Evaluation of the Local Immune Response to Hydatid Cysts in Sheep Liver
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Selection
2.2. Ethic Statement
2.3. Liver Macroscopic Examination
2.4. Histopathological Examination
2.5. Immunohistochemistry
2.6. Real-Time Reverse-Transcription Polymerase Chain Reaction (RT-PCR) Analysis
2.7. Statistical Analysis
3. Results
3.1. Gross Examination
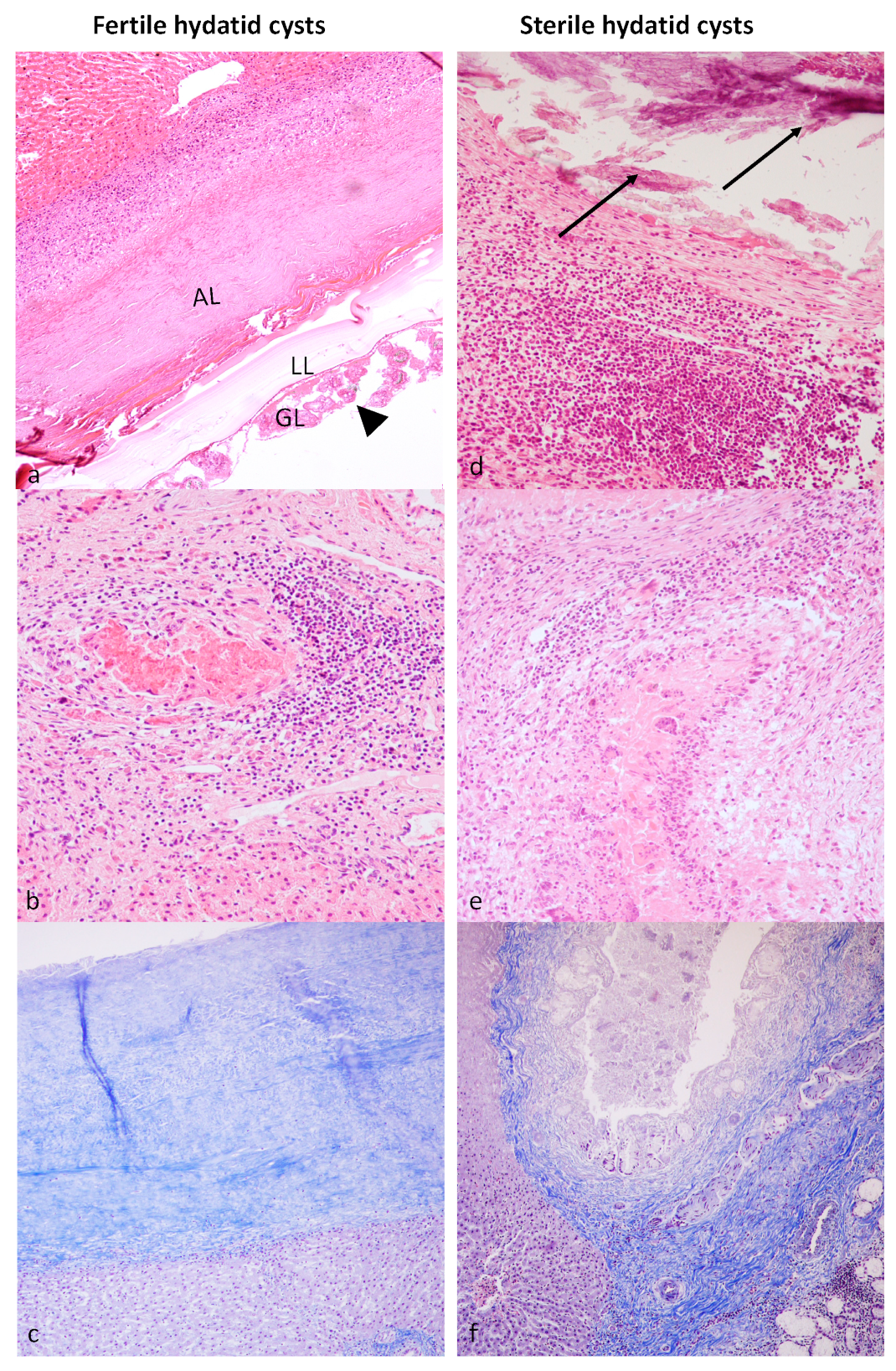
3.2. Histological Examination
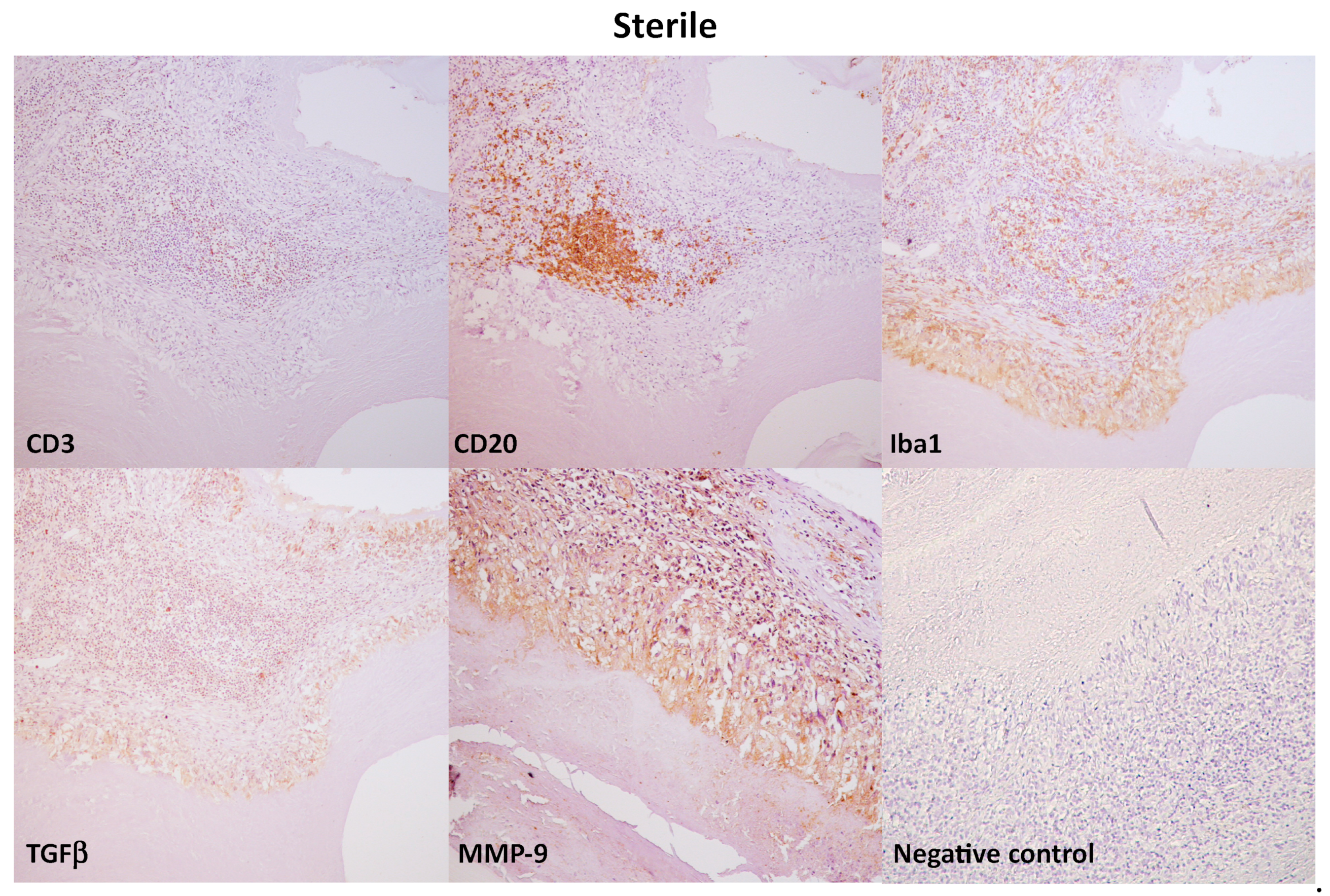
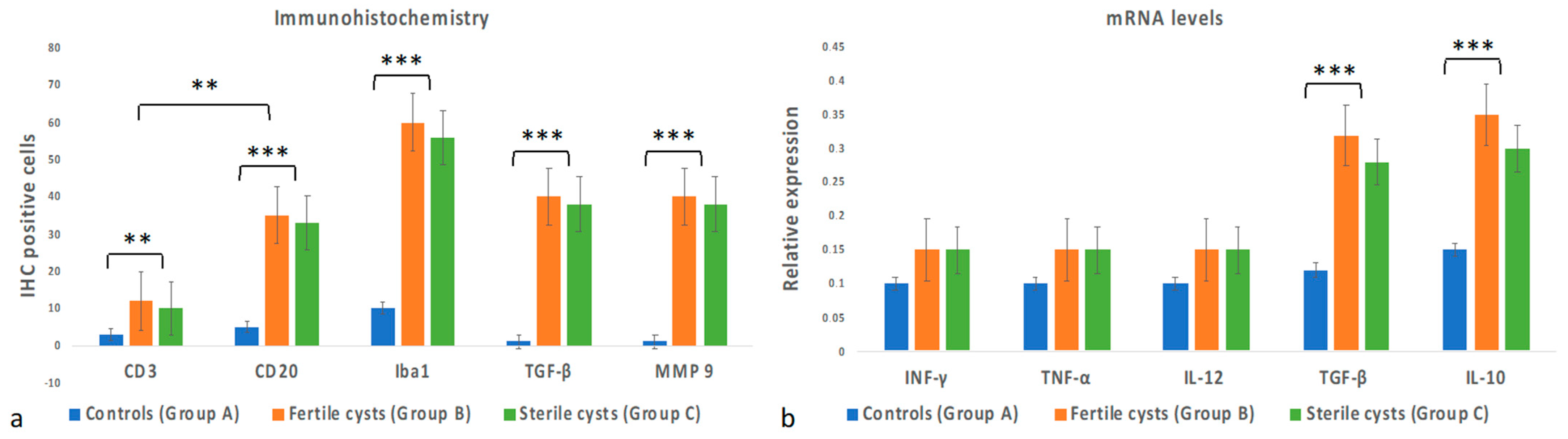
3.3. Immunohistochemical Evaluation
3.4. Cytokine Gene Expression Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bosco, A.; Alves, L.C.; Cociancic, P.; Amadesi, A.; Pepe, P.; Morgoglione, M.E.; Maurelli, M.P.; Ferrer-Miranda, E.; Santoro, K.R.; Nascimento Ramos, R.A.; et al. Epidemiology and spatial distribution of Echinococcus granulosus in sheep and goats slaughtered in a hyperendemic European Mediterranean area. Parasites Vectors 2021, 14, 421. [Google Scholar] [CrossRef]
- Atmaca, H.T.; Gazyagci, A.N.; Terzi, O.S.; Sumer, T. Role of stellate cells in hepatic echinococcosis in cattle. J. Parasit. Dis. 2019, 43, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, M.; Stoore, C.; Hidalgo, C.; Correa, F.; Hernandez, M.; Benavides, J.; Ferreras, M.C.; Saenz, L.; Paredes, R. Lymphocyte populations in the adventitial layer of hydatid cysts in cattle: Relationship with cyst fertility status and Fasciola hepatica co-infection. Vet. Pathol. 2020, 57, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Beigh, A.B.; Darzi, M.M.; Bashir, S.; Kashani, B.; Shah, A.; Shah, S.A. Gross and histopathological alterations associated with cystic echinococcosis in small ruminants. J. Parasit. Dis. 2017, 41, 1028–1033. [Google Scholar] [CrossRef]
- Atmaca, H.T. Determination of macrophage types by immunohistochemical methods in the local immune response to liver hydatid cysts in sheep. Acta Trop. 2022, 229, 106364. [Google Scholar] [CrossRef] [PubMed]
- Abo-Aziza, F.A.M.; Hendawy, S.H.M.; Oda, S.S.; Aboelsoued, D.; El Shanawany, E.E. Cell-mediated and humoral immune profile to hydatidosis among naturally infected farm animals. Vet. World 2020, 13, 214–221. [Google Scholar] [CrossRef]
