Outlook of Adipose-Derived Stem Cells: Challenges to Their Clinical Application in Horses
Abstract
Simple Summary
Abstract
1. Introduction
2. Discussion
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar] [PubMed]
- Reed, S.A.; Leahy, E.R. Growth and Development Symposium: Stem cell therapy in equine tendon injury. J. Anim. Sci. 2013, 91, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Shojaee, A.; Parham, A. Strategies of tenogenic differentiation of equine stem cells for tendon repair: Current status and challenges. Stem Cell Res. Ther. 2019, 10, 181. [Google Scholar] [CrossRef] [PubMed]
- Volk, S.W.; Theoret, C. Translating stem cell therapies: The role of companion animals in regenerative medicine. Wound Repair Regen. 2013, 21, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Arnhold, S.; Elashry, M.I.; Klymiuk, M.C.; Geburek, F. Investigation of stemness and multipotency of equine adipose-derived mesenchymal stem cells (ASCs) from different fat sources in comparison with lipoma. Stem Cell Res. Ther. 2019, 10, 309. [Google Scholar] [CrossRef] [PubMed]
- Konstanty-Kalandyk, J.; Sadowski, J.; Anna Kędziora, A.; Urbańczyk-Zawadzka, M.; Baran, J.; Banyś, P.; Bogusław Kapelak, B.; Piątek, J. Functional Recovery after Intramyocardial Injection of Adipose-Derived Stromal Cells Assessed by Cardiac Magnetic Resonance Imaging. Stem Cells Int. 2021, 2021, 5556800. [Google Scholar] [CrossRef]
- Baer, P.C.; Geiger, H. Adipose-derived mesenchymal stromal/stem cells: Tissue localization, characterization, and heterogeneity. Stem Cells Int. 2012, 2012, 812693. [Google Scholar] [CrossRef]
- Vidal, M.A.; Kilroy, G.E.; Lopez, M.J.; Johnson, J.R.; Moore, R.M.; Gimble, J.M. Characterization of equine adipose tissue-derived stromal cells: Adipogenic and osteogenic capacity and comparison with bone marrow-derived mesenchymal stromal cells. Vet. Surg. 2007, 36, 613–622. [Google Scholar] [CrossRef]
- Semon, J.A.; Catherine Maness, C.; Zhang, X.; Sharkey, S.A.; Beuttler, M.M.; Shah, F.S.; Pandey, A.C.; Gimble, J.M.; Zhang, S.; Scruggs, B.A.; et al. Comparison of human adult stem cells from adipose tissue and bone marrow in the treatment of experimental autoimmune encephalomyelitis. Stem Cell Res. Ther. 2014, 5, 2. [Google Scholar] [CrossRef]
- Renzi, S.; Riccò, S.; Dotti, S.; Sesso, L.; Grolli, S.; Cornali, M.; Carlin, S.; Patruno, M.; Cinotti, S.; Ferrari, M. Autologous bone marrow mesenchymal stromal cells for regeneration of injured equine ligaments and tendons: A clinical report. Res. Vet. Sci. 2013, 95, 272–277. [Google Scholar] [CrossRef]
- De Ugarte, D.A.; Morizono, K.; Elbarbary, A.; Alfonso, Z.; Zuk, P.A.; Zhu, M.; Dragoo, J.L.; Ashjian, P.; Thomas, B.; Benhaim, P.; et al. Comparison of Multi-Lineage Cells from Human Adipose Tissue and Bone Marrow. Cells Tissues Organs 2003, 174, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Bongso, A.; Richards, M. History and perspective of stem cell research. Best Pract. Res. Clin. Obstet. Gynaecol. 2004, 18, 827–842. [Google Scholar] [CrossRef] [PubMed]
- Smith, A. A glossary for stem-cell biology. Nature 2006, 441, 1060. [Google Scholar] [CrossRef]
- Reyes, M.; Verfaillie, C.M. Characterization of Multipotent Adult Progenitor Cells, a Subpopulation of Mesenchymal Stem Cells. Ann. N. Y. Acad. Sci. 2001, 938, 231–235. [Google Scholar] [CrossRef]
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef]
