Simple Summary
Chemical signals are essential for communication between living organisms. Dogs possess two sensory organs enabling chemical communication: the main olfactory system and the vomeronasal organ (VNO). Additionally, contact chemoreception is also pertinent, by which non-volatile molecules, including but not limited to proteins, are recognized as chemical signals. However, non-volatile chemical signals have been sparsely studied in dogs. Therefore, we aimed to examine the urinary proteins of female domestic dogs during the estrus and anestrus phases to detect and identify such non-volatile chemical signals.
Abstract
The presence and identity of non-volatile chemical signals remain elusive in canines. In this study, we aim to evaluate the urinary proteins of female domestic dogs in the estrus and anestrus phases to evidence the presence of non-volatile chemical signals and to elucidate their identities. We collected urine samples from eight female dogs in the estrus and anestrus phases. A total of 240 proteins were identified in the urine samples using liquid chromatography–mass spectrometry (LC–MS analysis). The comparison of the proteins revealed a significant difference between the estrus and anestrus urine. We identified proteins belonging to the lipocalin family of canines (beta-lactoglobulin-1 and beta-lactoglobulin-2, P33685 and P33686, respectively), one of whose function was the transport of pheromones and which was present only in the estrus urine samples. Moreover, proteins such as Clusterin (CLU), Liver-expressed antimicrobial peptide 2 (LEAP2), and Proenkephalin (PENK) were more abundant in the estrus urine when compared to the anestrus urine. LEAP2 was recently described as a ghrelin receptor antagonist and implicated in regulating food intake and body weight in humans and mice. Proenkephalin, a polypeptide hormone cleaved into opioid peptides, was also recognized as a candidate to determine kidney function. As of yet, none of these have played a role in chemical communication. Clusterin, an extracellular chaperone protecting from protein aggregation implicated in stress-induced cell apoptosis, is a plausible candidate in chemical communication, which is a claim that needs to be ascertained further. Data are available via ProteomeXchange with the identifier PXD040418.
1. Introduction
Communication using chemical signals is widespread in the world of living organisms. A variety of information regarding different physiological and pathological conditions in animals is transmitted through this mode [1,2]. Semiochemical communication using sex pheromones is one of the best-known examples of this mechanism. In dogs, sex pheromones secreted by a female during the proestrus and estrus phases are responsible for the modulation of physiology and behavior of male dogs [3,4,5,6,7]. While the composition of volatile compounds in the urine during various stages of the ovarian cycle in female canines has been extensively studied, current studies also emphasize the role of less volatile compounds [7,8,9,10,11]. Such compounds, present in the female secretions and collected by males during direct contact with the female (licking a vulva), may be involved in inter-individual communication and recognition of sexual attractiveness [11].
Like many other vertebrates, dogs use two sense organs for chemical communication: the main olfactory system and the vomeronasal organ (VNO). Both of these organs were reported to function in chemical signaling; however, the molecules perceived by the two systems vary. For instance, the MOS detects general odorants; whereas, the VNO, in some species, is reported in the detection of pheromones. However, in dogs, the vomeronasal type 2 receptor is missing, which raises the question of whether the VNO is functional [1,2,12,13,14]. Evaluation of the detected chemical compounds (including odors) helps assess the reproductive status of the signal emitter. It is also important to note that the chemical signals are used by the estrus females to attract males. However, exhibition of consummatory sexual behaviors is a multi-step process [6]. Similar to other species, male dogs approach, sniff, and lick the vulva of the females. The peculiar behavior is that the males also lick the urine of females [7,11,15,16], which could facilitate the transfer of volatile and non-volatile compounds. Indeed, the volatile and non-volatile compounds play an important role in estrus detection [11].
Urinary proteins are unequivocally vital in chemical communication. For instance, MUPs and α2u-globulins are the best-studied chemical signals reported in the urine of mice and rats, respectively. MUPs are lipocalins (proteins that transport small hydrophobic molecules) that bind with pheromones [13]. In mice, urine-derived scent marks have multiple roles in communication, mainly because of MUPs and other pheromones [17]. MUPs provide stability to the bound pheromones and can also function as pheromones themselves. It has been shown that MUPs modulate the behavior of males in that it promotes inter-male aggression [18,19]. Additionally, MUPs also stimulate reproductive behaviors and accelerate puberty attainment in females. Some of these functions are postulated as not being unique to rats and mice alone [20]. Mentioned above, Logan et al. [20], in evaluating parallel expansions of non-rodent MUP clusters, found that apart from other species, such as pigs, baboons, chimpanzees, bush babies, and orangutans, dogs also have a single MUP gene. Studies on rats by Rajkumar et al. [21] detected alpha (2u)-globulin and demonstrated its functional analogy in the Indian commensal rat (Rattus rattus). Changes in the composition and concentration of the volatile compounds are considered to develop estrus detection methods in some species [10,22,23,24,25]. Evaluation of the urinary protein profile (unique proteins at a particular phase or the difference in the concentration) was also conducted in several species [25,26,27,28,29]. However, information about urinary proteomes that may have implications in estrus detection is scanty for domestic dogs.
