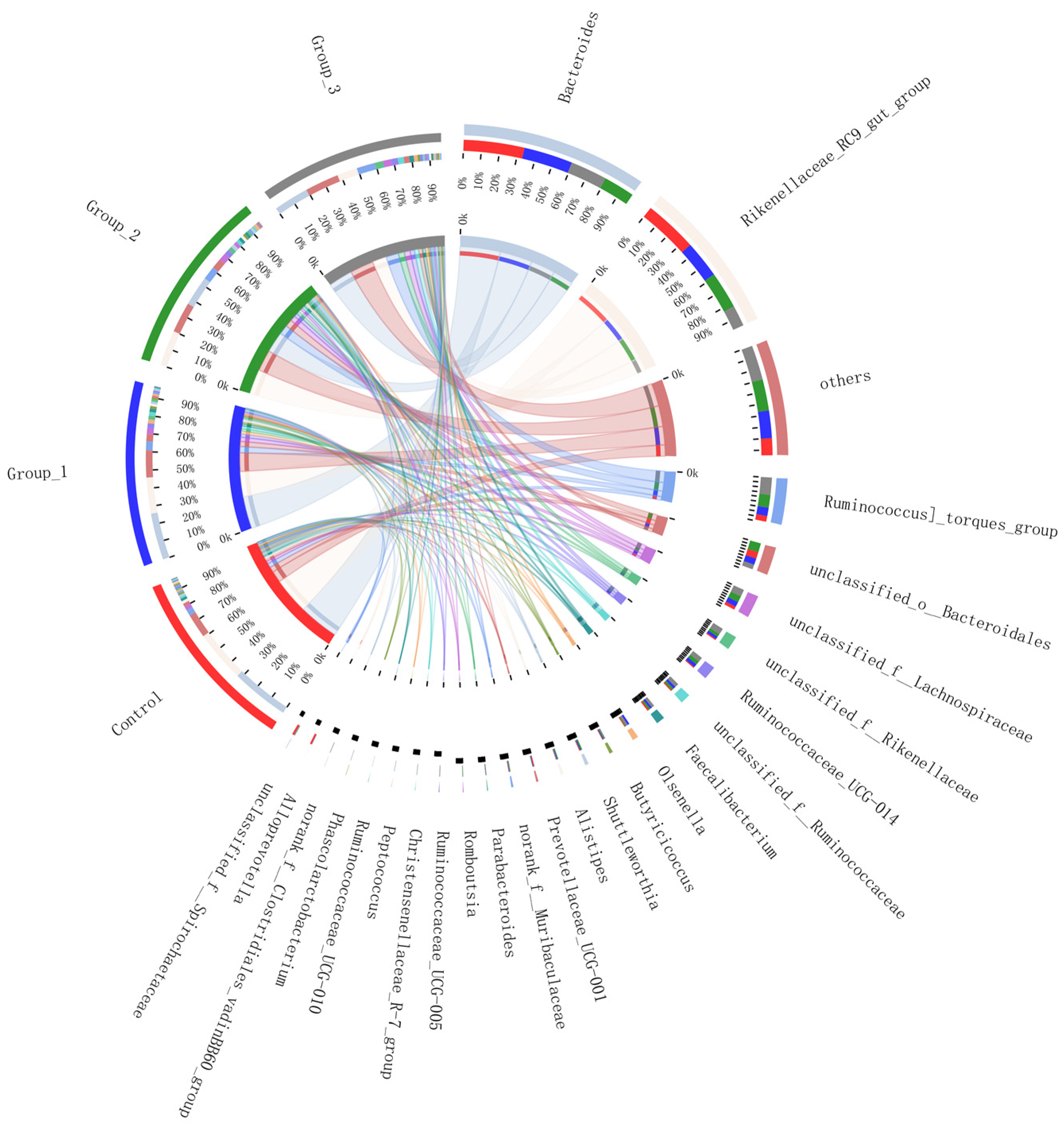
3.2.3. Circos Samples and Species Relationship Map
3.2.4. Beta Diversity Analysis
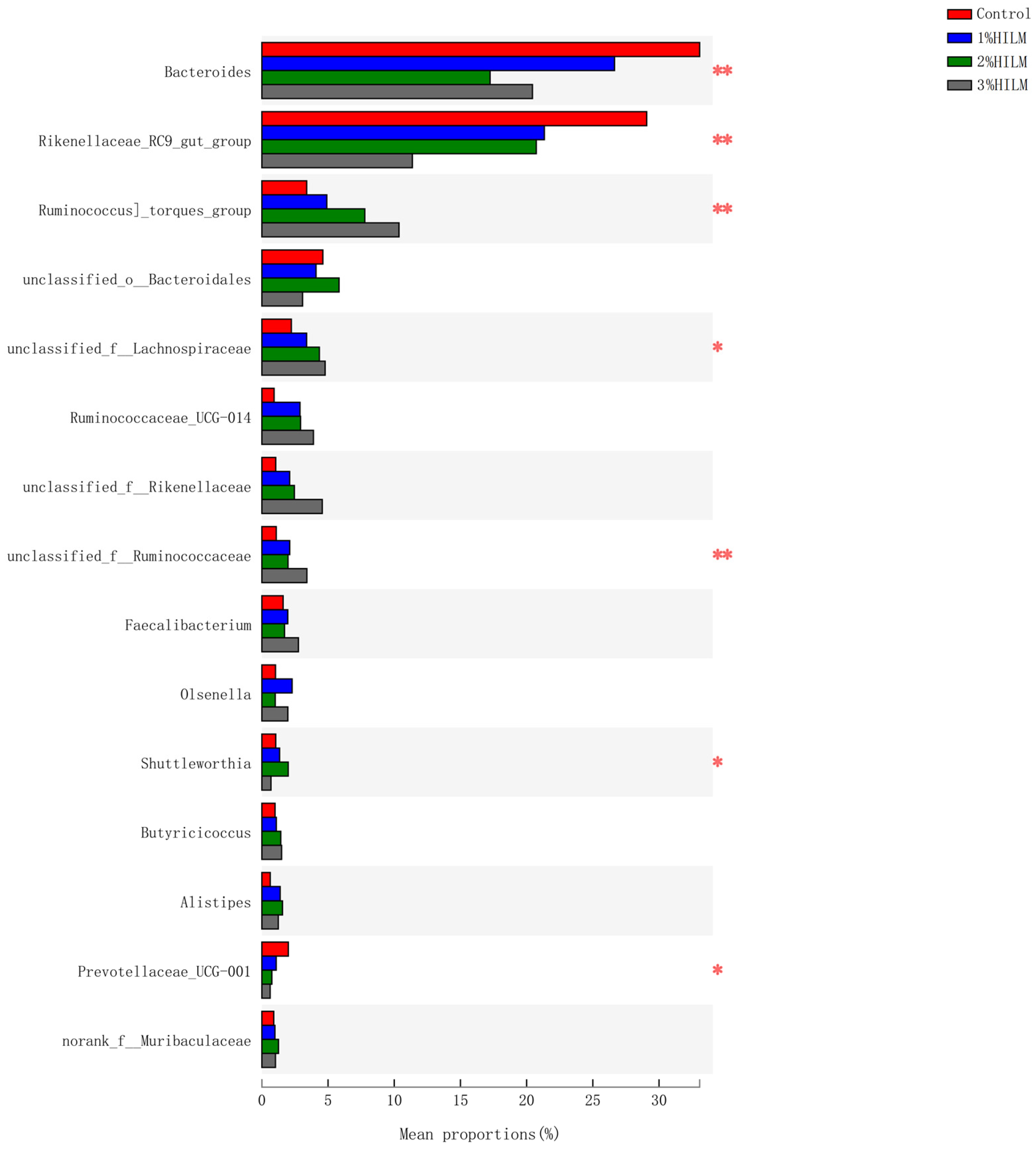
3.2.5. Species Difference Analysis
4. Discussion
4.1. Effect of Hermetia illucens Larvae Meal on the Production Performance of Laying Hens
4.2. Effect of Hermetia illucens Larvae Meal on the Cecal Microflora of Laying Hens
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rumpold, B.A.; Schlüter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef] [PubMed]
- Van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security; FAO: Rome, Italy, 2013. [Google Scholar]
- James, M.T. The genus Hermetia in the United States (Diptera: Stratiomyidae). Bull. Brooklyn Entomol. Soc. 1935, 30, 165–170. [Google Scholar]
- Lu, S.; Taethaisong, N.; Meethip, W.; Surakhunthod, J.; Sinpru, B.; Sroichak, T.; Archa, P.; Thongpea, S.; Paengkoum, S.; Purba, R.A.; et al. Nutritional Composition of Black Soldier Fly Larvae (Hermetia illucens L.) and Its Potential Uses as Alternative Protein Sources in Animal Diets: A Review. Insects 2022, 13, 831. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Wu, L.; Li, B.; Zhang, D. Reproductive potential and nutritional composition of Hermetia illucens (Diptera: Stratiomyidae) prepupae reared on different organic wastes. J. Econ. Entomol. 2020, 113, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Hou, Y.; Li, W.; Yang, S.; Li, Q.; Yu, Z. Biodiesel production from rice straw and restaurant waste employing black soldier fly assisted by microbes. Energy 2012, 47, 225–229. [Google Scholar] [CrossRef]
- Lopes, I.G.; Lalander, C.; Vidotti, R.M.; Vinnerås, B. Using Hermetia illucens larvae to process biowaste from aquaculture production. J. Clean. Prod. 2020, 251, 119753. [Google Scholar] [CrossRef]
- Truzzi, C.; Giorgini, E.; Annibaldi, A.; Antonucci, M.; Illuminati, S.; Scarponi, G.; Riolo, P.; Isidoro, N.; Conti, C.; Zarantoniello, M. Fatty acids profile of black soldier fly (Hermetia illucens): Influence of feeding substrate based on coffee-waste silverskin enriched with microalgae. Anim. Feed Sci. Technol. 2020, 259, 114309. [Google Scholar] [CrossRef]
- Spranghers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Deboosere, S.; De Meulenaer, B.; Michiels, J.; Eeckhout, M.; De Clercq, P.; De Smet, S. Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substrates. J. Sci. Food Agric. 2017, 97, 2594–2600. [Google Scholar] [CrossRef] [PubMed]
- Barragan-Fonseca, K.B.; Dicke, M.; van Loon, J.J. Nutritional value of the black soldier fly (Hermetia illucens L.) and its suitability as animal feed—A review. J. Insects Food Feed 2017, 3, 105–120. [Google Scholar] [CrossRef]
- Kawasaki, K.; Hashimoto, Y.; Hori, A.; Kawasaki, T.; Hirayasu, H.; Iwase, S.-i.; Hashizume, A.; Ido, A.; Miura, C.; Miura, T. Evaluation of black soldier fly (Hermetia illucens) larvae and pre-pupae raised on household organic waste, as potential ingredients for poultry feed. Animals 2019, 9, 98. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Shelomi, M. Review of Black Soldier Fly (Hermetia illucens) as Animal Feed and Human Food. Foods 2017, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Mohan, K.; Sathishkumar, P.; Rajan, D.K.; Rajarajeswaran, J.; Ganesan, A.R. Black soldier fly (Hermetia illucens) larvae as potential feedstock for the biodiesel production: Recent advances and challenges. Sci. Total Environ. 