Influenza A Virus Weakens the Immune Response of Mice to Toxoplasma gondii, Thereby Aggravating T. gondii Infection
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Parasite Strain, Viruses, Cell Lines, and Mice
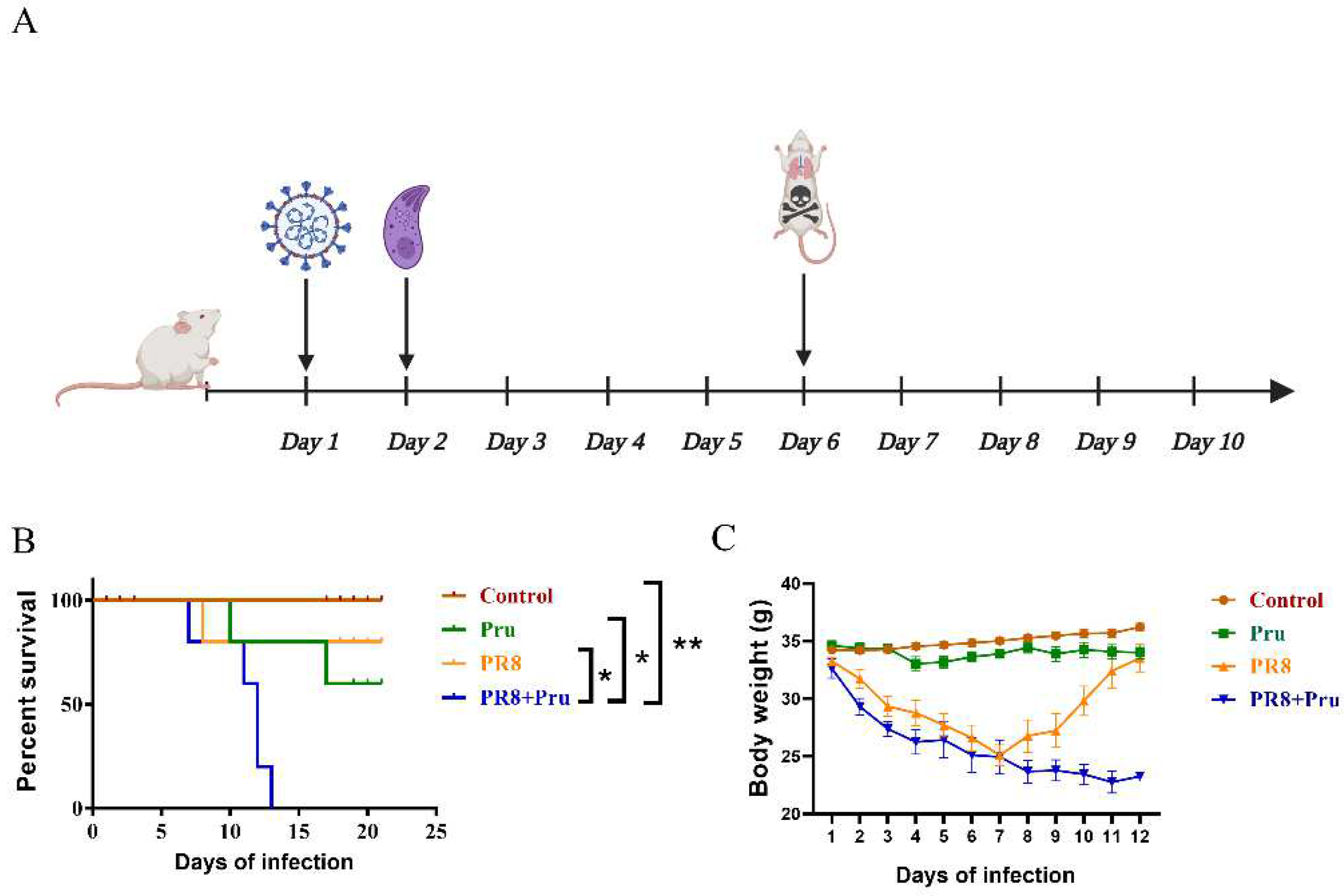
2.2. Animal Experimental Procedures
2.3. Establishment of Standard Curves of Pru and PR8
2.4. Quantitative Real-Time PCR
2.5. Flow Cytometry
2.6. Histological Analysis
2.7. Indirect Immunofluorescent Assay
2.8. Statistical Analysis
3. Results
3.1. PR8 Virus Administration Increases the Pathogenicity of Pru in Infected Mice
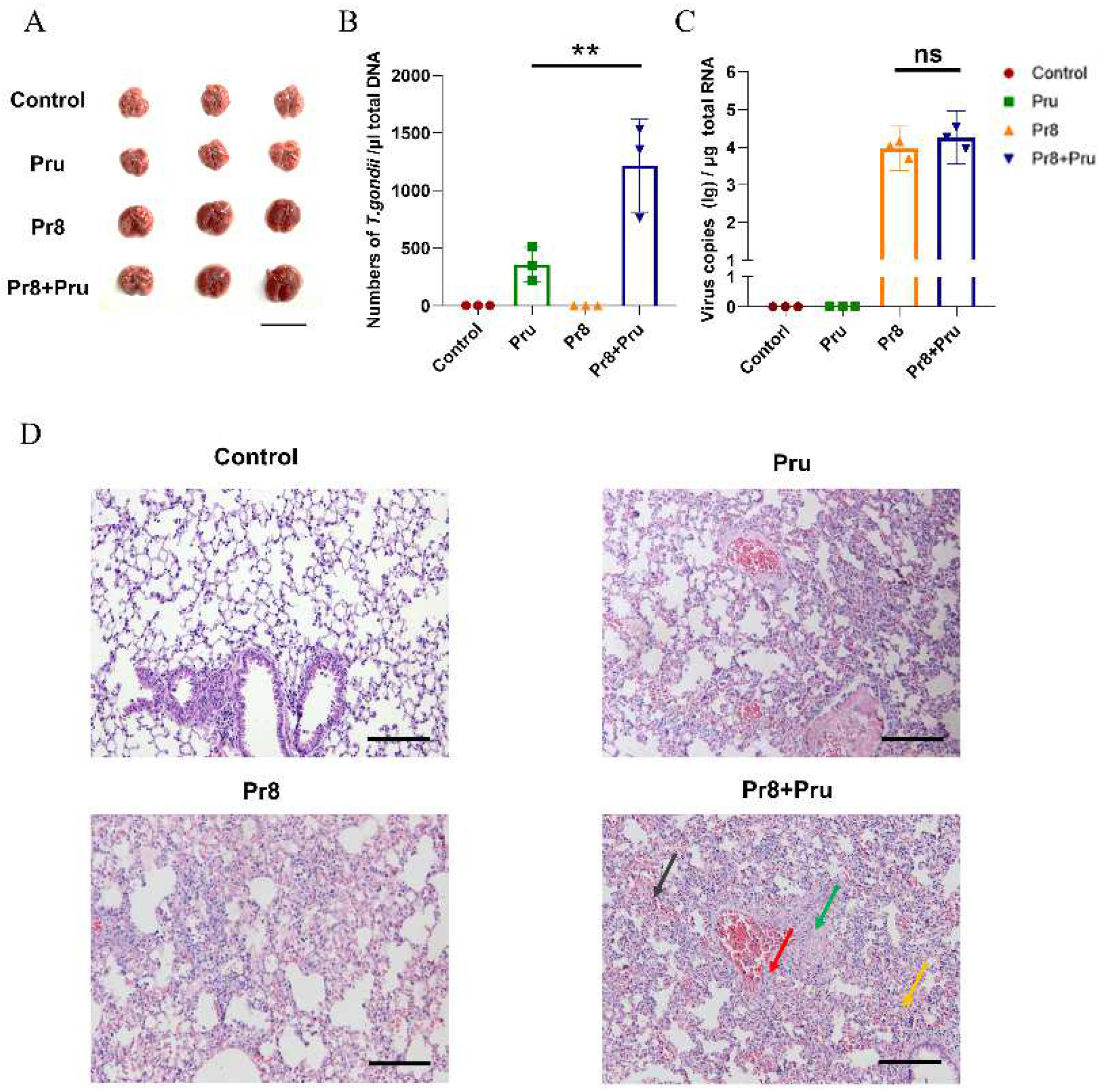
3.2. Co-Infection Promotes T. gondii (Pru) Colonization and Aggravates Lung Pathology
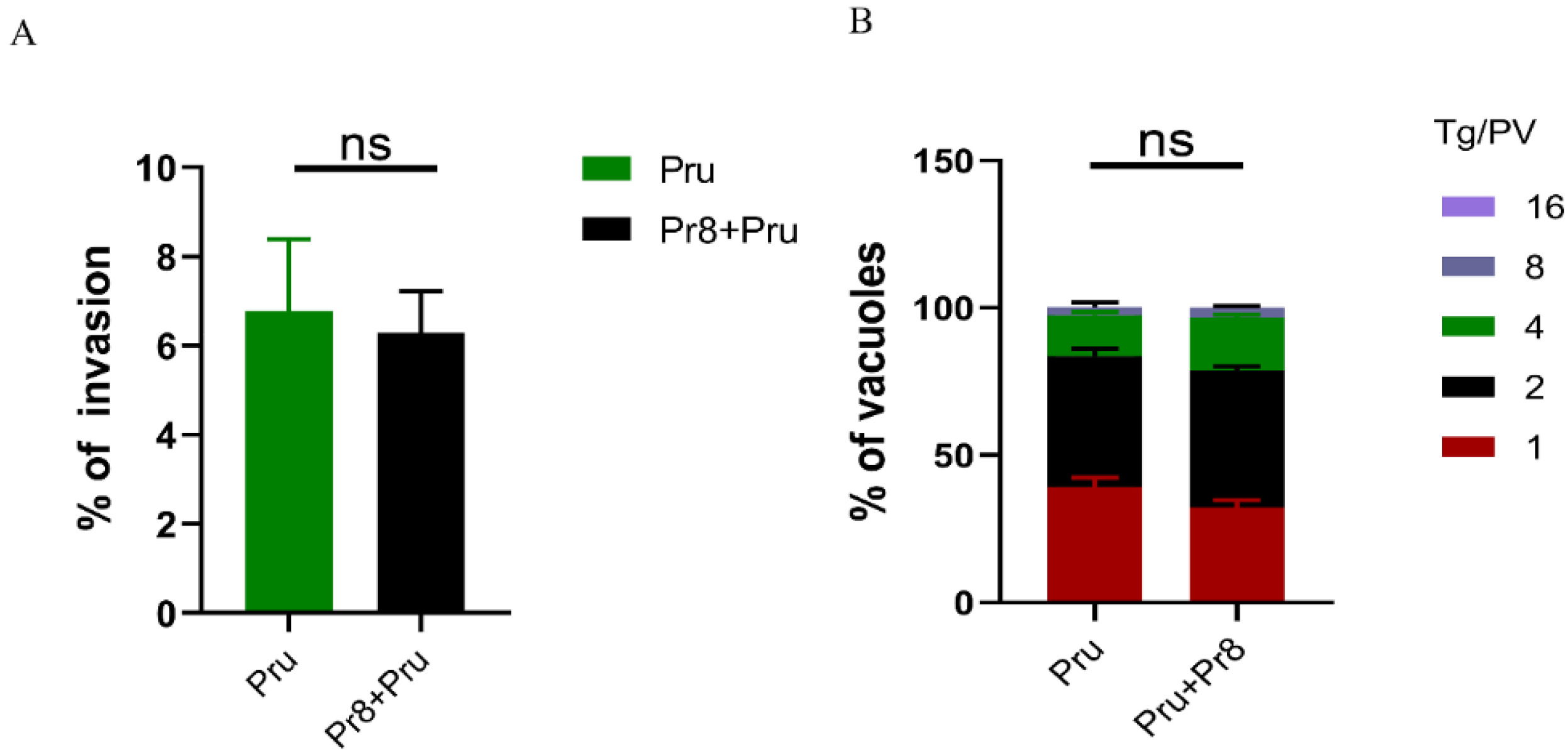
3.3. In Vitro Invasion and Vacuoles of T. gondii (Pru) Showed No Significant Changes
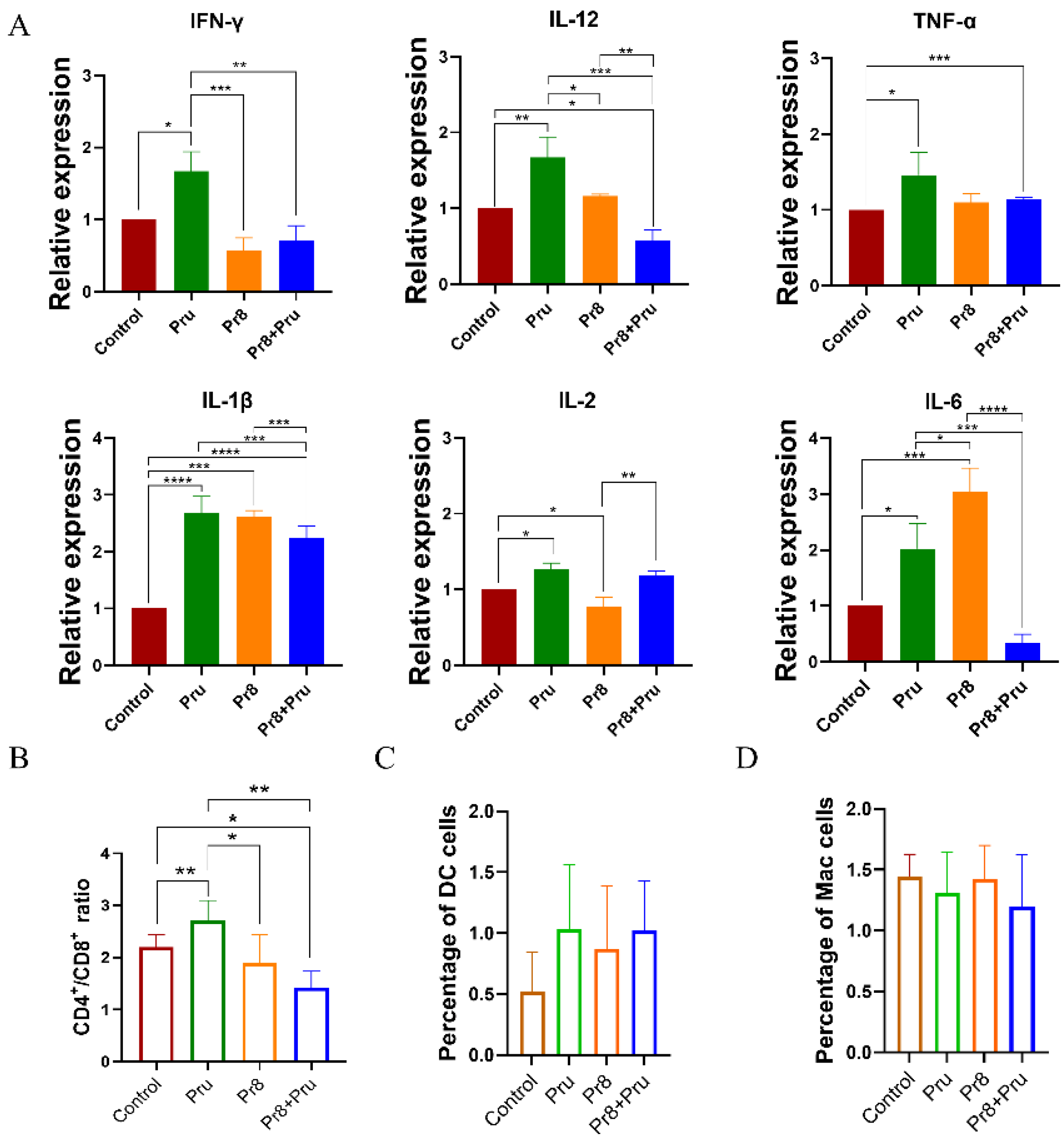
3.4. Co-Infected Mice Showed Abnormal Expression of Cytokines and Abnormal Lymphocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, K.H.; Lal, S.K. Alternative antiviral approaches to combat influenza A virus. Virus Genes 2022, 59, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Buonocore, S.M.; van der Most, R.G. Narcolepsy and H1N1 influenza immunology a decade later: What have we learned? Front. Immunol. 2022, 13, 902840. [Google Scholar] [CrossRef] [PubMed]
- Dunning, J.; Thwaites, R.S.; Openshaw, P.J.M. Seasonal and pandemic influenza: 100 years of progress, still much to learn. Mucosal Immunol. 2020, 13, 566–573. [Google Scholar] [CrossRef]