Ferlizza et al. [30] stated that urine is an ideal sample for the studies of proteomics and metabolomics; however, specific urinary biomarkers are currently lacking in dogs. The urinary proteins were investigated in regard to kidney diseases, emphasizing the potential of a dog as an animal model for human disease states [28,30,31]. A recent study [32] also stated that the body fluids of dogs still need to be better characterized in the context of proteomic studies.
The serum proteome was mapped using 2D PAGE in humans [33], horses [34], and dogs [35]. Altherton et al. [36] also characterized the canine serum proteome using mass spectrometry; wherein, 32 proteins were identified, and the reference ranges for albumin and globulin sub-fractions were established in 17 dogs. In a subsequent study, the changes in the canine serum proteome were attributed to the homeostatic disturbance resulting from various diseases [37]. Interestingly, Szczubiał et al. [38] described plasma proteins in pregnant and non-pregnant female dogs. In contrast, Dąbrowski and Franco Martínez described the roles of blood and salivary acute proteins in the condition known as pyometra [39,40]. Despite this, neither urinary proteomes nor reproduction-related aspects of proteomes have been investigated in canines.
Apart from blood, other body fluids (e.g., cerebrospinal fluid (CSF), follicular fluid), as well as tissues (e.g., parietal cortex) were investigated in dogs in the context of proteome evaluation [41]. In the aforementioned study, in both genders, the urine contained proteins derived from the serum through ultrafiltration and incomplete reabsorption. For instance, albumin was a predominant protein in the urine. Authors also identified unique proteins in the urine of males and females, confirming the sexual dimorphism urinary proteins in canines. Teinfalt et al. [42] identified a prostate-specific protein (PSP; 30 kDa) in the urine of intact males, which was absent in females and castrated males. This study indicated that the composition of the urine collected during natural micturition and cystocentesis varied and underscored the involvement of the prostate gland in regulating the urinary constituents.
Studies on chemical communication in canines hitherto mainly focused on volatile compounds. Taking this into account, in this study, we aimed to evaluate the changes in the composition of the female canine urinary proteome during various stages of the ovarian cycle to ascertain any relevant biomarkers of estrus.
2. Materials and Methods
2.1. Ethics Statement and Animals
The study was conducted following the regulations on animal experimentation and guidelines for the use of animals in research. The experimental protocol was approved by the 2nd Local Commission for Animal Experimentation in Wrocław, Poland (permission no. 17/2017). The animals used in the experiments were Beagle breed dogs belonging to the Local Experimental Kennel and patients of the local clinic of reproduction.
2.2. Sample Collection
Urine Sampling
Midstream urine samples from the healthy female donor dogs were collected in the morning during spontaneous urination using a sterile steel ladle. Samples were stored in glass vials at −20 °C. The experiment was conducted with eight individuals, and twelve samples were collected. The samples after pooling constituted nine experiments:
- One animal in estrus only; single sample (Female 1).
- Two animals in estrus; two samples each (Female 2 and Female 3):
- ○
- One estrus sample of Female 3 contributed to pool P1.
- ○
- Additional sample of Female 2 in anestrus, out of which a portion was analyzed independently and the remaining contributed to pool P2.
- One animal in estrus and anestrus; one sample each (Female 4):
- ○
- Estrus sample was used as part of pool P1.
- ○
- Due to low volume, anestrus sample was used as part of pool P2.
- Four immature ones (constituting experimental pools P3: Female 5 and Female 6; P4: Female 7 and Female 8).
- Exact sample coding is described in Supplementary Materials along with the specific protein results. Sample scheme is also further described in Table 1.
 Table 1. Samples included in the study.
Table 1. Samples included in the study.