- Rickard, M.D.; Williams, J.F. Hydatidosis/cysticercosis: Immune mechanisms and immunization against infection. Adv. Parasitol. 1982, 21, 229–296. [Google Scholar] [CrossRef]
- Tamarozzi, F.; Mariconti, M.; Neumayr, A.; Brunetti, E. The intermediate host immune response in cystic echinococcosis. Parasite Immunol. 2016, 38, 170–181. [Google Scholar] [CrossRef]
- Zhang, W.; Wen, H.; Li, J.; Lin, R.; McManus, D.P. Immunology and immunodiagnosis of cystic echinococcosis: An update. Clin. Dev. Immunol. 2012, 2012, 101895. [Google Scholar] [CrossRef]
- Siracusano, A.; Delunardo, F.; Teggi, A.; Ortona, E. Host-parasite relationship in cystic echinococcosis: An evolving story. Clin. Dev. Immunol. 2012, 2012, 639362. [Google Scholar] [CrossRef]
- Díaz, Á. Immunology of cystic echinococcosis (hydatid disease). Br. Med. Bull. 2017, 124, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Heath, D.D.; Jensen, O.; Lightowlers, M.W. Progress in control of hydatidosis using vaccination—A review of formulation and delivery of the vaccine and recommendations for practical use in control programmes. Acta Trop. 2003, 85, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Vismarra, A.; Mangia, C.; Passeri, B.; Brundu, D.; Masala, G.; Ledda, S.; Mariconti, M.; Brindani, F.; Kramer, L.; Bacci, C. Immuno-histochemical study of ovine cystic echinococcosis (Echinococcus granulosus) shows predominant T cell infiltration in established cysts. Vet. Parasitol. 2015, 209, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Cringoli, G.; Pepe, P.; Bosco, A.; Maurelli, M.P.; Baldi, L.; Ciaramella, P.; Musella, V.; Buonanno, M.L.; Capuano, F.; Corrado, F.; et al. An integrated approach to control Cystic Echinococcosis in southern Italy. Vet. Parasitol. 2021, 290, 109347. [Google Scholar] [CrossRef] [PubMed]
- Deplazes, P.; Rinaldi, L.; Alvarez Rojas, C.A.; Torgerson, P.R.; Harandi, M.F.; Romig, T.; Antolova, D.; Schurer, J.M.; Lahmar, S.; Cringoli, G.; et al. Global Distribution of Alveolar and Cystic Echinococcosis. J. Adv. Parasitol. 2017, 95, 315–493. [Google Scholar]
- Borriello, G.; Guccione, J.; Di Loria, A.; Bosco, A.; Pepe, P.; Prisco, F.; Cringoli, G.; Paciello, O.; Rinaldi, L.; Ciaramella, P. Fast Focus Ultrasound Liver Technique for the Assessment of Cystic Echinococcosis in Sheep. Animals 2021, 11, 452. [Google Scholar] [CrossRef]