- Unwin, R.J. The 1918 Influenza Pandemic: Back to the Future? Kidney Blood Press. Res. 2021, 46, 639–646. [Google Scholar] [CrossRef]
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J.; et al. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet 2018, 391, 1285–1300. [Google Scholar] [CrossRef]
- Yi, T.; Ding, W.; Hao, Y.; Cen, L.; Li, J.; Shi, X.; Wang, T.; Chen, D.; Zhu, H. Neutrophil extracellular traps mediate severe lung injury induced by influenza A virus H1N1 in mice coinfected with Staphylococcus aureus. Microb. Pathog. 2022, 166, 105558. [Google Scholar] [CrossRef]
- Okahashi, N.; Sumitomo, T.; Nakata, M.; Kawabata, S. Secondary streptococcal infection following influenza. Microbiol. Immunol. 2022, 66, 253–263. [Google Scholar] [CrossRef]
- Park, Y.; Chin, B.S.; Han, S.H.; Yun, Y.; Kim, Y.J.; Choi, J.Y.; Kim, C.O.; Song, Y.G.; Kim, J.M. Pandemic Influenza (H1N1) and Mycobacterium tuberculosis Co-infection. Tuberc. Respir. Dis. 2014, 76, 84–87. [Google Scholar] [CrossRef]
- Wang, Z.-D.; Wang, S.-C.; Liu, H.-H.; Ma, H.-Y.; Li, Z.-Y. Prevalence and burden of Toxoplasma gondii infection in HIV-infected people: A systematic review and meta-analysis. Lancet HIV 2017, 4, e177–e188. [Google Scholar] [CrossRef]
- Segerstrom, S.C.; Reed, R.G.; Karr, J.E. Cytomegalovirus and Toxoplasma gondii Serostatus Prospectively Correlated With Problems in Self-Regulation but not Executive Function Among Older Adults. Psychosom. Med. 2022, 84, 603–611. [Google Scholar] [CrossRef]