- Ranera, B.; Ordovás, L.; Lyahyai, J.; Bernal, M.L.; Fernandes, F.; Remacha, A.R.; Romero, A.; Vázquez, F.J.; Osta, R.; Cons, C.; et al. Comparative study of equine bone marrow and adipose tissue-derived mesenchymal stromal cells. Equine Vet. J. 2012, 44, 33–42. [Google Scholar] [CrossRef]
- Reed, S.A.; Johnson, S.E. Refinement of Culture Conditions for Maintenance of Undifferentiated Equine Umbilical Cord Blood Stem Cells. J. Equine Vet. Sci. 2012, 32, 360–366. [Google Scholar] [CrossRef]
- Koerner, J.; Nesic, D.; Romero, J.D.; Brehm, W.; Mainil-Varlet, P.; Grogan, S.P. Equine Peripheral Blood-Derived Progenitors in Comparison to Bone Marrow-Derived Mesenchymal Stem Cells. Stem Cells 2006, 24, 1613–1619. [Google Scholar] [CrossRef]
- Ahern, B.J.; Schaer, T.P.; Terkhorn, S.P.; Jackson, K.V.; Mason, N.J.; Hankenson, K.D. Evaluation of equine peripheral blood apheresis product, bone marrow, and adipose tissue as sources of mesenchymal stem cells and their differentiation potential. Am. J. Vet. Res. 2011, 72, 127–133. [Google Scholar] [CrossRef]
- Da Silva, M.L.; Chagastelles, P.C.; Nardi, N.B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 2006, 119, 2204–2213. [Google Scholar]
- Chen, Y.; Shao, J.; Xiang, L.; Dong, X.; Zhang, G. Mesenchymal stem cells: A promising candidate in regenerative medicine. Int. J. Biochem. Cell Biol. 2008, 40, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, R.J.; Deasy, B.M.; Huard, J. Muscle-derived stem cells. Gene Ther. 2002, 9, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970, 3, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Javazon, E.; Colter, D.; Schwarz, E.; Prockop, D. Rat marrow stromal cells are more sensitive to plating density and expand more rapidly from single-cell-derived colonies than human marrow stromal cells. Stem Cells 2001, 19, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Alipour, F.; Parham, A.; Mehrjerdi, H.K.; Dehghani, H. Equine adipose-derived mesenchymal stem cells: Phenotype and growth characteristics, gene expression profile and differentiation potentials. J. Cell 2015, 16, 456–465. [Google Scholar]
- Dominici, M.; Le Blanc, K.; Müller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, W.; Rubin, J.P.; Marra, K.G. Adipose-derived stem cells: Implications in tissue regeneration. World J. Stem Cells 2014, 6, 312–321. [Google Scholar] [CrossRef]
- Ranera, B.; Lyahyai, J.; Romero, A.; Vázquez, F.J.; Remacha, A.R.; Bernal, M.L.; Zaragoza, P.; Rodellar, C.; Martín-Burriel, I. Immunophenotype and gene expression profiles of cell surface markers of mesenchymal stem cells derived from equine bone marrow and adipose tissue. Vet. Immunol. Immunopathol. 2011, 144, 147–154. [Google Scholar] [CrossRef]
- Mitchell, J.B.; McIntosh, K.; Zvonic, S.; Garrett, S.; Floyd, Z.E.; Kloster, A.; Halvorsen, Y.D.; Storms, R.W.; Goh, B.; Kilroy, G.; et al. Immunophenotype of human adipose-derived cells: Temporal changes in stromal-associated and stem cell-associated markers. Stem Cells 2006, 24, 376–385. [Google Scholar] [CrossRef]
- Bundgaard, L.; Stensballe, A.; Elbæk, K.J.; Berg, L.C. Mapping of equine mesenchymal stromal cell surface proteomes for identification of specific markers using proteomics and gene expression analysis: An in vitro cross-sectional study. Stem Cell Res. Ther. 2018, 9, 288. [Google Scholar] [CrossRef]
- González-Garza, M.T.; Cruz-Vega, D.E.; Cárdenas-Lopez, A.; de la Rosa, R.M.; Moreno-Cuevas, J.E. Comparing stemness gene expression between stem cell subpopulations from peripheral blood and adipose tissue. Am. J. Stem Cells 2018, 7, 38–47. [Google Scholar] [PubMed]