2023, 859, 160235. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Tawwab, M.; Khalil, R.H.; Metwally, A.A.; Shakweer, M.S.; Khallaf, M.A.; Abdel-Latif, H.M. Effects of black soldier fly (Hermetia illucens L.) larvae meal on growth performance, organs-somatic indices, body composition, and hemato-biochemical variables of European sea bass, Dicentrarchus labrax. Aquaculture 2020, 522, 735136. [Google Scholar] [CrossRef]
- Li, Y.; Kortner, T.M.; Chikwati, E.M.; Belghit, I.; Lock, E.-J.; Krogdahl, Å. Total replacement of fish meal with black soldier fly (Hermetia illucens) larvae meal does not compromise the gut health of Atlantic salmon (Salmo salar). Aquaculture 2020, 520, 734967. [Google Scholar] [CrossRef]
- Cullere, M.; Tasoniero, G.; Giaccone, V.; Acuti, G.; Marangon, A.; Dalle Zotte, A. Black soldier fly as dietary protein source for broiler quails: Meat proximate composition, fatty acid and amino acid profile, oxidative status and sensory traits. Animal 2018, 12, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Cullere, M.; Tasoniero, G.; Giaccone, V.; Miotti-Scapin, R.; Claeys, E.; De Smet, S.; Dalle Zotte, A. Black soldier fly as dietary protein source for broiler quails: Apparent digestibility, excreta microbial load, feed choice, performance, carcass and meat traits. Animal 2016, 10, 1923–1930. [Google Scholar] [CrossRef] [PubMed]
- Dalle Zotte, A.; Singh, Y.; Michiels, J.; Cullere, M. Black soldier fly (Hermetia illucens) as dietary source for laying quails: Live performance, and egg physico-chemical quality, sensory profile and storage stability. Animals 2019, 9, 115. [Google Scholar] [CrossRef]
- Loponte, R.; Nizza, S.; Bovera, F.; De Riu, N.; Fliegerova, K.; Lombardi, P.; Vassalotti, G.; Mastellone, V.; Nizza, A.; Moniello, G. Growth performance, blood profiles and carcass traits of Barbary partridge (Alectoris barbara) fed two different insect larvae meals (Tenebrio molitor and Hermetia illucens). Res. Vet. Sci. 2017, 115, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Secci, G.; Moniello, G.; Gasco, L.; Bovera, F.; Parisi, G. Barbary partridge meat quality as affected by Hermetia illucens and Tenebrio molitor larva meals in feeds. Food Res. Int. 2018, 112, 291–298. [Google Scholar] [CrossRef]
- Gariglio, M.; Dabbou, S.; Biasato, I.; Capucchio, M.T.; Colombino, E.; Hernández, F.; Madrid, J.; Martínez, S.; Gai, F.; Caimi, C. Nutritional effects of the dietary inclusion of partially defatted Hermetia illucens larva meal in Muscovy duck. J. Anim. Sci. Biotechnol. 2019, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Gariglio, M.; Dabbou, S.; Crispo, M.; Biasato, I.; Gai, F.; Gasco, L.; Piacente, F.; Odetti, P.; Bergagna, S.; Plachà, I. Effects of the dietary inclusion of partially defatted black soldier fly (Hermetia illucens) meal on the blood chemistry and tissue (Spleen, Liver, Thymus, and Bursa of Fabricius) histology of muscovy ducks (Cairina moschata domestica). Animals 2019, 9, 307. [Google Scholar] [CrossRef]
