Technical Validation of Ultrasound Assessment of the Thyroid Gland in Cattle
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Observers
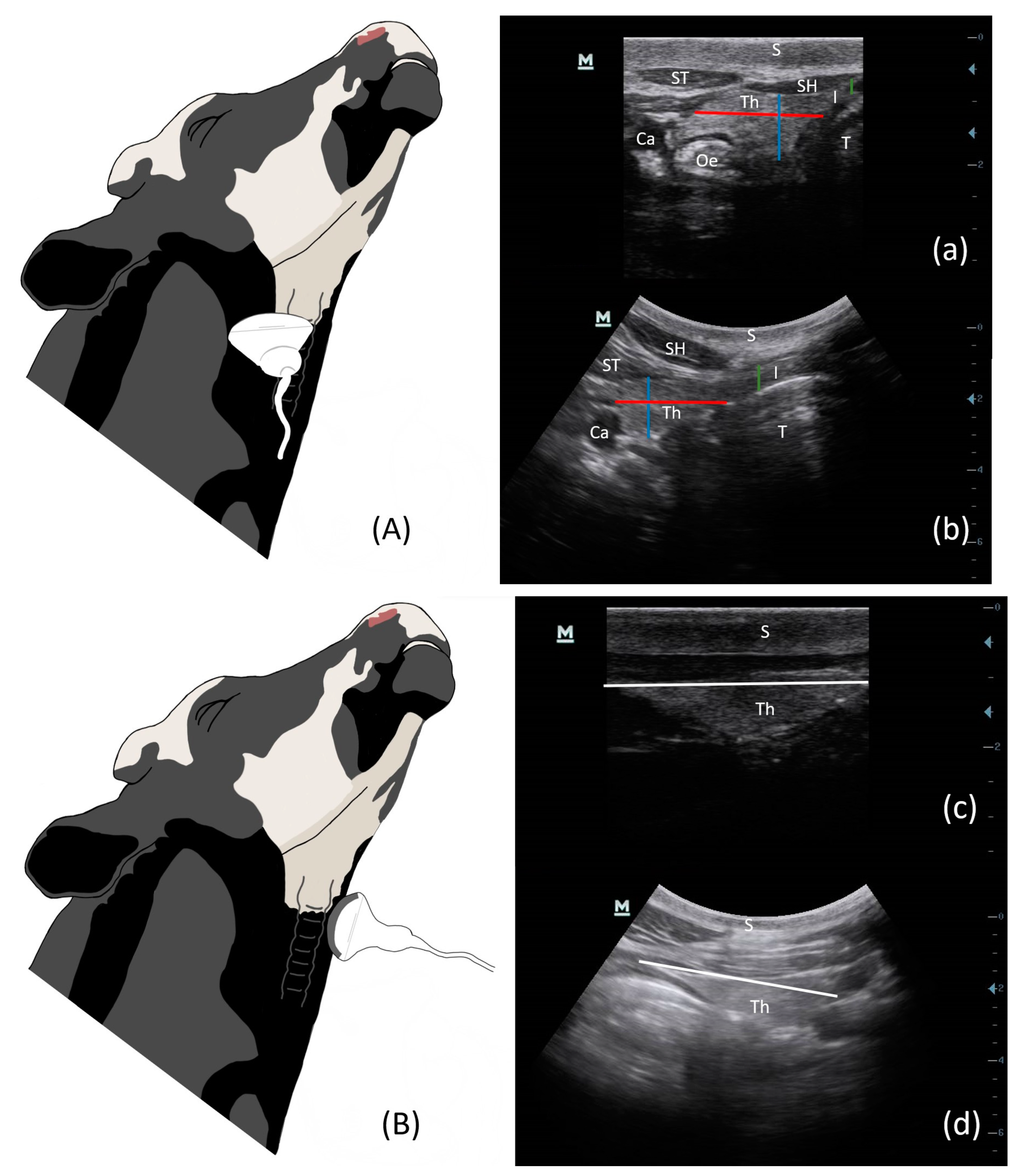
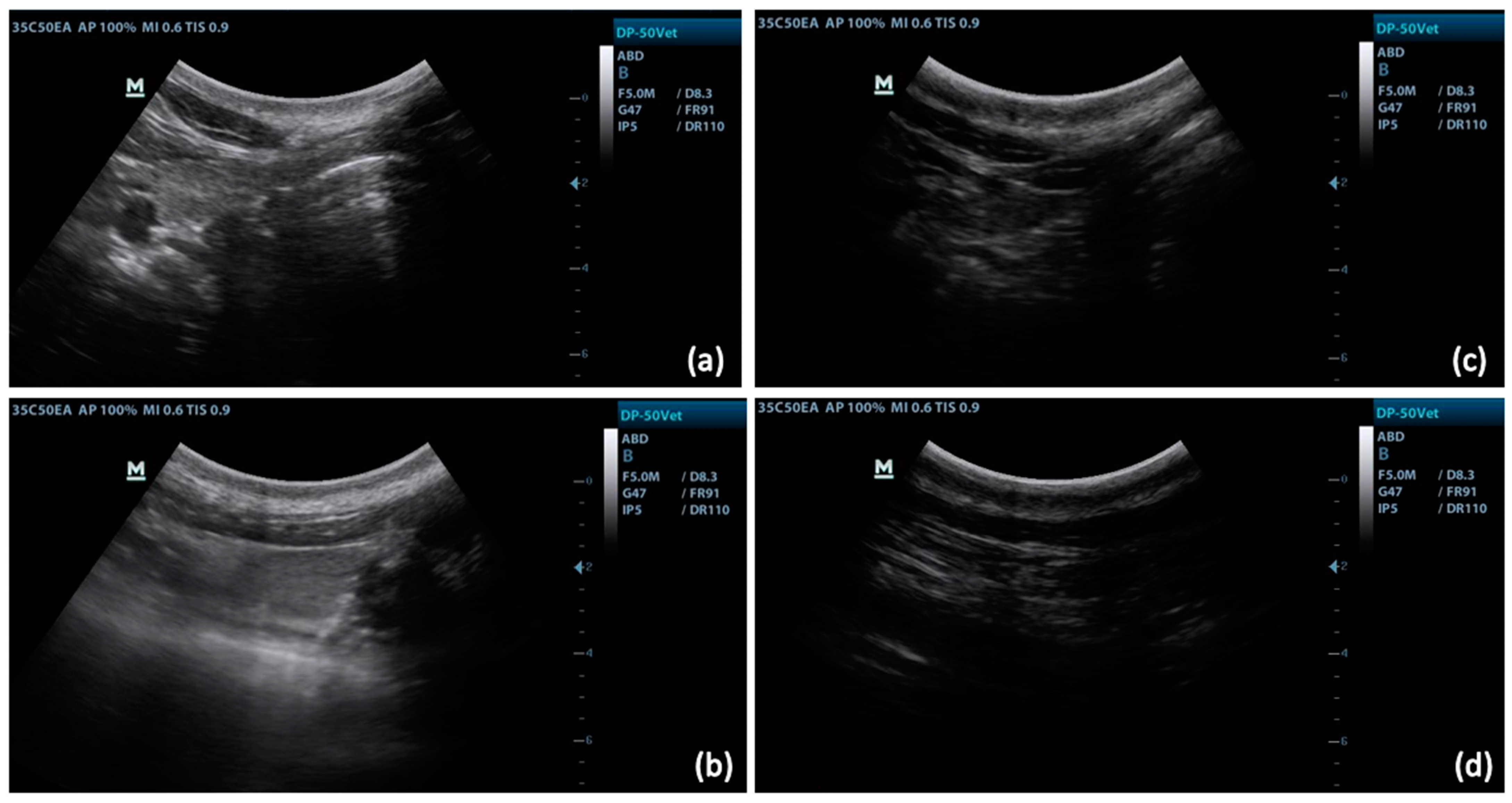
2.3. Ultrasonography
2.4. Statistics
3. Results
3.1. Ultrasound Technique and Measurements
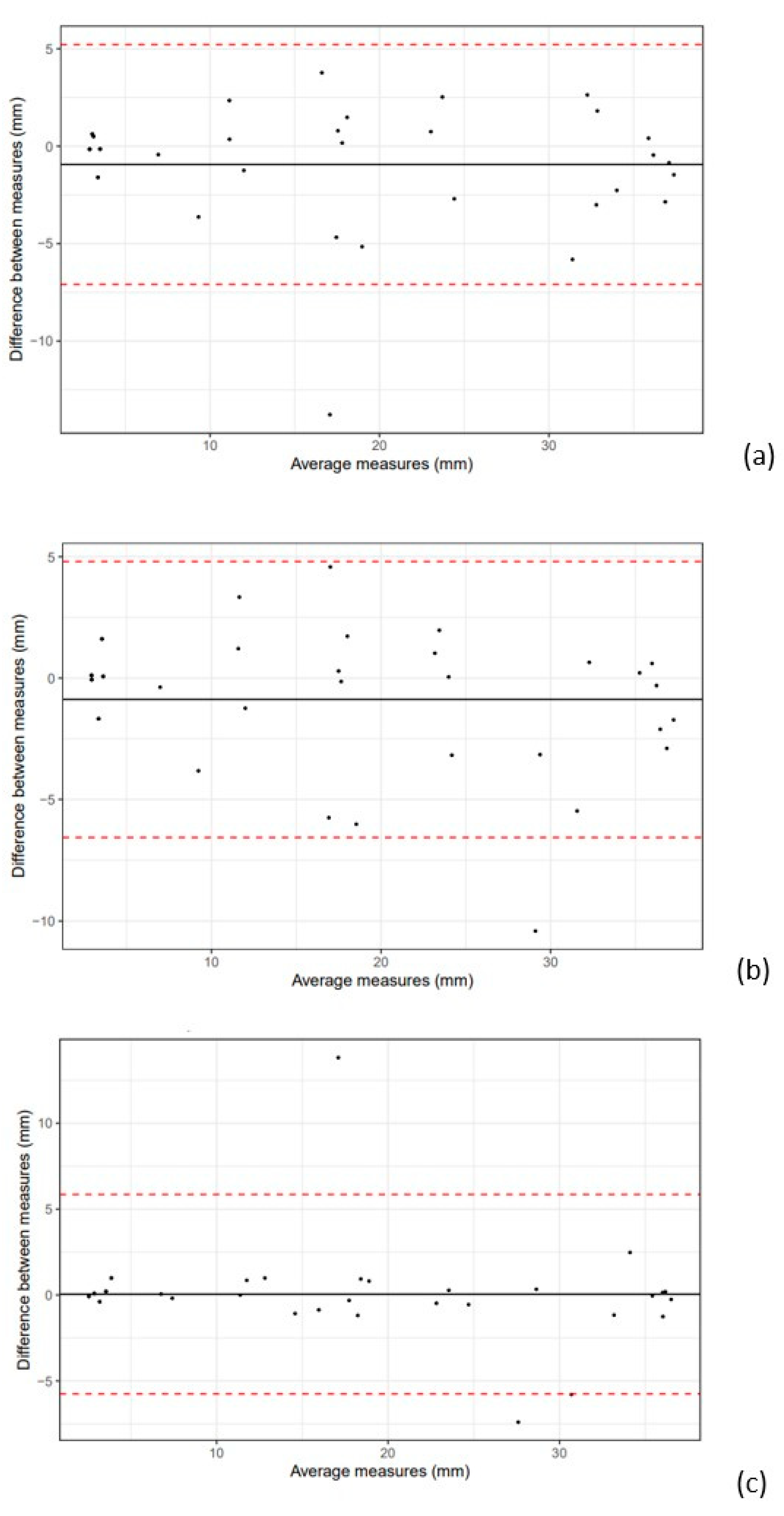
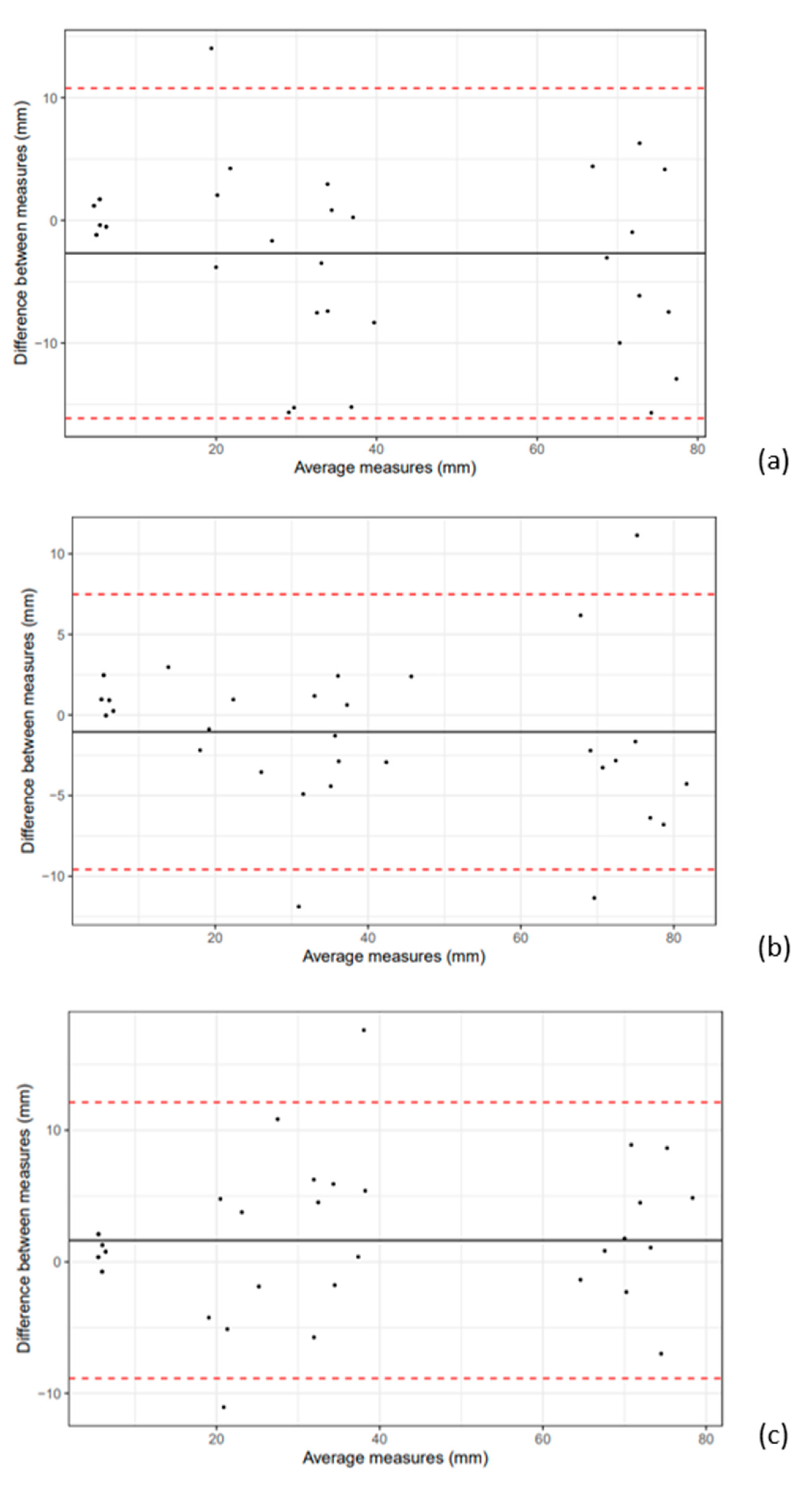
3.2. Intra- and Interobserver Variability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gy, H.; Kulcszr, M.; Rudas, P. Clinical endocrinology of thyroid gland function in ruminants. Vet. Med. 2002, 47, 199–210. [Google Scholar] [CrossRef]
- Kunz, P.L.; Blum, J.W. Relationships between energy balances and blood levels of hormones and metabolites in dairy cows during late pregnancy and early lactation. Z. Für Tierphysiol. Tierernährung Und Futterm. 1985, 54, 239–248. [Google Scholar] [CrossRef]
- Reist, M.; Erdin, D.K.; von Euw, D.; Tschümperlin, K.M.; Leuenberger, H.; Hammon, H.M.; Morel, C.; Philipona, C.; Zbinden, Y.; Künzi, N.; et al. Postpartum reproductive function: Association with energy, metabolic and endocrine status in high yielding dairy cows. Theriogenology 2002, 59, 1707–1723. [Google Scholar] [CrossRef]
- Castro, N.; Kawashima, C.; van Dorland, H.; Morel, I.; Miyamoto, A.; Bruckmaier, R. Metabolic and energy status during the dry period is crucial for the resumption of ovarian activity postpartum in dairy cows. J. Dairy Sci. 2012, 95, 5804–5812. [Google Scholar] [CrossRef] [PubMed]