- Mieczkowska, A.; Schumacher, A.; Filipowicz, N.; Wardowska, A.; Zielinski, M.; Madanecki, P.; Nowicka, E.; Langa, P.; Deptuła, M.; Zielinski, J.; et al. Immunophenotyping and transcriptional profiling of in vitro cultured human adipose tissue derived stem cells. Sci. Rep. 2018, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Bausch-Fluck, D.; Hofmann, A.; Bock, T.; Frei, A.P.; Cerciello, F.; Jacobs, A.; Wollscheid, B. A mass spectrometric-derived cell surface protein atlas. PLoS ONE 2015, 10, e0121314. [Google Scholar] [CrossRef] [PubMed]
- Fujinaka, C.M.; Waas, M.; Gundry, R.L. Mass Spectrometry-Based Identification of Extracellular Domains of Cell Surface N-Glycoproteins: Defining the Accessible Surfaceome for Immunophenotyping Stem Cells and Their Derivatives. Methods Mol. Biol. 2018, 1722, 57–78. [Google Scholar]
- Li, X.; Ma, T.; Sun, J.; Shen, M.; Xue, X.; Chen, Y.; Zhanget, Z. Harnessing the secretome of adipose-derived stem cells in the treatment of ischemic heart diseases. Stem Cell Res. Ther. 2019, 10, 196. [Google Scholar] [CrossRef]
- Wankhade, U.D.; Shen, M.; Kolhe, R.; Fulzele, S. Advances in adipose-derived stem cells isolation, characterization, and application in regenerative tissue engineering. Stem Cells Int. 2016, 2016, 3206807. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef]
- Yoshimura, K.; Shigeura, T.; Matsumoto, D.; Sato, T.; Takaki, Y.; Aiba-Kojima, E.; Sato, K.; Inoue, K.; Nagase, T.; Koshima, I.; et al. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J. Cell. Physiol. 2006, 208, 64–76. [Google Scholar] [CrossRef]
- Gimble, J.M.; Katz, A.J.; Bunnell, B.A. Adipose-derived stem cells for regenerative medicine. Circ. Res. 2007, 100, 1249–1260. [Google Scholar] [CrossRef]
- Carelli, S.; Messaggio, F.; Canazza, A.; Hebda, D.M.; Caremoli, F.; Latorre, E.; Grimoldi, M.G.; Colli, M.; Bulfamante, G.; Tremolada, C.; et al. Characteristics and Properties of Mesenchymal Stem Cells Derived from Microfragmented Adipose Tissue. Cell Transplant. 2015, 24, 1233–1252. [Google Scholar] [CrossRef] [PubMed]
- Guilak, F.; Lott, K.E.; Awad, H.A.; Cao, Q.; Hicok, K.C.; Fermor, B.; Gimble, J.M. Clonal analysis of the differentiation potential of human adipose-derived adult stem cells. J. Cell. Physiol. 2006, 206, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Gonda, K.; Shigeura, T.; Sato, T.; Matsumoto, D.; Suga, H.; Inoue, K.; Aoi, N.; Kato, H.; Sato, K.; Murase, S.; et al. Preserved proliferative capacity and multipotency of human adipose-derived stem cells after long-term cryopreservation. Plast. Reconstr. Surg. 2008, 121, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Mohamed-Ahmed, S.; Fristad, I.; Lie, S.A.; Suliman, S.; Mustafa, K.; Vindenes, H.; Idris, S.B. Adipose-derived and bone marrow mesenchymal stem cells: A donor-matched comparison. Stem Cell Res. Ther. 2018, 9, 168. [Google Scholar] [CrossRef]
- Choi, W.Y.; Poss, K.D. Cardiac regeneration. Curr. Top. Dev. Biol. 2012, 100, 319–344. [Google Scholar]
- Grıgorova, N.; Gócza, E.; Vachkova, E. Pilot study on cardiogenic differentiation capability of rabbit mesenchymal stem cells. Ank. Üniv. Vet. Fak. Derg. 2020, 67, 407–412. [Google Scholar] [CrossRef]
- Caplan, A.I.; Correa, D. The MSC: An injury drugstore. Cell Stem Cell 2011, 9, 11–15. [Google Scholar] [CrossRef]
- Murphy, M.B.; Moncivais, K.; Caplan, A.I. Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Exp. Mol. Med. 2013, 45, e54. [Google Scholar] [CrossRef]