- Pieterse, E.; Erasmus, S.W.; Uushona, T.; Hoffman, L.C. Black soldier fly (Hermetia illucens) pre-pupae meal as a dietary protein source for broiler production ensures a tasty chicken with standard meat quality for every pot. J. Sci. Food Agric. 2019, 99, 893–903. [Google Scholar] [CrossRef]
- Schiavone, A.; Dabbou, S.; De Marco, M.; Cullere, M.; Biasato, I.; Biasibetti, E.; Capucchio, M.; Bergagna, S.; Dezzutto, D.; Meneguz, M. Black soldier fly larva fat inclusion in finisher broiler chicken diet as an alternative fat source. Animal 2018, 12, 2032–2039. [Google Scholar] [CrossRef] [PubMed]
- Schiavone, A.; Dabbou, S.; Petracci, M.; Zampiga, M.; Sirri, F.; Biasato, I.; Gai, F.; Gasco, L. Black soldier fly defatted meal as a dietary protein source for broiler chickens: Effects on carcass traits, breast meat quality and safety. Animal 2019, 13, 2397–2405. [Google Scholar] [CrossRef] [PubMed]
- Sypniewski, J.; Kierończyk, B.; Benzertiha, A.; Mikołajczak, Z.; Pruszyńska-Oszmałek, E.; Kołodziejski, P.; Sassek, M.; Rawski, M.; Czekała, W.; Józefiak, D. Replacement of soybean oil by Hermetia illucens fat in turkey nutrition: Effect on performance, digestibility, microbial community, immune and physiological status and final product quality. Br. Poult. Sci. 2020, 61, 294–302. [Google Scholar] [CrossRef]
- Al-Qazzaz, M.F.A.; Ismail, D.; Akit, H.; Idris, L.H. Effect of using insect larvae meal as a complete protein source on quality and productivity characteristics of laying hens. Rev. Bras. Zootec. 2016, 45, 518–523. [Google Scholar] [CrossRef]
- Marono, S.; Loponte, R.; Lombardi, P.; Vassalotti, G.; Pero, M.; Russo, F.; Gasco, L.; Parisi, G.; Piccolo, G.; Nizza, S. Productive performance and blood profiles of laying hens fed Hermetia illucens larvae meal as total replacement of soybean meal from 24 to 45 weeks of age. Poult. Sci. 2017, 96, 1783–1790. [Google Scholar] [CrossRef]
- Moniello, G.; Ariano, A.; Panettieri, V.; Tulli, F.; Olivotto, I.; Messina, M.; Randazzo, B.; Severino, L.; Piccolo, G.; Musco, N. Intestinal morphometry, enzymatic and microbial activity in laying hens fed different levels of a Hermetia illucens larvae meal and toxic elements content of the insect meal and diets. Animals 2019, 9, 86. [Google Scholar] [CrossRef] [PubMed]
- Secci, G.; Bovera, F.; Nizza, S.; Baronti, N.; Gasco, L.; Conte, G.; Serra, A.; Bonelli, A.; Parisi, G. Quality of eggs from Lohmann Brown Classic laying hens fed black soldier fly meal as substitute for soya bean. Animal 2018, 12, 2191–2197. [Google Scholar] [CrossRef] [PubMed]
- Star, L.; Arsiwalla, T.; Molist, F.; Leushuis, R.; Dalim, M.; Paul, A. Gradual provision of live Black soldier fly (Hermetia illucens) larvae to older laying hens: Effect on production performance, egg quality, feather condition and behavior. Animals 2020, 10, 216. [Google Scholar] [CrossRef]
- Biasato, I.; Renna, M.; Gai, F.; Dabbou, S.; Meneguz, M.; Perona, G.; Martinez, S.; Lajusticia, A.C.B.; Bergagna, S.; Sardi, L. Partially defatted black soldier fly larva meal inclusion in piglet diets: Effects on the growth performance, nutrient digestibility, blood profile, gut morphology and histological features. J. Anim. Sci. Biotechnol. 2019, 10, 12. [Google Scholar] [CrossRef]