2.3. Determination of the Phases of Cycle
The estrus cycle stages’ determination was based on vaginal cytology and plasma progesterone concentrations. Additionally, the consummatory sexual behaviors of male dogs toward females and owners’ observations of the female dogs were also considered [10,43].
2.3.1. Vaginal Cytological Examination
Vaginal smear samples were stained with Haemacolor® (Merck KGaA, Darmstadt, Germany) stain, and based on the percentage of the cornified superficial cells, the estrus cycle stages were evaluated [44].
2.3.2. Progesterone Level Evaluation
The plasma progesterone concentration was determined by an enzyme-linked fluorescence assay (ELFA; mini VIDAS® Biomerieux, Marcy-L’étoile, France) with the mean level of progesterone 21.66 ng/mL (with a standard deviation of 0.823 ng/mL, i.e., coefficient of variation (CV) of 3.8%) [45].
2.4. Proteomic Approach
2.4.1. BCA Assay
The urine samples (n = 18) were centrifuged (1000× g, 10 min, 4 °C) to remove any insoluble particles and cell debris. The resulted supernatants were diluted 10-fold with 2 M urea and stored as aliquots of 1.5 mL at −80 °C to use in protein measurement. Protein concentration in the urine samples was determined using Pierce™ BCA Protein Assay Kit (Thermo Scientific, Waltham, MA, USA).
2.4.2. Sample Pooling
Some samples from the same stage (either estrus or anestrus) were pooled to facilitate experiments; whereas, several samples were analyzed individually. This also created the reference sample, which was used in every run for inter-run comparison and scaling.
2.4.3. Reduction, Alkylation, Digestion, and Tandem Mass Tag (TMT) Labeling
FASP (Filter-Aided Sample Preparation) was used to prepare samples for mass spectrometric analysis. In FASP, a mass weight cut-off of 3 kDa was used. The urine samples containing 80 µg of protein were treated with 8 M urea (Sigma-Aldrich, St. Louis, MO, USA) and 10 mM DTT (dithiothreitol, Sigma-Aldrich) (prepared in 100 mM trimethylammonium bicarbonate (TEAB)) for 1 h at 55 °C. Subsequently, the samples were alkylated with 17 mM iodoacetamide (Thermo Scientific) in 100 mM TEAB at room temperature for 30 min. After incubation, the samples were transferred to cut-off membrane-fitted tubes and centrifuged at 10,000× g for 30 min. The resulting flow-through was collected from the lower portion of the tube and digested with trypsin (sample: trypsin at a ratio of 40:1) in 50 mM TEAB (Promega) at 37 °C for 18 h. The peptides were labeled with the TMTsixplex (Tandem Mass Tags) Label Reagent Set (Thermo Scientific) according to the manufacturer’s instructions. The resulting samples were stored at −80 °C until further processing. All the samples were prepared in triplicates. To facilitate comparison between samples, we included the samples from Pool 1 in both TMT six-plexes. The sample division across six-plexes can be found in Supplementary Table S2.
2.4.4. LC–MS/MS
All the measurements were performed using LTQ Elite Orbitrap ETD (Thermo Scientific, USA) connected to the Easy nLC 1000 chromatograph (Thermo Scientific) at the Mass Spectrometry Laboratory of Łukasiewicz Research Network, Polish Center for Technology Development, Wrocław, Poland. Peptides were trapped using Acclaim PepMap C-18, 2 cm trap column (Thermo Scientific) and separated on Acclaim PepMap C-18 column (100 Å, 500 mm × 0.075 mm × 3 μm) (Thermo Scientific) in ambient temperature. Mobile phases A and B were 0.1% formic acid in water and in acetonitrile: water [90:10 (v/v)], respectively. Five microliters of the sample was injected at a flow rate of 300 nL/min with a gradient from 2 to 55% of phase B (during 200 min). The external calibration of mass spectrometer with LTQ Velos Positive calibration standard was employed, achieving the standard deviation of measurements below 1 ppm. Measurements were performed in positive ion mode with m/z ratio between 110 and 2000. The capillary voltage was 3 kV. Higher energy collisional dissociation (HCD) fragmentation of top 10 peaks was employed with normalized collision energy set to 35 eV in 1 m/z isolation window with minimum 2+ charge state of parent ion and dynamic exclusion for 30 s after two spectra.