- Trzyna, A.; Banaś-Ząbczyk, A. Adipose-Derived Stem Cells Secretome and Its Potential Application in “Stem Cell-Free Therapy”. Biomolecules 2021, 11, 878. [Google Scholar] [CrossRef]
- An, Y.; Zhao, J.; Nie, F.; Qin, Z.; Xue, H.; Wang, G.; Li, D. Exosomes from Adipose-Derived Stem Cells (ADSCs) Overexpressing miR-21 Promote Vascularization of Endothelial Cells. Sci. Rep. 2019, 9, 12861. [Google Scholar] [CrossRef]
- Koniusz, S.; Andrzejewska, A.; Muraca, M.; Srivastava, A.K.; Janowski, M.; Lukomska, B. Extracellular Vesicles in Physiology, Pathology, and Therapy of the Immune and Central Nervous System, with Focus on Extracellular Vesicles Derived from Mesenchymal Stem Cells as Therapeutic Tools. Front. Cell. Neurosci. 2016, 10, 109. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.; Mellows, B.; Sheard, J.; Antonioli, M.; Kretz, O.; Chambers, D.; Zeuner, M.T.; Tomkins, J.E.; Denecke, B.; Musante, L.; et al. Secretome of adipose-derived mesenchymal stem cells promotes skeletal muscle regeneration through synergistic action of extracellular vesicle cargo and soluble proteins. Stem Cell Res. Ther. 2019, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Guo, X. A review: Therapeutic potential of adipose-derived stem cells in cutaneous wound healing and regeneration. Stem Cell Res. Ther. 2018, 9, 302. [Google Scholar] [CrossRef] [PubMed]
- Riis, S.; Stensballe, A.; Emmersen, J.; Pennisi, C.P.; Birkelund, S.; Zachar, V.; Fink, T. Mass spectrometry analysis of adipose-derived stem cells reveals a significant effect of hypoxia on pathways regulating extracellular matrix. Stem Cell Res. Ther. 2016, 7, 52. [Google Scholar] [CrossRef]
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef]
- Salgado, A.J.; Reis, R.L.; Sousa, N.J.; Gimble, J.M. Adipose tissue derived stem cells secretome: Soluble factors and their roles in regenerative medicine. Curr. Stem Cell Res. Ther. 2010, 5, 103–110. [Google Scholar] [CrossRef]
- Ma, T.; Sun, J.; Zhao, Z.; Lei, W.; Chen, Y.; Wang, X.; Shen, Z. A brief review: Adipose-derived stem cells and their therapeutic potential in cardiovascular diseases. Stem Cell Res. Ther. 2017, 8, 124. [Google Scholar] [CrossRef]
- Salem, B.; Miner, S.; Hensel, N.F.; Battiwalla, M.; Keyvanfar, K.; Stroncek, D.F.; Gee, A.P.; Hanley, P.J.; Bollard, C.M.; Ito, S.; et al. Quantitative activation suppression assay to evaluate human bone marrow-derived mesenchymal stromal cell potency. Cytotherapy 2015, 17, 1675–1686. [Google Scholar] [CrossRef]
- Menard, C.; Tarte, K. Immunoregulatory properties of clinical grade mesenchymal stromal cells: Evidence, uncertainties, and clinical application. Stem Cell Res. Ther. 2013, 4, 64. [Google Scholar] [CrossRef]
- Tolar, J.; Le Blanc, K.; Keating, A.; Blazar, B.R. Concise review: Hitting the right spot with mesenchymal stromal cells. Stem Cells 2010, 28, 1446–1455. [Google Scholar] [CrossRef]
- Corcione, A.; Benvenuto, F.; Ferretti, E.; Giunti, D.; Cappiello, V.; Cazzanti, F.; Risso, M.; Gualandi, F.; Mancardi, G.L.; Pistoia, V.; et al. Human mesenchymal stem cells modulate B-cell functions. Blood 2006, 107, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Carrade, D.D.; Lame, M.W.; Kent, M.S.; Clark, K.S.; Walker, N.J.; Borjesson, D.L. Comparative analysis of the immunomodulatory properties of equine adult-derived mesenchymal stem cells. Cell Med. 2012, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Falomo, M.E.; Ferroni, L.; Tocco, I.; Gardin, C.; Zavan, B. Immunomodulatory Role of Adipose-Derived Stem Cells on Equine Endometriosis. Biomed. Res. Int. 2015, 2015, 141485. [Google Scholar] [CrossRef] [PubMed]