- Yu, M.; Li, Z.; Chen, W.; Rong, T.; Wang, G.; Li, J.; Ma, X. Use of Hermetia illucens larvae as a dietary protein source: Effects on growth performance, carcass traits, and meat quality in finishing pigs. Meat Sci. 2019, 158, 107837. [Google Scholar] [CrossRef]
- Yu, M.; Li, Z.; Chen, W.; Rong, T.; Wang, G.; Ma, X. Hermetia illucens larvae as a potential dietary protein source altered the microbiota and modulated mucosal immune status in the colon of finishing pigs. J. Anim. Sci. Biotechnol. 2019, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Li, Z.; Chen, W.; Rong, T.; Wang, G.; Wang, F.; Ma, X. Evaluation of full-fat Hermetia illucens larvae meal as a fishmeal replacement for weanling piglets: Effects on the growth performance, apparent nutrient digestibility, blood parameters and gut morphology. Anim. Feed Sci. Technol. 2020, 264, 114431. [Google Scholar] [CrossRef]
- Dalle Zotte, A.; Cullere, M.; Martins, C.; Alves, S.P.; Freire, J.P.; Falcão-e-Cunha, L.; Bessa, R.J. Incorporation of Black Soldier Fly (Hermetia illucens L.) larvae fat or extruded linseed in diets of growing rabbits and their effects on meat quality traits including detailed fatty acid composition. Meat Sci. 2018, 146, 50–58. [Google Scholar] [CrossRef]
- Martins, C.; Cullere, M.; Dalle Zotte, A.; Cardoso, C.; Alves, S.P.; Bessa, R.; Freire, J.P.B.; Falcao-e-Cunha, L. Incorporation of two levels of black soldier fly (Hermetia illucens L.) larvae fat or extruded linseed in diets of growing rabbits: Effects on growth performance and diet digestibility. Czech. J. Anim. Sci. 2018, 63, 356–362. [Google Scholar] [CrossRef]
- Maurer, V.; Holinger, M.; Amsler, Z.; Früh, B.; Wohlfahrt, J.; Stamer, A.; Leiber, F. Replacement of soybean cake by Hermetia illucens meal in diets for layers. J. Insects Food Feed 2016, 2, 83–90. [Google Scholar] [CrossRef]
- Liu, X.; Liu, X.; Yao, Y.; Qu, X.; Chen, J.; Xie, K.; Wang, X.; Qi, Y.; Xiao, B.; He, C. Effects of different levels of Hermetia illucens larvae meal on performance, egg quality, yolk fatty acid composition and oxidative status of laying hens. Ital. J. Anim. Sci 2021, 20, 256–266. [Google Scholar] [CrossRef]
- Lee, J.-A.; Kim, Y.-M.; Park, Y.-K.; Yang, Y.-C.; Jung, B.-G.; Lee, B.-J. Black soldier fly (Hermetia illucens) larvae enhances immune activities and increases survivability of broiler chicks against experimental infection of Salmonella Gallinarum. J. Vet. Med. Sci. 2018, 80, 736–740. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jin, J.; Hou, F.; Song, B.; Li, Z.; Zhao, Y. Effects of black soldier fly larvae oil on growth performance, immunity and antioxidant capacity, and intestinal function and microbiota of broilers. J. Appl. Poult. Res. 2022, 31, 100292. [Google Scholar] [CrossRef]
- Tang, Q.; Xu, E.; Wang, Z.; Xiao, M.; Cao, S.; Hu, S.; Wu, Q.; Xiong, Y.; Jiang, Z.; Wang, F.; et al. Dietary Hermetia illucens Larvae Meal Improves Growth Performance and Intestinal Barrier Function of Weaned Pigs Under the Environment of Enterotoxigenic Escherichia coli K88. Front. Nutr. 2022, 8, 812011. [Google Scholar] [CrossRef] [PubMed]
- Colombino, E.; Biasato, I.; Ferrocino, I.; Bellezza Oddon, S.; Caimi, C.; Gariglio, M.; Dabbou, S.; Caramori, M.; Battisti, E.; Zanet, S.; et al. Effect of Insect Live Larvae as Environmental Enrichment on Poultry Gut Health: Gut Mucin Composition, Microbiota and Local Immune Response Evaluation. Animals 2021, 11, 2819. [Google Scholar] [CrossRef] [PubMed]