2.4.5. Protein Identification and Quantification
Mass spectra were processed with Proteome Discoverer 2.4, and Sequest HT search engine was used to extract and annotate MS/MS spectra. To assign spectra to canine proteomes, we conducted a Sequest HT search against a custom database, which comprised SwissProt and TrEMBL protein sequences of Canis lupus familiars and its subspecies, which were extracted from UniProtKB 2022_12. Trypsin was selected as an enzyme with a maximum of two missed cleavages. Maximum precursor and fragment tolerances were set to 20 ppm and 0.1 Da, respectively, to permit for higher sensitivity of the experiment. We also explored a more stringent set of thresholds of 10 ppm/0.05 Da, as well as 10 ppm/0.02 Da. Modifications were set as follows: quantification—TMT 6plex; static modification—carbamidomethyl (C); and dynamic modifications—acetyl (protein N-term) and oxidation (M). Reporter ion quantification was performed using Proteome Discoverer 2.4. Spectra were normalized to the total amount of peptides in the sample and scaled to the reference sample channel (TMT 126 in urine samples). Unique and razor peptides were used for quantification with at least two peptide matches per protein. The false discovery rate was set to 0.01 (strict) and 0.05 (relaxed).
2.5. Statistical Methods
Statistical analysis of collected data was performed using the functionality implemented in Proteome Discoverer 2.5 [46]. Protein FDR rate was assessed at two levels: 0.01 (as a strict criterion) and 0.05 (relaxed criterion). The statistical significance of quantification values for proteins was tested with background-based multiple t-testing. This method considers the prior distribution of protein and peptide abundance rates, allowing for the estimation of a relative change for this background. As the samples came from comparable animals, the fundamental assumption of this approach, that the expected protein abundances between samples remain invariant, holds in this case. Protein ratio calculation was performed on protein abundance basis with a maximum fold change of 1000 allowed to avoid unduly confounding effects of outliers. For missing values, low abundance resampling was introduced.
PCA analysis and hierarchical clustering were performed using standard implementations of these methods, as available in Proteome Discoverer 2.5 software, using all measured samples, excluding pooled control. The inter-relationship of principal components #1 and #2, depicted in Figure 1, showed a clear separation between estrus and anestrus samples along the first principal component, with high dispersion for anestrus samples along the second component. The relationships within protein expression profiles were assessed using the l2 (Euclidean) norm as a distance metric with complete-linkage clustering, with results illustrated in Figure 2.
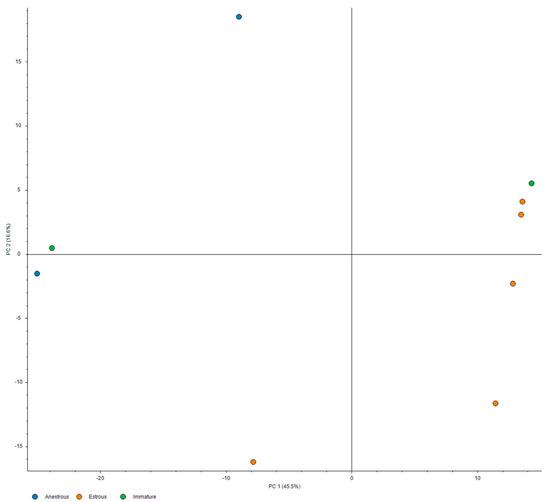
Figure 1.
PCA analysis of the identified urinary proteins. The orange circle indicates the proteins from estrus stage, and blue circle indicates the proteins from anestrus urine samples, while immature samples are labeled in green.
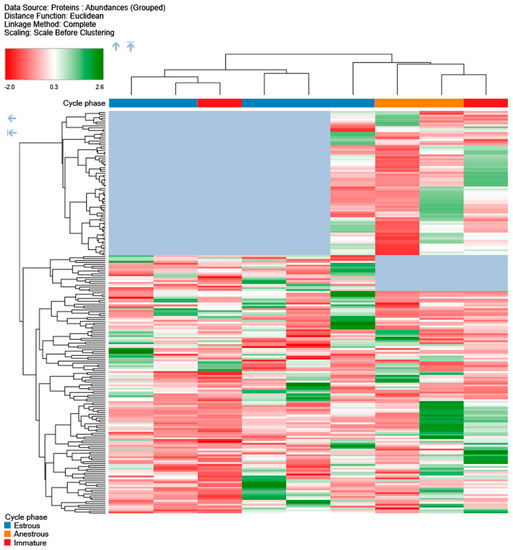
Figure 2.