- Montazeri, M.; Nakhaei, M.; Fakhar, M.; Pazoki, H.; Pagheh, A.S.; Nazar, E.; Zakariaei, Z.; Mirzaeian, H.; Sharifpour, A.; Banimostafavi, E.S.; et al. Exploring the Association Between Latent Toxoplasma gondii Infection and COVID-19 in Hospitalized Patients: First Registry-Based Study. Acta Parasitol. 2022, 67, 1172–1179. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A Simple Method of Estimating Fifty Percent Endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Daniel, K.; Howe, L.D.S. Toxoplasma gondii comprises three clonal lineages: Correlation of parasite genotype with human disease. J. Infect. Dis. 1995, 172, 6. [Google Scholar]
- Conn, C.A.; McClellan, J.L.; Maassab, H.F.; Smitka, C.W.; Majde, J.A.; Kluger, M.J. Cytokines and the acute phase response to influenza virus in mice. Am. J. Physiol. 1995, 268 Pt 2, R78–R84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Luo, J.; Alcorn, J.F.; Chen, K.; Fan, S.; Pilewski, J.; Liu, A.; Chen, W.; Kolls, J.K.; Wang, J. AIM2 Inflammasome Is Critical for Influenza-Induced Lung Injury and Mortality. J. Immunol. 2017, 198, 4383–4393. [Google Scholar] [CrossRef] [PubMed]
- Del Gobbo, V.; Villani, N.; Marini, S.; Balestra, E.; Caliò, R. Suppressor cells induced by influenza virus inhibit interleukin-2 production in mice. Immunology 1990, 69, 454–459. [Google Scholar]
- Jebbari, H.; Roberts, C.W.; Ferguson, D.J.; Bluethmann, H.; Alexander, J. A protective role for IL-6 during early infection with Toxoplasma gondii. Parasite Immunol. 1998, 20, 231–239. [Google Scholar] [CrossRef]