- Markoski, M.M. Advances in the Use of Stem Cells in Veterinary Medicine: From Basic Research to Clinical Practice. Scientifica 2016, 2016, 4516920. [Google Scholar] [CrossRef] [PubMed]
- Vachkova, E.; Grigorova, N.; Beev, G.; Tacheva, T.; Vlaykova, T. Possible effect of PUFAs on gelatinases and MMP-14 mRNA expression in rabbit differentiated adipose derived stem cells in vitro. In Proceedings of the CellFit Meeting, Hvar Island, Croatia, 1–4 October 2018; p. 86. [Google Scholar]
- Vachkova, E.; Grigorova, N.; Tacheva, T.; Vlaykova, T. Combined EPA-DHA treatment effects negatively leptin and obesity-related membrane-type MT1-MMP mRNA expression in rabbit subcutaneous ADSCs in vitro. In Proceedings of the 3rd CellFit Annual Meeting: The Extracellular Vesicles Paradigm of Intra and Intercellular Communication, Athens, Greece, 10–12 October 2019; p. 79. [Google Scholar]
- Mambelli, L.I.; Santos, E.J.; Frazao, P.J.; Chaparro, M.B.; Kerkis, A.; Zoppa, A.L.; Kerkis, I. Characterization of equine adipose tissue–derived progenitor cells before and after cryopreservation. Tissue Eng. Part C Methods 2009, 15, 87–94. [Google Scholar] [CrossRef]
- Pascucci, L.; Curina, G.; Mercati, F.; Marini, C.; Dall’Aglio, C.; Paternesi, B.; Ceccarelli, P. Flow cytometric characterization of culture expanded multipotent mesenchymal stromal cells (MSCs) from horse adipose tissue: Towards the definition of minimal stemness criteria. Vet. Immunol. Immunopathol. 2011, 144, 499–506. [Google Scholar] [CrossRef]
- Petrova, V.; Yonkova, P.; Simeonova, G.; Vachkova, E. Improvement of adipogenic protocol for equine subcutaneous ADSCs in vitro. In Proceedings of the 3rd CellFit Workshop, 3D3C: Towards a New Vista of In-Vitro 3-Dimensional Organization and Cell to Cell Communication, Jerusalem, Israel, 3–6 March 2020; pp. 84–85. [Google Scholar]
- Pezzanite, L.; Chow, L.; Griffenhagen, G.; Dow, S.; Goodrich, L. Impact of Three Different Serum Sources on Functional Properties of Equine Mesenchymal Stromal Cells. Front. Vet. Sci. 2021, 8, 634064. [Google Scholar] [CrossRef]
- Franke, J.; Abs, V.; Zizzadoro, C.; Abraham, G. Comparative study of the effects of fetal bovine serum versus horse serum on growth and differentiation of primary equine bronchial fibroblasts. BMC Vet. Res. 2014, 10, 119. [Google Scholar] [CrossRef]
- Dessels, C.; Potgieter, M.; Pepper, M.S. Making the Switch: Alternatives to Fetal Bovine Serum for Adipose-Derived Stromal Cell Expansion. Front. Cell Dev. Biol. 2016, 4, 115. [Google Scholar] [CrossRef]
- Naskou, M.C.; Sumner, S.M.; Chocallo, A.; Kemelmakher, H.; Thoresen, M.; Copland, I.; Galipeau, J.; Peroni, J.F. Platelet lysate as a novel serum-free media supplement for the culture of equine bone marrow-derived mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 75. [Google Scholar] [CrossRef]
- Lv, F.; Tuan, R.S.; Cheung, K.M.; Leung, V.Y. Concise Review. The Surface Markers and Identity of Human Mesenchymal Stem Cells. Stem Cells 2014, 32, 1408–1419. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.C.; Kol, A.; Shahbenderian, S.; Granick, J.L.; Walker, N.J.; Borjesson, D.L. Canine and Equine Mesenchymal Stem Cells Grown in Serum Free Media Have Altered Immunophenotype. Stem Cell Rev. Rep. 2016, 12, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.K.; Tan, C.S.; Chan, K.L.; Goesantoso, G.G.; Chan, X.H.; Chan, E.; Yin, J.; Yeo, C.R.; Khoo, C.M.; So, J.B.; et al. Identification of specific cell-surface markers of adipose-derived stem cells from subcutaneous and visceral fat depots. Stem Cell Rep. 2014, 2, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Vachkova, E.; Bosnakovski, D.; Yonkova, P.; Grigorova, N.; Ivanova Zh Todorov, P.; Penchev, G.; Milanova, A.; Simeonova, G.; Stanilova, S.; Penchev, I. Adipogenic potential of stem cells derived from rabbit subcutaneous and visceral adipose tissue in vitro. Vitr. Cell Dev. Biol. Anim. 2016, 52, 829–837. [Google Scholar] [CrossRef]