Hierarchical clustering of urine samples. Samples collected during estrus phase are indicated in orange color, and samples from anestrus phase are indicated in blue color, while samples from immature animals are in red.
3. Results
A total of 240 proteins were identified in the urine samples. The lists of identified and quantified proteins are provided in Supplementary Table S1a. We observe that our results are robust to the tightening of thresholds, as illustrated in Supplementary Table S1b, which comprises nearly the same proteins as these used in this work, but are identified with 0.05 Da fragment and 10 ppm maximum precursor tolerances. Increasing the threshold of fragment tolerance further to 0.02 Da results in the identification of no meaningful protein signal (as demonstrated in Supplementary Table S1c).
Use of classic evaluation techniques for elucidating signal in the identified proteins has yielded interesting results congruent with the expectations, yet not fully informative (shown in Table 2, Table 3, Table 4 and Table 5). Therefore, PCA analysis (Figure 1) and hierarchical clustering (Figure 2) were performed for the differentiation of specific biological states. Results are presented in the figures below.

Table 2.
Results of DAVID analysis. Clusters with an enrichment score of at least 1 were selected with a raw p-value and Benjamini–Hofberg adjusted metric (BH p-value).

Table 3.
List of proteins with significantly affected abundance rates in estrus vs. anestrus samples (identified as involved in response to external stimuli).

Table 4.
List of proteins significantly altered in estrus vs. anestrus samples, which were identified as involved in signaling and as secreted/extracellular.

Table 5.
List of other proteins significantly altered in estrus vs. anestrus samples.
From the above figure, it is evident that there is a clear separation between the estrus and anestrus samples. PC1 clearly separates the estrus samples from anestrus ones, and in conjunction with PC2, the separation is even clearer. We also observe that two immature pools (in green) tend to follow the anestrus samples in the PC2 dimension, but reside near opposite limits of the PC1 range in the vicinity of either of the estrus or anestrus groups. This suggests that the proteomic profile of the estrus urine may be convergent; meanwhile, there is a significant dispersion in the case of anestrus samples.
There is a clear separation at the first layer of the tree between the bulk of the estrus samples and the anestrus ones. Both clusterings appear to be influenced by the absence of certain proteins in the estrus and anestrus groups, respectively (indicated in blue).
We performed a BLAST search of known murine MUPs against dog proteomes with an e-value cut-off of 1e-10 to permit the identification of more distant homologs. Naturally, the most relevant hits included lipocalins predominantly present in saliva. However, canine beta-lactoglobulin-1 and beta-lactoglobulin-2 (P33685 and P33686), belonging to the lipocalin family and sharing 90% sequence identity, were identified with high confidence in the mass spectrometry experiment. Both beta-lactoglobulins were identified only in the estrus samples.
Another highly homologous hit to murine MUPs in our results was A0A8P0NKK8 (lipocln_cytosolic_FA-bd_dom domain-containing protein), whose close homologs can be identified uniquely in mammals (predominantly Eutheria) with more distant homologs present in Archelosauria. This protein was found across all samples and pools; its abundance in the estrus pool was 44.3% higher than in the anestrus one (anestrus: estrus ratio: 0.693).
4. Discussion
The results of our study show the presence of several proteins in the urine samples which are characteristic of the estrus female. Considering that urine is expected to be relatively devoid of protein with proteinuria being associated with pathology in dogs, the amount of identified proteins in the estrus urine was unexpectedly high.
Changes in the composition of urinary proteins in the context of the estrus/ovarian cycle were investigated in other species. Muthukumar et al. [47] studied urinary proteins in female house rats and evidenced a correlation of lipocalin concentration with the phases of the estrus cycle. In dogs, lipocalin proteins were primarily examined in the context of allergenic agents, and the source used was saliva [48]. The lipocalins are predominantly involved in the transport of small molecules, such as steroids; therefore, it is plausible that they are key molecules in chemical signaling. In fact, the major respiratory allergens of dogs, mice, rats, horses, and cows belong to the this group of proteins [48]. According to our best knowledge, there were no previous studies on identifying estrus-specific proteins in domestic dogs.
Promisingly, we identified proteins belonging to the lipocalin family of canines (beta-lactoglobulin-1 and beta-lactoglobulin-2, (P33685 and P33686, respectively)) in the estrus urine samples. However, due to a high degree of sequence similarity between the two proteins, it was impossible to ascertain which homolog was present in the urine. It is worth noticing that beta-lactoglobulins are small, very stable proteins that contain a hydrophobic pocket, and we postulated them as carriers of semiochemicals in dog urine. It is also interesting that beta-lactoglobulins were absent in the anestrus samples.