- Yap, G.; Pesin, M.; Sher, A. Cutting edge: IL-12 is required for the maintenance of IFN-gamma production in T cells mediating chronic resistance to the intracellular pathogen, Toxoplasma gondii. J. Immunol. 2000, 165, 628–631. [Google Scholar] [CrossRef]
- Grunvald, E.; Chiaramonte, M.; Hieny, S.; Wysocka, M.; Trinchieri, G.; Vogel, S.N.; Gazzinelli, R.T.; Sher, A. Biochemical characterization and protein kinase C dependency of monokine-inducing activities of Toxoplasma gondii. Infect. Immun. 1996, 64, 2010–2018. [Google Scholar] [CrossRef]
- O’Brien, K.B.; Schultz-Cherry, S.; Knoll, L.J. Parasite-Mediated Upregulation of NK Cell-Derived Gamma Interferon Protects against Severe Highly Pathogenic H5N1 Influenza Virus Infection. J. Virol. 2011, 85, 8680. [Google Scholar] [CrossRef]
- Suzuki, Y.; Orellana, M.A.; Schreiber, R.D.; Remington, J.S. Interferon-gamma: The major mediator of resistance against Toxoplasma gondii. Science 1988, 240, 516–518. [Google Scholar] [CrossRef]
- Suzuki, Y.; Wang, X.; Jortner, B.S.; Payne, L.; Ni, Y.; Michie, S.A.; Xu, B.; Kudo, T.; Perkins, S. Removal of Toxoplasma gondii cysts from the brain by perforin-mediated activity of CD8+ T cells. Am. J. Pathol. 2010, 176, 1607–1613. [Google Scholar] [CrossRef]
- Khan, I.A.; Hwang, S.; Moretto, M. Toxoplasma gondii: CD8 T Cells Cry for CD4 Help. Front. Cell. Infect. Microbiol. 2019, 9, 136. [Google Scholar] [CrossRef]