- Bukowska, J.; Szóstek-Mioduchowska, A.Z.; Kopcewicz, M.; Walendzik, K.; Machcińska, S.; Gawrońska-Kozak, B. Adipose-Derived Stromal/Stem Cells from Large Animal Models: From Basic to Applied Science. Stem Cell Rev. Rep. 2021, 17, 719–738. [Google Scholar] [CrossRef]
- Voga, M.; Adamic, N.; Vengust, M.; Majdic, G. Stem Cells in Veterinary Medicine-Current State and Treatment Options. Front. Vet. Sci. 2020, 7, 278. [Google Scholar] [CrossRef]
- FDA. CVM GFI #218 Cell-Based Products for Animal Use. 2015. Available online: https://www.fda.gov/media/88925/download (accessed on 15 February 2020).
- Debnath, T.; Chelluri, L.K. Standardization and quality assessment for clinical grade mesenchymal stem cells from human adipose tissue. Hematol. Transfus. Cell Ther. 2019, 41, 7–16. [Google Scholar] [CrossRef]
- Marx, C.; Silveira, M.D.; Nardi, N.B. Adipose-derived stem cells in veterinary medicine: Characterization and therapeutic applications. Stem Cells Dev. 2015, 24, 803–813. [Google Scholar] [CrossRef]
- Barrachina, L.; Romero, A.; Zaragoza, P.; Rodellar, C.; Vázquez, F.J. Practical considerations for clinical use of mesenchymal stem cells: From the laboratory to the horse. Vet. J. 2018, 238, 49–57. [Google Scholar] [CrossRef]
- Zayed, M.; Adair, S.; Ursini, T.; Schumacher, J.; Misk, N.; Dhar, M. Concepts and challenges in the use of mesenchymal stem cells as a treatment for cartilage damage in the horse. Res. Vet. Sci. 2018, 118, 317–323. [Google Scholar] [CrossRef]
- Shojaee, A.; Parham, A.; Ejeian, F.; Nasr Esfahani, M.H. Equine adipose mesenchymal stem cells (eq-ASCs) appear to have higher potential for migration and musculoskeletal differentiation. Res. Vet. Sci. 2019, 125, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Prządka, P.; Buczak, K.; Frejlich, E.; Gąsior, L.; Suliga, K.; Kiełbowicz, Z. The Role of Mesenchymal Stem Cells (MSCs) in Veterinary Medicine and Their Use in Musculoskeletal Disorders. Biomolecules 2021, 11, 1141. [Google Scholar] [CrossRef] [PubMed]
- Clegg, P.D. Musculoskeletal disease and injury, now and in the future. Part 2: Tendon and ligament injuries. Equine Vet. J. 2012, 44, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.J.; Jarazo, J. State of the art: Stem cells in equine regenerative medicine. Equine Vet. J. 2015, 47, 145–154. [Google Scholar] [CrossRef]
- Brandao, J.S.; Alvarenga, M.L.; Hubbe Pfeifer, J.P.; dos Santos, V.H.; Fonseca-Alves, C.E.; Rodrigues, M.; Laufer-Amorim, R.; Lucas Castillo, J.A.; Garcia Alves, A.L. Allogeneic mesenchymal stem cell transplantation in healthy equine superficial digital flexor tendon: A study of the local inflammatory response. Res. Vet. Sci. 2018, 118, 423–430. [Google Scholar] [CrossRef]
- Dakin, S.G. A review of the healing processes in equine superficial digital flexor tendinopathy. Equine Vet. Educ. 2016, 29, 516–520. [Google Scholar] [CrossRef]
- Romero, A.; Barrachina, L.; Ranera, B.; Remacha, A.; Moreno, B.; De Blas, I.; Sanz, A.; Vázquez, F.; Vitoria, A.; Junquera, C. Comparison of Autologous Bone Marrow and Adipose Tissue Derived Mesenchymal Stem Cells, and Platelet Rich Plasma, for Treating Surgically Induced Lesions of the Equine Superficial Digital Flexor Tendon. Vet. J. 2017, 224, 76–84. [Google Scholar] [CrossRef]