- Kafi, M.; Tamadon, A.; Saeb, M.; Mirzaei, A.; Ansari-Lari, M. Relationships between thyroid hormones and serum energy metabolites with different patterns of postpartum luteal activity in high-producing dairy cows. Animal 2012, 6, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Tiirats, T. Thyroxine, Triiodothyronine and Reverse-Triiodothyronine Concentrations in Blood Plasma in Relation to Lactational Stage, Milk Yield, Energy and Dietary Protein Intake in Estonian Dairy Cows. Acta Vet. Scand. 1997, 38, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Gueorguiev, I.P. Thyroxine and triiodothyronine concentrations during lactation in dairy cows. Ann. Res. 1999, 48, 477–480. [Google Scholar] [CrossRef]
- Steinhoff, L.; Jung, K.; Meyerholz, M.; Heidekorn-Dettmer, J.; Hoedemaker, M.; Schmicke, M. Thyroid hormone profiles and TSH evaluation during early pregnancy and the transition period in dairy cows. Theriogenology 2019, 129, 23–28. [Google Scholar] [CrossRef]
- Thrift, T.A.; Bernal, A.; Lewis, A.W.; Neuendorff, D.A.; Willard, C.C.; Randel, R.D. Effects of induced hypothyroidism or hyperthyroidism on growth and reproductive performance of Brahman heifers. J. Anim. Sci. 1999, 77, 1833–1843. [Google Scholar] [CrossRef]
- Bernal, A.; DeMoraes, G.V.; Thrift, T.A.; Willard, C.C.; Randel, R.D. Effects of induced hypothyroidism on ovarian response to superovulation in Brahman (Bos indicus) cows. J. Anim. Sci. 1999, 77, 2749–2756. [Google Scholar] [CrossRef]
- Subcommittee on Beef Cattle Nutrition; Committee on Animal Nutrition; Board on Agriculture; National Research Council Minerals. Nutrient Requirements of Beef Cattle, 7th ed.; The National Academies Press: Washington, DC, USA, 2000; p. 68. [Google Scholar] [CrossRef]