- Huyben, D.; Vidaković, A.; Hallgren, S.W.; Langeland, M. High-throughput sequencing of gut microbiota in rainbow trout (Oncorhynchus mykiss) fed larval and pre-pupae stages of black soldier fly (Hermetia illucens). Aquaculture 2019, 500, 485–491. [Google Scholar] [CrossRef]
- Rinttilä, T.; Apajalahti, J. Intestinal microbiota and metabolites—Implications for broiler chicken health and performance1 1Presented as a part of the Informal Nutrition Symposium “Metabolic Responses to Nutrition and Modifiers” at the Poultry Science Association’s annual meeting in Athens, Georgia, July 9, 2012. J. Appl. Poult. Res. 2013, 22, 647–658. [Google Scholar] [CrossRef]
- Torok Valeria, A.; Ophel-Keller, K.; Loo, M.; Hughes Robert, J. Application of Methods for Identifying Broiler Chicken Gut Bacterial Species Linked with Increased Energy Metabolism. Appl. Environ. Microbiol. 2008, 74, 783–791. [Google Scholar] [CrossRef]
- Charlton, A.; Dickinson, M.; Wakefield, M.; Fitches, E.; Kenis, M.; Han, R.; Zhu, F.; Kone, N.; Grant, M.; Devic, E. Exploring the chemical safety of fly larvae as a source of protein for animal feed. J. Insects Food Feed 2015, 1, 7–16. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Poultry: 1994; National Academies Press: Washington, DC, USA, 1994. [Google Scholar]
- Bovera, F.; Loponte, R.; Pero, M.E.; Cutrignelli, M.I.; Calabrò, S.; Musco, N.; Vassalotti, G.; Panettieri, V.; Lombardi, P.; Piccolo, G. Laying performance, blood profiles, nutrient digestibility and inner organs traits of hens fed an insect meal from Hermetia illucens larvae. Res. Vet. Sci. 2018, 120, 86–93. [Google Scholar] [CrossRef]
- Widjastuti, T.; Wiradimadja, R.; Rusmana, D. The Effect of Substitution of Fish Meal by Black Soldier Fly (Hermetia illucens) Maggot Meal in the Diet on Production Performance of Quail (Coturnix coturnix japonica); Animal Science—The International Session of Scientific Communications of the Faculty of Animal Science: Padjadjaran, Indonesia, 2014. [Google Scholar]
- Scott, K.P.; Gratz, S.W.; Sheridan, P.O.; Flint, H.J.; Duncan, S.H. The influence of diet on the gut microbiota. Pharmacol. Res. 2013, 69, 52–60. [Google Scholar] [CrossRef]
- Walugembe, M.; Hsieh, J.C.; Koszewski, N.J.; Lamont, S.J.; Persia, M.E.; Rothschild, M.F. Effects of dietary fiber on cecal short-chain fatty acid and cecal microbiota of broiler and laying-hen chicks. Poult. Sci. 2015, 94, 2351–2359. [Google Scholar] [CrossRef]
- Borrelli, L.; Coretti, L.; Dipineto, L.; Bovera, F.; Menna, F.; Chiariotti, L.; Nizza, A.; Lembo, F.; Fioretti, A. Insect-based diet, a promising nutritional source, modulates gut microbiota composition and SCFAs production in laying hens. Sci. Rep. 2017, 7, 16269. [Google Scholar] [CrossRef]
- Spranghers, T.; Michiels, J.; Vrancx, J.; Ovyn, A.; Eeckhout, M.; De Clercq, P.; De Smet, S. Gut antimicrobial effects and nutritional value of black soldier fly (Hermetia illucens L.) prepupae for weaned piglets. Anim. Feed Sci. Technol. 2018, 235, 33–42. [Google Scholar] [CrossRef]