- Mathewos, M.; Dawa, D.; Yirgalem, M.; Denano, T.; Fesseha, H. Cystic echinococcosis in cattle slaughtered at a slaughterhouse in Gessa, southern Ethiopia. Parasite Epidemiol. Control 2022, 18, e00262. [Google Scholar] [CrossRef]
- Piegari, G.; Pepe, P.; De Biase, D.; d’Aquino, I.; Bosco, A.; Cringoli, G.; Papparella, S.; Rinaldi, L.; Paciello, O. Immunopathological Response, Histological Changes, Parasitic Burden, and Egg Output in Sheep Naturally Infected by Dicrocoelium dendriticum. Animals 2021, 11, 546. [Google Scholar] [CrossRef]
- De Biase, D.; Piegari, G.; Prisco, F.; Cimmino, I.; Pirozzi, C.; Mattace Raso, G.; Oriente, F.; Grieco, E.; Papparella, S.; Paciello, O. Autophagy and NLRP3 inflammasome crosstalk in neuroinflammation in aged bovine brains. J. Cell. Physiol. 2020, 235, 5394–5403. [Google Scholar] [CrossRef]
- Grossman, P.C.; Schneider, D.A.; Herndon, D.R.; Knowles, D.P.; Highland, M.A. Differential pulmonary immunopathology of domestic sheep (Ovis aries) and bighorn sheep (Ovis canadensis) with Mycoplasma ovipneumoniae infection: A retrospective study. Comp. Immunol. Microbiol. Infect. Dis. 2021, 76, 101641. [Google Scholar] [CrossRef]
- Dekker, S.; van Geemen, D.; van den Bogaerdt, A.J.; Driessen-Mol, A.; Aikawa, E.; Smits, A.I.P.M. Sheep-Specific Immunohistochemical Panel for the Evaluation of Regenerative and Inflammatory Processes in Tissue-Engineered Heart Valves. Front. Cardiovasc. Med. 2018, 15, 105. [Google Scholar] [CrossRef]
- Hewitt, S.M.; Baskin, D.G.; Frevert, C.W.; Stahl, W.L.; Rosa-Molinar, E. Controls for immunohistochemistry: The Histochemical Society’s standards of practice for validation of immunohistochemical assays. J. Histochem. Cytochem. 2014, 62, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Budhia, S.; Haring, L.F.; McConnell, I. and Blacklaws, B.A. Quantitation of ovine cytokine mRNA by real-time RT-PCR. J. Immunol. Methods 2006, 309, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Haçariz, O.; Sayers, G.; Flynn, R.J.; Lejeune, A.; Mulcahy, G. IL-10 and TGF-β1 are associated with variations in fluke burdens following experimental fasciolosis in sheep. Parasite Immunol. 2009, 31, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Gruttadauria, S.; Biondi, A.; Marventano, S.; Mistretta, A. Worldwide epidemiology of liver hydatidosis including the Mediterranean area. World J. Gastroenterol. 2012, 18, 1425–1437. [Google Scholar] [CrossRef] [PubMed]