- Guyot, H.; Lebreton, P.; Alves De Oliveira, L.; Sulon, J.; Beckers, J.F.; Rollin, F. Thyrotropin in newborn calves as a tool for diagnosing hypothyroidism. Cattle Pract. 2007, 15, 271–275. [Google Scholar]
- Fish, R.; Swanson, E. Effects of Excessive Intakes of Iodine upon Growth and Thyroid Function of Growing Holstein Heifers. J. Dairy Sci. 1982, 65, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, K.; Hidiroglou, M. Effects of Elevated Iodine in Milk Replacer on Calf Performance. J. Dairy Sci. 1990, 73, 804–807. [Google Scholar] [CrossRef] [PubMed]
- Bleul, U. Respiratory Distress Syndrome in Calves. Vet. Clin. N. Am. Food Anim. Pract. 2009, 25, 179–193. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Ahmed, N.H. History of disorders of thyroid dysfunction. East. Mediterr. Health J. 2005, 11, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Amino, N.; Arata, N. Thyroid dysfunction following pregnancy and implications for breastfeeding. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101438. [Google Scholar] [CrossRef]
- Zgliczynska, M.; Ostrowska, M.; Szymusik, I.; Ciebiera, M.; Kosinska-Kaczynska, K. Maternal thyroid function in multiple pregnancies—A systematic review. Front. Endocrinol. 2023, 13, 1044655. [Google Scholar] [CrossRef]
- Geng, X.; Chen, Y.; Li, S.; Wang, W.; Wu, W.; Sun, C.; Li, N.; Wang, L. Systematic review and meta-analysis on the influence of thyroid dysfunction in early pregnancy on pregnancy outcomes under ultrasound guidance. Ann. Palliat. Med. 2022, 11, 1001–1016. [Google Scholar] [CrossRef]