- Mead, G. Microbes of the avian cecum: Types present and substrates utilized. J. Exp. Zool. 1989, 252, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Sergeant, M.J.; Constantinidou, C.; Cogan, T.A.; Bedford, M.R.; Penn, C.W.; Pallen, M.J. Extensive microbial and functional diversity within the chicken cecal microbiome. PLoS ONE 2014, 9, e91941. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.Y.; Zhong, T.; Pandya, Y.; Joerger, R.D. 16S rRNA-based analysis of microbiota from the cecum of broiler chickens. Appl. Environ. Microbiol. 2002, 68, 124–137. [Google Scholar] [CrossRef]
- Yan, W.; Sun, C.; Yuan, J.; Yang, N. Gut metagenomic analysis reveals prominent roles of Lactobacillus and cecal microbiota in chicken feed efficiency. Sci. Rep. 2017, 7, 45308. [Google Scholar] [CrossRef] [PubMed]
- Callaway, T.R.; Dowd, S.E.; Wolcott, R.D.; Sun, Y.; McReynolds, J.L.; Edrington, T.S.; Byrd, J.A.; Anderson, R.C.; Krueger, N.; Nisbet, D.J. Evaluation of the bacterial diversity in cecal contents of laying hens fed various molting diets by using bacterial tag-encoded FLX amplicon pyrosequencing. Poult. Sci. 2009, 88, 298–302. [Google Scholar] [CrossRef]
- Lumpkins, B.; Batal, A.; Lee, M. Evaluation of the bacterial community and intestinal development of different genetic lines of chickens. Poult. Sci. 2010, 89, 1614–1621. [Google Scholar] [CrossRef]
- Hume, M.; Kubena, L.; Edrington, T.; Donskey, C.; Moore, R.; Ricke, S.; Nisbet, D. Poultry digestive microflora biodiversity as indicated by denaturing gradient gel electrophoresis. Poult. Sci. 2003, 82, 1100–1107. [Google Scholar] [CrossRef]
- Videnska, P.; Sedlar, K.; Lukac, M.; Faldynova, M.; Gerzova, L.; Cejkova, D.; Sisak, F.; Rychlik, I. Succession and replacement of bacterial populations in the caecum of egg laying hens over their whole life. PLoS ONE 2014, 9, e115142. [Google Scholar] [CrossRef]
- Polansky, O.; Sekelova, Z.; Faldynova, M.; Sebkova, A.; Sisak, F.; Rychlik, I. Important metabolic pathways and biological processes expressed by chicken cecal microbiota. Appl. Environ. Microbiol. 2016, 82, 1569–1576. [Google Scholar] [CrossRef]
- Van der Wielen, P.W.; Biesterveld, S.; Notermans, S.; Hofstra, H.; Urlings, B.A.; van Knapen, F. Role of volatile fatty acids in development of the cecal microflora in broiler chickens during growth. Appl. Environ. Microbiol. 2000, 66, 2536–2540. [Google Scholar] [CrossRef] [PubMed]
- Van Immerseel, F.; Boyen, F.; Gantois, I.; Timbermont, L.; Bohez, L.; Pasmans, F.; Haesebrouck, F.; Ducatelle, R. Supplementation of coated butyric acid in the feed reduces colonization and shedding of Salmonella in poultry. Poult. Sci. 2005, 84, 1851–1856. [Google Scholar] [CrossRef] [PubMed]
- Apajalahti, J. Comparative Gut Microflora, Metabolic Challenges, and Potential Opportunities. J. Appl. Poult. Res. 2005, 14, 444–453. [Google Scholar] [CrossRef]
- Kien, C.L.; Blauwiekel, R.; Bunn, J.Y.; Jetton, T.L.; Frankel, W.L.; Holst, J.J. Cecal infusion of butyrate increases intestinal cell proliferation in piglets. J. Nutr. 2007, 137, 916–922. [Google Scholar] [CrossRef]