- Rogan, M.T.; Bodell, A.J.; Craig, P.S. Post-encystment/established immunity in cystic echinococcosis: Is it really that simple? Parasite Immunol. 2015, 37, 1–9. [Google Scholar] [CrossRef]
- Hidalgo, C.; Stoore, C.; Strull, K.; Franco, C.; Corrêa, F.; Jiménez, M.; Hernández, M.; Lorenzatto, K.; Ferreira, H.B.; Galanti, N.; et al. New insights of the local immune response against both fertile and infertile hydatid cysts. PLoS ONE 2019, 14, e0211542. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, F.; Hidalgo, C.; Stoore, C.; Jiménez, M.; Hernández, M.; Paredes, R. Cattle co-infection of Echinococcus granulosus and Fasciola hepatica results in a different systemic cytokine profile than single parasite infection. PLoS ONE 2020, 15, e0238909. [Google Scholar] [CrossRef]
- Paredes, R.; Godoy, P.; Rodríguez, B.; García, M.P.; Cabezón, C.; Cabrera, G.; Jiménez, V.; Hellman, U.; Sáenz, L.; Ferreira, A.; et al. Bovine (Bos taurus) humoral immune response against Echinococcus granulosus and hydatid cyst infertility. J. Cell. Biochem. 2011, 112, 189–199. [Google Scholar] [CrossRef]
- Gago da Graça, C.; van Baarsen, L.G.M.; Mebius, R.E. Tertiary Lymphoid Structures: Diversity in Their Development, Composition, and Role. J. Immunol. 2021, 206, 273–281. [Google Scholar] [CrossRef]
- Maizels, R.M.; McSorley, H.J. Regulation of the host immune system by helminth parasites. J. Allergy Clin. Immunol. 2016, 138, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Xu, H.W.; Hao, W.T.; Sun, F.F.; Qin, Y.F.; Hao, S.S.; Liu, H.; Cao, J.P.; Shen, Y.J.; Zheng, K.Y. The excretory-secretory products of Echinococcus granulosus protoscoleces stimulated IL-10 production in B cells via TLR-2 signaling. BMC Immunol. 2018, 19, 29. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, T.; Tedder, T.F. Identifying regulatory B cells (B10 cells) that produce IL-10 in mice. Methods Mol. Biol. 2011, 677, 99–111. [Google Scholar] [PubMed]
- Mourglia-Ettlin, G.; Marqués, J.M.; Chabalgoity, J.A.; Dematteis, S. Early peritoneal immune response during Echinococcus granulosus establishment displays a biphasic behavior. PLoS Negl. Trop. Dis. 2011, 5, e1293. [Google Scholar] [CrossRef] [PubMed]