- Burk, J.; Badylak, S.F.; Kelly, J.; Brehm, W. Equine cellular therapy--from stall to bench to bedside? Cytom. A 2013, 83, 103–113. [Google Scholar] [CrossRef]
- Beerts, C.; Suls, M.; Broeckx, S.Y.; Seys, B.; Vandenberghe, A.; Declercq, J.; Duchateau, L.; Vidal, M.A.; Spaas, J.H. Tenogenically Induced Allogeneic Peripheral Blood Mesenchymal Stem Cells in Allogeneic Platelet-Rich Plasma: 2-Year Follow-up after Tendon or Ligament Treatment in Horses. Front. Vet. Sci. 2017, 4, 158. [Google Scholar] [CrossRef]
- Ricco, S.; Renzi, S.; Del Bue, M.; Conti, V.; Merli, E.; Ramoni, R.; Lucarelli, E.; Gnudi, G.; Ferrari, M.; Grolli, S. Allogeneic adipose tissue-derived mesenchymal stem cells in combination with platelet rich plasma are safe and effective in the therapy of superficial digital flexor tendonitis in the horse. Int. J. Immunopathol. Pharmacol. 2013, 26 (Suppl. 1), 61–68. [Google Scholar] [CrossRef]
- Carvalho, A.M.; Badial, P.R.; Álvarez, L.E.; Yamada, A.L.; Borges, A.S.; Deffune, E.; Hussni, C.A.; Garcia Alves, A.L. Equine Tendonitis Therapy Using Mesenchymal Stem Cells and Platelet Concentrates: A Randomized Controlled Trial. Stem Cell Res. Ther. 2013, 4, 85. [Google Scholar] [CrossRef] [PubMed]
- Guercio, A.; Di Marco, P.; Casella, S.; Russotto, L.; Puglisi, F.; Majolino, C.; Giudice, E.; Di Bella, S.; Purpari, G.; Cannella, V.; et al. Mesenchymal stem cells derived from subcutaneous fat and platelet-rich plasma used in athletic horses with lameness of the superficial digital flexor tendon. J. Equine Vet. Sci. 2015, 35, 19–26. [Google Scholar] [CrossRef]
- Magri, C.; Schramme, M.; Febre, M.; Cauvin, E.; Labadie, F.; Saulnier, N.; François, I.; Lechartier, A.; Aebischer, D.; Moncelet, A.S. Comparison of Efficacy and Safety of Single versus Repeated Intra-Articular Injection of Allogeneic Neonatal Mesenchymal Stem Cells for Treatment of Osteoarthritis of the Metacarpophalangeal/Metatarsophalangeal Joint in Horses: A Clinical Pilot Study. PLoS ONE 2019, 14, e0221317. [Google Scholar] [CrossRef] [PubMed]
- Nicpon, J.; Marycz, K.; Grzesiak, J. Therapeutic effect of adipose-derived mesenchymal stem cell injection in horses suffering from bone spavin. Pol. J. Vet. Sci. 2013, 16, 753–754. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Juan, W.; Xin, Z.; Zehuan, X.; Jiajia, Z.; Ran, Y.; Fang, H.; Handong, Z.; Lili, C. Exosomes derived from human adipose mesenchymal stem cells accelerate cutaneous wound healing by optimizing fibroblasts’ characteristics. Sci. Rep. 2016, 6, 32993. [Google Scholar]
- Chicharro, D.; Carrillo, J.M.; Rubio, M.; Cugat, R.; Cuervo, B.; Guil, S.; Forteza, J.; Moreno, V.; Vilar, J.M.; Sopena, J. Combined plasma rich in growth factors and adipose-derived mesenchymal stem cells promotes cutaneous wound healing in rabbits. BMC Vet. Res. 2018, 14, 288. [Google Scholar] [CrossRef] [PubMed]
- Marycz, K.; Szłapka-Kosarzewska, J.; Geburek, F.; Kornicka-Garbowska, K. Systemic Administration of Rejuvenated Adipose-Derived Mesenchymal Stem Cells Improves Liver Metabolism in Equine Metabolic Syndrome (EMS)-New Approach in Veterinary Regenerative Medicine. Stem Cell Rev. Rep. 2019, 15, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Vackova, E.; Bosnakovski, D.; Bjørndal, B.; Yonkova, P.; Grigorova, N.; Ivanova, Z.; Penchev, G.; Simeonova, G.; Miteva, L.; Milanova, A.; et al. n-3 polyunsaturated fatty acids provoke a specific transcriptional profile in rabbit adipose-derived stem cells in vitro. J. Anim. Physiol. Anim. Nutr. 2019, 103, 925–934. [Google Scholar] [CrossRef]