- Janssen, E.M.; Droin, N.M.; Lemmens, E.E.; Pinkoski, M.J.; Bensinger, S.J.; Ehst, B.D.; Griffith, T.S.; Green, D.R.; Schoenberger, S.P. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature 2005, 434, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Bennett, S.R.; Carbone, F.R.; Karamalis, F.; Miller, J.F.; Heath, W.R. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J. Exp. Med. 1997, 186, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.A.; Green, W.R.; Kasper, L.H.; Green, K.A.; Schwartzman, J.D. Immune CD8(+) T cells prevent reactivation of Toxoplasma gondii infection in the immunocompromised host. Infect. Immun. 1999, 67, 5869–5876. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Xie, J.; Zhao, J.; Cao, D.; Liang, Y.; Hou, X.; Wang, L.; Li, Z. Mechanisms of Severe Mortality-Associated Bacterial Co-infections Following Influenza Virus Infection. Front. Cell. Infect. Microbiol. 2017, 7, 338. [Google Scholar] [CrossRef]
- Ifijen, I.H.; Atoe, B.; Ekun, R.O.; Ighodaro, A.; Odiachi, I.J. Treatments of Mycobacterium tuberculosis and Toxoplasma gondii with Selenium Nanoparticles. BioNanoScience 2023, 13, 249–277. [Google Scholar] [CrossRef]
- Buguliskis, J.S.; Brossier, F.; Shuman, J.; Sibley, L.D. Rhomboid 4 (ROM4) affects the processing of surface adhesins and facilitates host cell invasion by Toxoplasma gondii. PLoS Pathog. 2010, 6, e1000858. [Google Scholar] [CrossRef]
- Daher, W.; Plattner, F.; Carlier, M.F.; Soldati-Favre, D. Concerted action of two formins in gliding motility and host cell invasion by Toxoplasma gondii. PLoS Pathog. 2010, 6, e1001132. [Google Scholar] [CrossRef]