- Dighe, M.; Barr, R.; Bojunga, J.; Cantisani, V.; Chammas, M.C.; Cosgrove, D.O.; Cui, X.W.; Dong, Y.; Fenner, F.; Radzina, M.; et al. Thyroid Ultrasound: State of the Art Part 1–Thyroid Ultrasound reporting and Diffuse Thyroid Diseases. Med. Ultrason. 2017, 19, 79–93. [Google Scholar] [CrossRef]
- Gharib, H.; Papini, E.; Garber, J.R.; Duick, D.S.; Harrell, R.M.; Hegedüs, L.; Paschke, R.; Valcavi, R.; Vitti, P. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical Guidelines for Clinical Practice for the Diagnosis and Management of Thyroid Nodules-2016 Update Appendix. Endocr. Pract. 2016, 22 (Suppl. S1), 1–60. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Brenta, G.; Vaisman, M.; Sgarbi, J.; Bergoglio, L.M.; De Andrada, N.C.; Bravo, P.P.; Orlandi, A.M.; Graf, H. Clinical practice guidelines for the management of hypothyroidism. Arq. Bras. de Endocrinol. Metabol. 2013, 57, 265–291. [Google Scholar] [CrossRef] [PubMed]
- Wisner, E.R.; Nyland, T.G. Ultrasonography of the Thyroid and Parathyroid Glands. Vet. Clin. N. Am. Small Anim. Pract. 1998, 28, 973–991. [Google Scholar] [CrossRef] [PubMed]
- Brömel, C.; Pollard, R.E.; Kass, P.H.; Samii, V.F.; Davidson, A.P.; Nelson, R.W. Comparison of ultrasonographic characteristics of the thyroid gland in healthy small-, medium-, and large-breed dogs. Am. J. Vet. Res. 2006, 67, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.R.; Assis, M.M.Q.; Doiche, D.P.; Souza, L.P.; Pizzigatti, D.; Mamprim, M.J. Do Thyroid Ultrasonographic Features Change According to Age in Euthyroid Dogs? J. Vet. Med. Ser. C Anat. Histol. Embryol. 2014, 43, 468–473. [Google Scholar] [CrossRef]