- Wei, H.J.; Zeng, R.; Lu, J.H.; Lai, W.F.; Che, W.H.; Liu, H.Y.; Chang, Y.T.; Deng, W.P. Adipose-derived stem cells promote tumor initiation and accelerate tumor growth by interleukin-6 production. Oncotarget 2015, 6, 7713–7726. [Google Scholar] [CrossRef]
- Gibson, M.A.; Brown, S.G.; Brown, N.O. Semitendinosus myopathy and treatment with adipose-derived stem cells in working German shepherd police dogs. Can. Vet. J. 2017, 58, 241–246. [Google Scholar]
- Arzi, B.; Peralta, S.; Fiani, N.; Vapniarsky, N.; Taechangam, N.; Delatorre, U.; Clark, K.C.; Walker, N.J.; Loscar, M.R.; Lommer, M.J.; et al. A multicenter experience using adipose-derived mesenchymal stem cell therapy for cats with chronic, non-responsive gingivostomatitis. Stem Cell Res. Ther. 2020, 11, 115. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Merino, E.M.; Usón-Casaús, J.M.; Duque-Carrasco, J.; Zaragoza-Bayle, C.; Mariñas-Pardo, L.; Hermida-Prieto, M.; Vilafranca-Compte, M.; Barrera-Chacón, R.; Gualtieri, M. Safety and efficacy of allogeneic adipose tissue-derived mesenchymal stem cells for treatment of dogs with inflammatory bowel disease: Endoscopic and histological outcomes. Vet. J. 2015, 206, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Webb, T.L.; Webb, C.B. Stem cell therapy in cats with chronic enteropathy: A proof-of-concept study. J. Feline Med. Surg. 2015, 17, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Teshima, T.; Matsumoto, H.; Michishita, M.; Matsuoka, A.; Shiba, M.; Nagashima, T.; Koyama, H. Allogenic adipose tissue-derived mesenchymal stem cells ameliorate acute hepatic injury in dogs. Stem Cells Int. 2017, 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Escalhão, C.C.M.I.; Ramos, I.P.; Hochman-Mendez, C.; Brunswick, T.H.K.; Souza, S.A.L.; Gutfilen, B.; Dos Santos Goldenberg, R.C.; Coelho-Sampaio, T. Safety of allogeneic canine adipose tissue-derived mesenchymal stem cell intraspinal transplantation in dogs with chronic spinal cord injury. Stem Cells Int. 2017, 2017, 3053759. [Google Scholar] [CrossRef] [PubMed]
- Bittencourt, M.K.W.; Barros, M.A.; Martins, J.F.P.; Vasconcellos, J.P.C.; Morais, B.P.; Pompeia, C.; Bittencourt, M.D.; Evangelho, K.D.S.; Kerkis, I.; Wenceslau, C.V. Allogeneic Mesenchymal Stem Cell Transplantation in Dogs with Keratoconjunctivitis Sicca. Cell Med. 2016, 8, 63–77. [Google Scholar] [CrossRef]
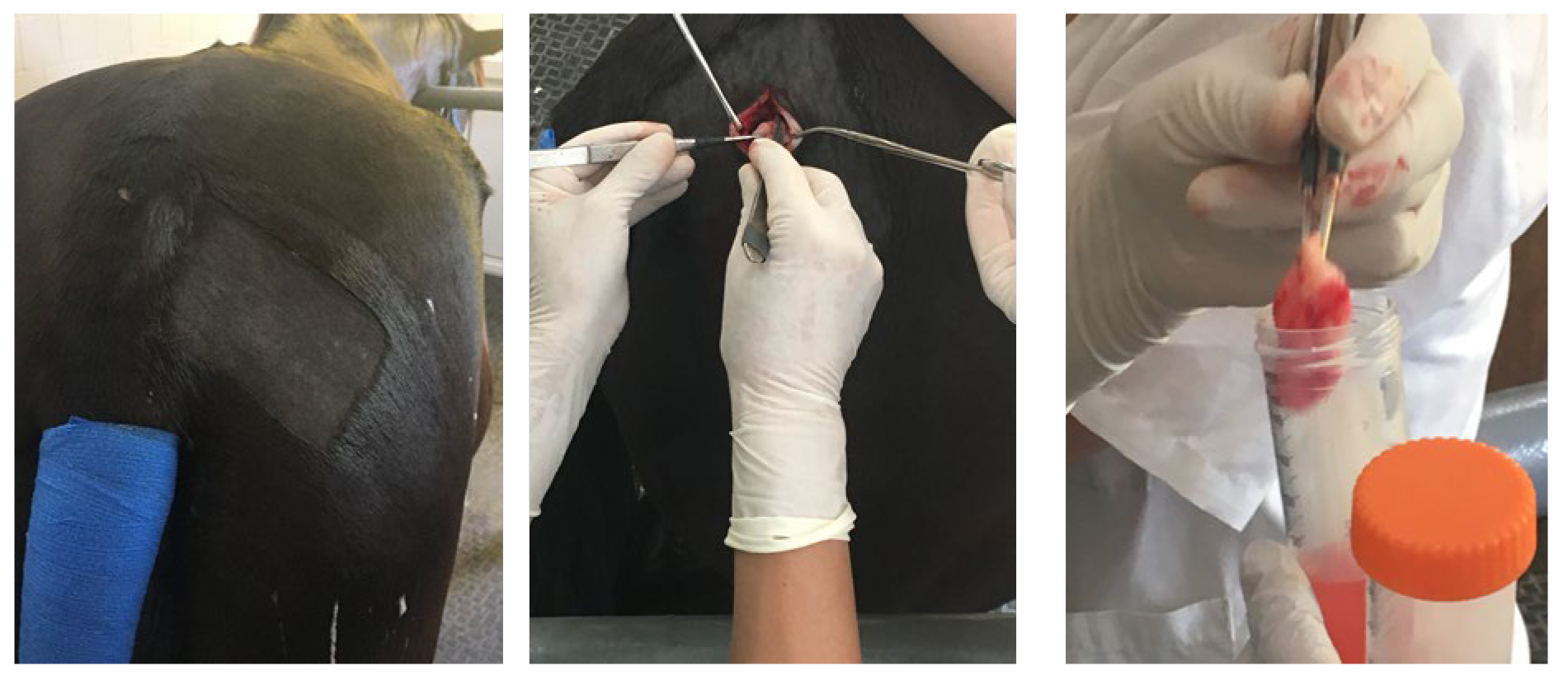

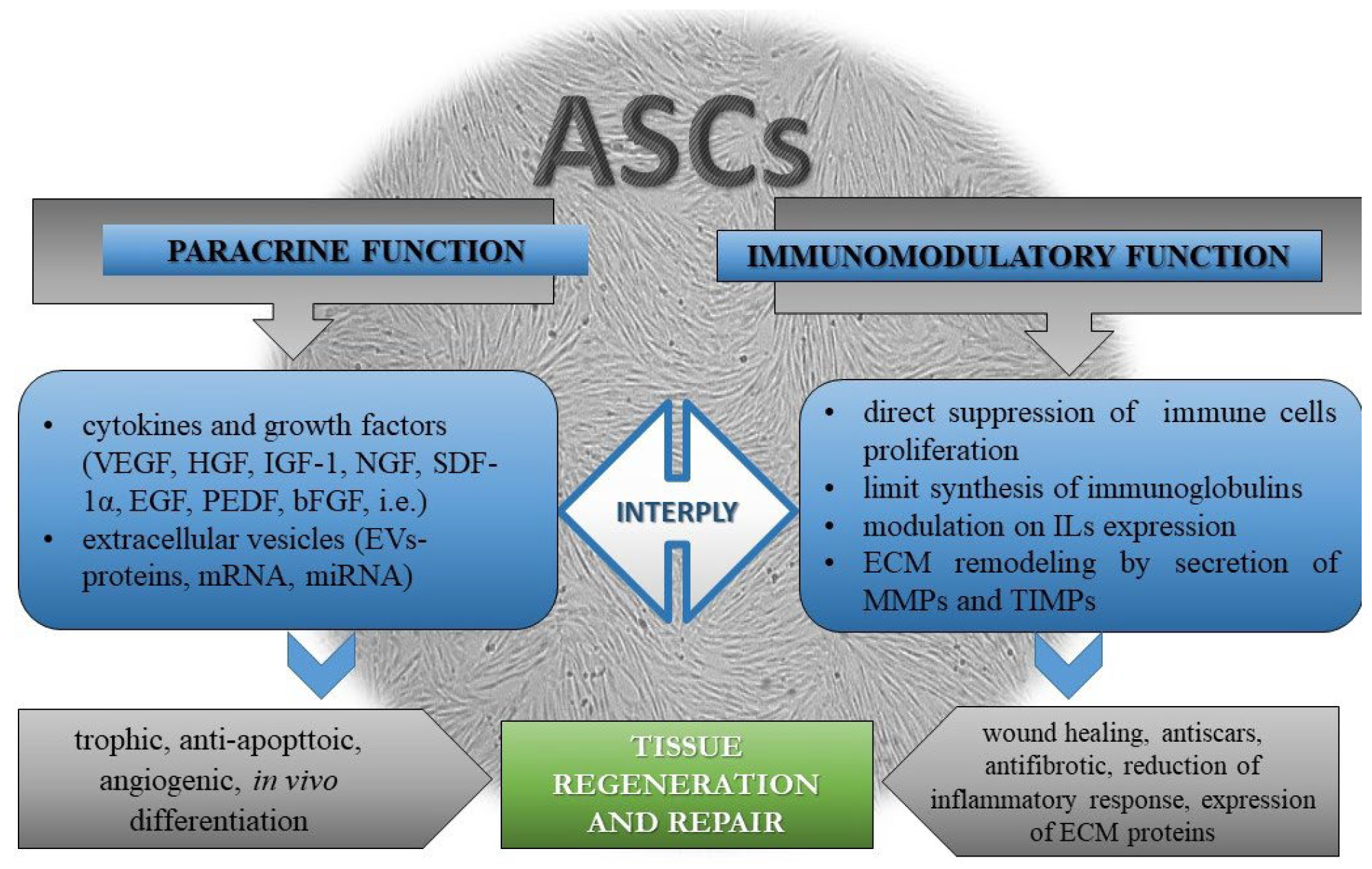
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrova, V.; Vachkova, E. Outlook of Adipose-Derived Stem Cells: Challenges to Their Clinical Application in Horses. Vet. Sci. 2023, 10, 348. https://doi.org/10.3390/vetsci10050348
Petrova V, Vachkova E. Outlook of Adipose-Derived Stem Cells: Challenges to Their Clinical Application in Horses. Veterinary Sciences. 2023; 10(5):348. https://doi.org/10.3390/vetsci10050348
Chicago/Turabian StylePetrova, Valeria, and Ekaterina Vachkova. 2023. "Outlook of Adipose-Derived Stem Cells: Challenges to Their Clinical Application in Horses" Veterinary Sciences 10, no. 5: 348. https://doi.org/10.3390/vetsci10050348
APA StylePetrova, V., & Vachkova, E. (2023). Outlook of Adipose-Derived Stem Cells: Challenges to Their Clinical Application in Horses. Veterinary Sciences, 10(5), 348. https://doi.org/10.3390/vetsci10050348