- Lampropoulou, V.; Hoehlig, K.; Roch, T.; Neves, P.; Calderón Gómez, E.; Sweenie, C.H.; Hao, Y.; Freitas, A.A.; Steinhoff, U.; Anderton, S.M.; et al. TLR-activated B cells suppress T cell-mediated autoimmunity. J. Immunol. 2008, 180, 4763–4773. [Google Scholar] [CrossRef]
- Saraiva, M.; O’Garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef]
- Yanaba, K.; Bouaziz, J.D.; Haas, K.M.; Poe, J.C.; Fujimoto, M.; Tedder, T.F. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity 2008, 28, 639–650. [Google Scholar] [CrossRef]
- Mondragón-de-la-Peña, C.; Ramos-Solís, S.; Barbosa-Cisneros, O.; Rodríguez-Padilla, C.; Tavizón-García, P.; Herrera-Esparza, R. Echinococcus granulosus down regulates the hepatic expression of inflammatory cytokines IL-6 and TNF alpha in BALB/c mice. Parasite 2002, 9, 351–356. [Google Scholar] [CrossRef]
- Amri, M.; Mezioug, D.; Touil-Boukoffa, C. Involvement of IL-10 and IL-4 in evasion strategies of Echinococcus granulosus to host immune response. Eur. Cytokine Netw. 2009, 20, 63–68. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, C.; Li, L.; Bi, X.; Li, L.; Yang, S.; Zhang, N.; Wang, H.; Yang, N.; Abulizi, A.; et al. The local immune response during Echinococcus granulosus growth in a quantitative hepatic experimental model. Sci. Rep. 2019, 9, 19612. [Google Scholar] [CrossRef]
- Riganò, R.; Buttari, B.; Profumo, E.; Ortona, E.; Delunardo, F.; Margutti, P.; Mattei, V.; Teggi, A.; Sorice, M.; Siracusano, A. Echinococcus granulosus antigen B impairs human dendritic cell differentiation and polarizes immature dendritic cell maturation towards a Th2 cell response. Infect. Immun. 2007, 75, 1667–1678. [Google Scholar] [CrossRef] [PubMed]
- Baz, A.; Ettlin, G.M.; Dematteis, S. Complexity and function of cytokine responses in experimental infection by Echinococcus granulosus. Immunobiology 2006, 211, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Siracusano, A.; Delunardo, F.; Teggi, A.; Ortona, E. Cystic echinococcosis: Aspects of immune response, immunopathogenesis and immune evasion from the human host. Endocr. Metab. Immune Disord. Drug Targets 2012, 12, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Amri, M.; Aissa, S.A.; Belguendouz, H.; Mezioug, D.; Touil-Boukoffa, C. In vitro antihydatic action of IFN- gamma is dependent on the nitric oxide pathway. J. Interferon Cytokine Res. 2007, 27, 781–787. [Google Scholar] [CrossRef]