- Reese, S.; Breyer, U.; Deeg, C.; Kraft, W.; Kaspers, B. Thyroid Sonography as an Effective Tool to Discriminate between Euthyroid Sick and Hypothyroid Dogs. J. Vet. Intern. Med. 2005, 19, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Rathmanner, M.; Rijkenhuizen, A.B.M. Ultrasonography of the upper cervical region (EUCR) in the horse. Pferdeheilkunde 2012, 28, 575–582. [Google Scholar] [CrossRef]
- Viana, G.F.; Carandina, L.; Hataka, A.; Midon, M.; Sarkis, C.A.; Filho, J.N.P.; Machado, V.M. Ultrasonographic features of the normal thyroid gland in adult horses. Pesqui. Vet. Bras. 2019, 39, 923–931. [Google Scholar] [CrossRef]
- Pankowski, F.; Paśko, S.; Bonecka, J.; Szaluś-Jordanow, O.; Mickiewicz, M.; Moroz, A.; Bartyzel, B.J. Ultrasonographic and anatomical examination of normal thyroid and internal parathyroid glands in goats. PLoS ONE 2020, 15, e0233685. [Google Scholar] [CrossRef]
- Pankowski, F.; Bartyzel, B.J.; Paśko, S.; Moroz, A.; Mickiewicz, M.; Szaluś-Jordanow, O.; Bonecka, J. CT appearance and measurements of the normal thyroid gland in goats. BMC Vet. Res. 2021, 17, 337. [Google Scholar] [CrossRef]
- Braun, U.; Föhn, J.; Pusterla, N. Ultrasonographic examination of the ventral neck region in cows. Am. J. Vet. Res. 1994, 55, 14–21. [Google Scholar] [PubMed]
- Noro, M.; Araneda, P.; Mieres, L.; Rudorf, H.; Chihuailaf, R.; Wittwer, F. Evaluación ultrasonográfica de la tiroides en crías de vacas lecheras tratadas con yodo en el preparto. Arch. Med. Vet. 2013, 45, 299–303. [Google Scholar] [CrossRef]
- Metzner, M.; Uebelhack, S.; Reese, S.; Klee, W.; Sauter-Louis, C. Developing an Accurate Method for Estimating Thyroid Volume in Calves Using Ultrasonography. Vet. Radiol. Ultrasound 2014, 56, 301–306. [Google Scholar] [CrossRef]