- Ma, J.; Wu, M.; Wang, Z.; Yang, D.; Hou, S.; Cheng, Y.; Wang, H.; Yan, Y.; Sun, J. Pre-exposure to Streptococcus suis improved survival of influenza virus co-infection in mice. Vet. Microbiol. 2021, 258, 109071. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Wang, X.; Li, J.; Sun, L.; Chen, X.; Chu, Z.; Zhang, Z.; Wu, H.; Zhao, X.; Li, H.; et al. Influenza A Virus Weakens the Immune Response of Mice to Toxoplasma gondii, Thereby Aggravating T. gondii Infection. Vet. Sci. 2023, 10, 354. https://doi.org/10.3390/vetsci10050354
Chen J, Wang X, Li J, Sun L, Chen X, Chu Z, Zhang Z, Wu H, Zhao X, Li H, et al. Influenza A Virus Weakens the Immune Response of Mice to Toxoplasma gondii, Thereby Aggravating T. gondii Infection. Veterinary Sciences. 2023; 10(5):354. https://doi.org/10.3390/vetsci10050354
Chicago/Turabian StyleChen, Junpeng, Xiaoli Wang, Jinxuan Li, Lingyu Sun, Xiao Chen, Ziyu Chu, Zhenzhao Zhang, Hongxia Wu, Xiaomin Zhao, Hongmei Li, and et al. 2023. "Influenza A Virus Weakens the Immune Response of Mice to Toxoplasma gondii, Thereby Aggravating T. gondii Infection" Veterinary Sciences 10, no. 5: 354. https://doi.org/10.3390/vetsci10050354
APA StyleChen, J., Wang, X., Li, J., Sun, L., Chen, X., Chu, Z., Zhang, Z., Wu, H., Zhao, X., Li, H., & Zhang, X. (2023). Influenza A Virus Weakens the Immune Response of Mice to Toxoplasma gondii, Thereby Aggravating T. gondii Infection. Veterinary Sciences, 10(5), 354. https://doi.org/10.3390/vetsci10050354