- Liu, J.; Bayir, H.; Cosby, D.; Cox, N.; Williams, S.; Fowler, J. Evaluation of encapsulated sodium butyrate on growth performance, energy digestibility, gut development, and Salmonella colonization in broilers. Poult. Sci. 2017, 96, 3638–3644. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Belenguer, A.; Holtrop, G.; Johnstone, A.M.; Flint, H.J.; Lobley, G.E. Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate-producing bacteria in feces. Appl. Environ. Microbiol. 2007, 73, 1073–1078. [Google Scholar] [CrossRef]
- Cutrignelli, M.I.; Messina, M.; Tulli, F.; Randazzo, B.; Olivotto, I.; Gasco, L.; Loponte, R.; Bovera, F. Evaluation of an insect meal of the Black Soldier Fly (Hermetia illucens) as soybean substitute: Intestinal morphometry, enzymatic and microbial activity in laying hens. Res. Vet. Sci. 2018, 117, 209–215. [Google Scholar] [CrossRef]
- Lan, Y.; Williams, B.A.; Tamminga, S.; Boer, H.; Akkermans, A.; Erdi, G.; Verstegen, M.W. In vitro fermentation kinetics of some non-digestible carbohydrates by the caecal microbial community of broilers. Anim. Feed Sci. Technol. 2005, 123, 687–702. [Google Scholar] [CrossRef]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Lucey, A.J.; Humphreys, M.; Hogan, A.; Hayes, P.; O’Reilly, M.; Jeffery, I.B.; Wood-Martin, R. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 2014, 63, 1913–1920. [Google Scholar] [CrossRef]
- Waśko, A.; Bulak, P.; Polak-Berecka, M.; Nowak, K.; Polakowski, C.; Bieganowski, A. The first report of the physicochemical structure of chitin isolated from Hermetia illucens. Int. J. Biol. Macromol. 2016, 92, 316–320. [Google Scholar] [CrossRef]
- Terada, A.; Hara, H.; Sato, D.; Higashi, T.; Nakayama, S.; Tsuji, K.; Sakamoto, K.; Ishioka, E.; Maezaki, Y.; Tsugita, T. Effect of dietary chitosan on faecal microbiota and faecal metabolites of humans. Microb. Ecol. Health Dis. 1995, 8, 15–21. [Google Scholar] [CrossRef]
- Neyrinck, A.M.; Possemiers, S.; Verstraete, W.; De Backer, F.; Cani, P.D.; Delzenne, N.M. Dietary modulation of clostridial cluster XIVa gut bacteria (Roseburia spp.) by chitin–glucan fiber improves host metabolic alterations induced by high-fat diet in mice. J. Nutr. Biochem. 2012, 23, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Purkayastha, D.; Sarkar, S. Physicochemical structure analysis of chitin extracted from pupa exuviae and dead imago of Wild Black Soldier Fly (Hermetia illucens). J. Polym. Environ. 2020, 28, 445–457. [Google Scholar] [CrossRef]
- Wang, H.; ur Rehman, K.; Feng, W.; Yang, D.; ur Rehman, R.; Cai, M.; Zhang, J.; Yu, Z.; Zheng, L. Physicochemical structure of chitin in the developing stages of black soldier fly. Int. J. Biol. Macromol. 2020, 149, 901–907. [Google Scholar] [CrossRef]
- Caimi, C.; Renna, M.; Lussiana, C.; Bonaldo, A.; Gariglio, M.; Meneguz, M.; Dabbou, S.; Schiavone, A.; Gai, F.; Elia, A.C. First insights on Black Soldier Fly (Hermetia illucens L.) larvae meal dietary administration in Siberian sturgeon (Acipenser baerii Brandt) juveniles. Aquaculture 2020, 515, 734539. [Google Scholar] [CrossRef]
- Tabata, E.; Kashimura, A.; Wakita, S.; Ohno, M.; Sakaguchi, M.; Sugahara, Y.; Kino, Y.; Matoska, V.; Bauer, P.O.; Oyama, F. Gastric and intestinal proteases resistance of chicken acidic chitinase nominates chitin-containing organisms for alternative whole edible diets for poultry. Sci. Rep. 2017, 7, 6662. [Google Scholar] [CrossRef]