- Edmonson, A.J.; Lean, I.J.; Weaver, L.D.; Farver, T.; Webster, G. A Body Condition Scoring Chart for Holstein Dairy Cows. J. Dairy Sci. 1989, 72, 68–78. [Google Scholar] [CrossRef]
- Petrossians, P.; Petignot, S.; Benoit, A.; Beckers, A. Echographie de la Thyroïde; Graphmed SPRL: Liège, Belgium, 2015; 114. [Google Scholar]
- Ozgen, A.; Erol, C.; Kaya, A.; Ozmen, M.; Akata, D.; Akhan, O. Interobserver and intraobserver variations in sonographic measurement of thyroid volume in children. Eur. J. Endocrinol. 1999, 140, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Krönke, M.; Eilers, C.; Dimova, D.; Köhler, M.; Buschner, G.; Schweiger, L.; Konstantinidou, L.; Makowski, M.; Nagarajah, J.; Navab, N.; et al. Tracked 3D ultrasound and deep neural network-based thyroid segmentation reduce interobserver variability in thyroid volumetry. PLoS ONE 2022, 17, e0268550. [Google Scholar] [CrossRef]
- Taeymans, O.; Duchateau, L.; Schreurs, E.; Kramer, M.; Daminet, S.; Saunders, J.H. Intra- and Interobserver Variability of Ultrasonographic Measurements of the Thyroid Gland in Healthy Beagles. Vet. Radiol. Ultrasound 2005, 46, 139–142. [Google Scholar] [CrossRef]
- Jeeji, A.K.; Ekstein, S.F.; Ifelayo, O.I.; Oyemade, K.A.; Tawfic, S.S.; Hyde, R.J.; Laughlin, M.J.L., Jr.; Lohse, C.M.; Ma, A.F.M.; Kummer, T.; et al. Increased body mass index is associated with decreased imaging quality of point-of-care abdominal aortic ultrasonography. J. Clin. Ultrasound 2020, 49, 328–333. [Google Scholar] [CrossRef]
- Maar, M.; Lee, J.; Tardi, A.; Zheng, Y.-Y.; Wong, C.; Gao, J. Inter-transducer variability of ultrasound image quality in obese adults: Qualitative and quantitative comparisons. Clin. Imaging 2022, 92, 63–71. [Google Scholar] [CrossRef]