- Trinchieri, G. Interleukin-12: A cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood 1994, 84, 4008–4027. [Google Scholar] [CrossRef]
- Del Vecchio, M.; Bajetta, E.; Canova, S.; Lotze, M.T.; Wesa, A.; Parmiani, G.; Anichini, A. Interleukin-12: Biological properties and clinical application. Clin. Cancer Res. 2007, 13, 4677–4685. [Google Scholar] [CrossRef]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-gamma: An overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004, 75, 163–189. [Google Scholar] [CrossRef]
- Scott, P. IL-12: Initiation cytokine for cell-mediated immunity. Science 1993, 260, 496–497. [Google Scholar] [CrossRef]
- Zeghir-Bouteldja, R.; Amri, M.; Bouaziz, S.; Mezioug, D.; Touil-Boukoffa, C. Comparative study of nitric oxide (NO) production during human hydatidosis: Relationship with cystic fluid fertility. Parasitol. Res. 2013, 112, 649–654. [Google Scholar] [CrossRef]
- Faridnia, R.; Tolouei, S.; Khanahmad-Shahreza, H.; Kalani, H.; Yousefi-Darani, H. Evaluating the gene expression level of IL-12 in the fibrous layer of hepatic hydatid cysts isolated from slaughtered animals. Comp. Clin. Pathol. 2015, 24, 1193–1196. [Google Scholar] [CrossRef]
- Marco, M.; Baz, A.; Fernandez, C.; Gonzalez, G.; Hellman, U.; Salinas, G.; Nieto, A. A relevant enzyme in granulomatous reaction, active matrix metalloproteinase-9, found in bovine Echinococcus granulosus hydatid cyst wall and fluid. Parasitol. Res. 2006, 100, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Boubaker, G.; Hemphill, A.; Huber, C.O.; Spiliotis, M.; Babba, H.; Gottstein, B. Prevention and Immunotherapy of Secondary Murine Alveolar Echinococcosis Employing Recombinant EmP29 Antigen. PLoS Negl. Trop. Dis. 2015, 9, e0003795. [Google Scholar] [CrossRef] [PubMed]


| Antibody | Specificity | Epitope Demasking | Dilution | Reference |
|---|---|---|---|---|
| CD3 (IS503, rabbit polyclonal antibody, DAKO). | Pan T cell marker | Citrate pH 6, 20 min | 1:200 | [18,20] |