- Kumar, M.N.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Z.; Wang, Y.; Yan, S.; Shi, B. Effects of chitosan as growth promoter on diarrhea, nutrient apparent digestibility, fecal microbiota and immune response in weaned piglets. J. Appl. Anim. Res. 2018, 46, 1437–1442. [Google Scholar] [CrossRef]


| Items | Hermetia illucens Larvae Meal |
|---|---|
| DM | 90.02 |
| CP | 37.60 |
| ME/(MJ/kg) | 8.74 |
| EE | 36.00 |
| crude ash | 6.20 |
| Met | 0.69 |
| Lys | 2.18 |
| Ca | 0.96 |
| P | 0.83 |
| Items | Control | 1% HILM | 2% HILM | 3% HILM |
|---|---|---|---|---|
| Ingredients | ||||
| Corn | 62.00 | 61.63 | 61.25 | 60.88 |
| Soybean meal | 24.00 | 23.37 | 22.75 | 22.12 |
| Wheat bran | 2.00 | 2.00 | 2.00 | 2.00 |
| Limestone | 8.00 | 8.00 | 8.00 | 8.00 |
| Soybean oil | 1.00 | 1.00 | 1.00 | 1.00 |
| Hermetia illucens larva meal | 0.00 | 1.00 | 2.00 | 3.00 |
| Premix * | 3.00 | 3.00 | 3.00 | 3.00 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 |
| Nutrient levels & | ||||
| ME/(MJ/kg) | 11.39 | 11.39 | 11.40 | 11.40 |
| DM | 89.86 | 89.67 | 89.78 | 89.84 |
| CP | 15.80 | 15.81 | 15.85 | 15.89 |
| EE | 3.36 | 3.70 | 4.05 | 4.40 |
| Met | 0.25 | 0.26 | 0.26 | 0.27 |
| Lys | 0.76 | 0.78 | 0.79 | 0.80 |
| Trp | 0.20 | 0.19 | 0.23 | 0.24 |
| Phe | 0.58 | 0.60 | 0.63 | 0.64 |
| Thr | 0.62 | 0.64 | 0.65 | 0.68 |
| Ile | 0.72 | 0.74 | 0.75 | 0.77 |
| Leu | 1.07 | 1.10 | 1.13 | 1.4 |
| Val | 0.62 | 0.62 | 0.63 | 0.65 |
| Na | 0.3 | 0.3 | 0.3 | 0.3 |
| Ca | 3.50 | 3.5 | 3.5 | 3.5 |
| AP | 0.34 | 0.34 | 0.34 | 0.34 |
| TP | 0.50 | 0.5 | 0.5 | 0.5 |
| Items | Control # | 1% HILM # | 2% HILM # | 3% HILM # | SEM * | p-Value | ||
|---|---|---|---|---|---|---|---|---|
| Overall | Linear | Quadratic | ||||||
| Egg laying rate/(%) | 85.36 c | 85.95 bc | 86.52 ab | 87.51 a | 1.02 | 0.018 | 0.037 | 0.54 |
| Feed/egg | 2.22 a | 2.18 ab | 2.13 bc | 2.10 c | 0.08 | 0.041 | 0.032 | 0.306 |
| cracked-egg rate/(%) | 0.68 a | 0.54 ab | 0.46 ab | 0.39 b | 0.79 | 0.024 | 0.041 | 0.114 |
| Average egg weight/(g) | 67.17 | 67.10 | 66.93 | 66.98 | 0.42 | 0.616 | 0.379 | 0.787 |
| ADFI/(g) | 126.73 | 126.79 | 123.67 | 125.41 | 3.68 | 0.531 | 0.756 | 0.306 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.; Zhang, J.; Chen, X.; Wang, Z. Effects of Black Soldier Fly Larvae (Hermetia illucens Larvae) Meal on the Production Performance and Cecal Microbiota of Hens. Vet. Sci. 2023, 10, 364. https://doi.org/10.3390/vetsci10050364
Yan Y, Zhang J, Chen X, Wang Z. Effects of Black Soldier Fly Larvae (Hermetia illucens Larvae) Meal on the Production Performance and Cecal Microbiota of Hens. Veterinary Sciences. 2023; 10(5):364. https://doi.org/10.3390/vetsci10050364
Chicago/Turabian StyleYan, Yan, Jinjin Zhang, Xiaochen Chen, and Zhanbin Wang. 2023. "Effects of Black Soldier Fly Larvae (Hermetia illucens Larvae) Meal on the Production Performance and Cecal Microbiota of Hens" Veterinary Sciences 10, no. 5: 364. https://doi.org/10.3390/vetsci10050364
APA StyleYan, Y., Zhang, J., Chen, X., & Wang, Z. (2023). Effects of Black Soldier Fly Larvae (Hermetia illucens Larvae) Meal on the Production Performance and Cecal Microbiota of Hens. Veterinary Sciences, 10(5), 364. https://doi.org/10.3390/vetsci10050364