- Brömel, C.; Pollard, R.E.; Kass, P.H.; Samii, V.F.; Davidson, A.P.; Nelson, R.W. Ultrasonographic Evaluation of the Thyroïd Gland in Healthy, Hypothyroid, and Euthyroid Golden Retrievers with Nonthyroïdal Illness. J. Chem. Inf. Model. 2005, 53, 1689–1699. [Google Scholar] [CrossRef]
| Calves (n = 5) | Cows (n = 5) | |||
|---|---|---|---|---|
| LL | RL | LL | RL | |
| Height (mm) | 10.1 ± 2.4 | 8.8 ± 1.8 | 21 ± 4.6 | 22.8 ± 4.2 |
| Width (mm) | 20.4 ± 4.2 | 18.7 ± 3.2 | 37.2 ± 5.6 | 31.7 ± 5.5 * |
| Length (mm) | 33.5 ± 3.8 | 34.6 ± 2.8 | 72.5 ± 6.7 | 72.9 ± 5.4 |
| Isthmus thickness (mm) | 3.2 ± 0.6 | 5.7 ± 1.04 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eppe, J.; Petrossians, P.; Busoni, V.; Rollin, F.; Guyot, H. Technical Validation of Ultrasound Assessment of the Thyroid Gland in Cattle. Vet. Sci. 2023, 10, 322. https://doi.org/10.3390/vetsci10050322
Eppe J, Petrossians P, Busoni V, Rollin F, Guyot H. Technical Validation of Ultrasound Assessment of the Thyroid Gland in Cattle. Veterinary Sciences. 2023; 10(5):322. https://doi.org/10.3390/vetsci10050322
Chicago/Turabian StyleEppe, Justine, Patrick Petrossians, Valeria Busoni, Frédéric Rollin, and Hugues Guyot. 2023. "Technical Validation of Ultrasound Assessment of the Thyroid Gland in Cattle" Veterinary Sciences 10, no. 5: 322. https://doi.org/10.3390/vetsci10050322
APA StyleEppe, J., Petrossians, P., Busoni, V., Rollin, F., & Guyot, H. (2023). Technical Validation of Ultrasound Assessment of the Thyroid Gland in Cattle. Veterinary Sciences, 10(5), 322. https://doi.org/10.3390/vetsci10050322