| CD20 (ACR3004B, rabbit polyclonal antibody, Biocompare) | Pan B cell marker | Citrate pH 6, 20 min | 1:50 | [20] |
| Iba-1 (019_19741, rabbit polyclonal antibody, WAKO). | Macrophages | Citrate pH 6, 20 min | 1:800 | [20] |
| TGFβ (ab9758, rabbit polyclonal antibody, AbCam) | Macrophages | Citrate pH 6, 20 min | 1:200 | [21] |
| MMP-9 (ab38898, rabbit polyclonal antibody, Abcam). | Macrophages, Fibroblasts | Citrate pH 6, 20 min | 1:200 | [21] |
| Ovine Gene Target | Primer Sequences | Reference |
|---|---|---|
| TNF-α | Forward: GGTGCCTCAGCCTCTTCTC | [23] |
| Reverse: GAACCAGAGGCCTGTTGAAG | [23] | |
| INF-γ | Forward: CAAATTCCGGTGGATGATCTG | [24] |
| Reverse: GCGACAGGTCATTCATCACCTT | [24] | |
| IL-12 | Forward: TCTCGGCAGGTGGAAGTCA | [23] |
| Reverse: ACTTTGGCTGAGGTTTGGTCTG | [23] | |
| IL-10 | Forward: CCAGGATGGTGACTCGACTAGAC | [24] |
| Reverse: TGGCTCTGCTCTCCCAGAAC | [24] | |
| TGF-β | Forward: AAGCGGAAGGGCATCGA | [24] |
| Reverse: CGAGCCGAAGTTTGGACAAA | [24] | |
| GAPDH | Forward: GGCGTGAACCACGAGAAGTATAA | [23] |
| Reverse: CCCTCCACGATGCCAAAGT | [23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Biase, D.; Prisco, F.; Pepe, P.; Bosco, A.; Piegari, G.; d’Aquino, I.; Russo, V.; Papparella, S.; Maurelli, M.P.; Rinaldi, L.; et al. Evaluation of the Local Immune Response to Hydatid Cysts in Sheep Liver. Vet. Sci. 2023, 10, 315. https://doi.org/10.3390/vetsci10050315
De Biase D, Prisco F, Pepe P, Bosco A, Piegari G, d’Aquino I, Russo V, Papparella S, Maurelli MP, Rinaldi L, et al. Evaluation of the Local Immune Response to Hydatid Cysts in Sheep Liver. Veterinary Sciences. 2023; 10(5):315. https://doi.org/10.3390/vetsci10050315
Chicago/Turabian StyleDe Biase, Davide, Francesco Prisco, Paola Pepe, Antonio Bosco, Giuseppe Piegari, Ilaria d’Aquino, Valeria Russo, Serenella Papparella, Maria Paola Maurelli, Laura Rinaldi, and et al. 2023. "Evaluation of the Local Immune Response to Hydatid Cysts in Sheep Liver" Veterinary Sciences 10, no. 5: 315. https://doi.org/10.3390/vetsci10050315
APA StyleDe Biase, D., Prisco, F., Pepe, P., Bosco, A., Piegari, G., d’Aquino, I., Russo, V., Papparella, S., Maurelli, M. P., Rinaldi, L., & Paciello, O. (2023). Evaluation of the Local Immune Response to Hydatid Cysts in Sheep Liver. Veterinary Sciences, 10(5), 315. https://doi.org/10.3390/vetsci10050315