It is worth mentioning that A0A8P0NKK8 was present across all the samples and pools. In particular, its abundance (43% higher) in the estrus pool was higher than in the anestrus pool (anestrus: estrus ratio—0.693). Close homologs of this protein are also found among human lipocalins. LCNL1 and LCNL15 (UniProt accession IDs: Q6ZST4 and Q6UWW0) are preferentially expressed in the testes and seminal ducts implicating them in reproductive processes. It could also be the same function the proteins exhibit in canines.
Although they were not yet proven to be related to semiochemical or chemical signals, the other proteins identified in the female urine during estrus (Clusterin, Proenkephalin (PENK), and Liver-expressed antimicrobial peptide 2 (LEAP2)) are also worth focusing on. Clusterin, a heterodimeric glycoprotein, was isolated from the ram’s rete testis fluid that elicited the clustering of Sertoli cells [49]. Clusterin is also produced by various tissues and identified in biological fluids [50]. In dogs, Clusterin was identified concerning kidney and urinary tract disorders, infections, and injuries [51]. Clusterin, on the other hand, is involved in tissue remodeling, immune defense, and the transport of biologically active peptides. Interestingly, Clusterin is also detected in the ovary where it may facilitate sperm–ovum interactions. It is crucial to note the involvement of Clusterin in sperm maturation [50].
Taking into account the presence of Clusterin in many body fluids and a wide array of species, its involvement in the process of semiochemical communication remains elusive. However, Clusterin was found in greater concentrations in follicular fluids than in plasma. It was also suggested that it may likely play a role in follicle physiology and ovarian activity at the pre-ovulatory stage [52]. Clusterin was also identified in the estrus saliva of buffaloes [47]. A study by Zwain et al. [53] proved the association of Clusterin with programmed cell death (apoptosis) and follicular atresia in rats.
In the ovaries and uteri of rodents, the expression of the gene Proenkephalin (PENK) is significantly altered during the estrus cycle, wherein the highest concentrations are found during estrus [54]. Given the changes in the concentration, the association of Proenkephalin within the female reproductive system is expected. However, the interaction between the reproductive hormones (estrogens and progesterone) and Proenkephalin gene expression varies among species [55]. Proenkephalin is cleaved into opioid peptides (met-enkephalin and leu-enkephalin), whose role in nociception is widely studied. The role of the estrus cycle in opioid antinociception in dogs has not been systematically studied. However, similar investigations in rats demonstrate that opioids are least potent during estrus compared to metestrus and proestrus. Konturek et al. [56] showed that enkephalins inhibit pancreatic bicarbonate and protein (somatostatin) secretion during endogenous or exogenous stimulation (secretin or cholecystokinin-octapeptide) in dogs. Therefore, the presence of PENK in urine is expected due to the influence of sex steroid hormones on insulin homeostasis.
Liver-expressed antimicrobial peptide 2 (LEAP2) is a 40-residue cationic peptide that exhibits antimicrobial activity. It is highly expressed in the liver but also produced by other tissues and organs, such as the kidney [57]. It was proposed that antimicrobial peptides (AMPs) in domestic animals have beneficial effects on immune regulation and the reproductive system [58]. LEAP2 is also postulated to be involved in the control of sperm maturation [58]. Furthermore, LEAP2 is an endogenous antagonist of the ghrelin receptor; meanwhile, the spikes of ghrelin expression in estrus are an established phenomenon.
The role of bacteria in the process of synthesis of semiochemical signals has been discussed in many animals. Microbes are potential regulators of chemical signals, as evidenced by their presence in the vaginas of canines [59,60]. It could also be possible that the microbes facilitate the attraction of males toward females through the compounds they synthesize [61,62,63]. In this purview, the presence of infection-associated proteins (GUSB, GZMB, LEAP2, LY6D) in estrus urine is interesting.
Non-volatile compounds, such as proteins, can play various roles in chemical communication. The proteins may themselves act as pheromones or carry specific volatile ligands that act as pheromones. The proteins, in addition, can also be involved in other physiological processes. In cats, a major allergen (Fel d 1-tetrameric glycoprotein of the secretoglobin superfamily) is responsible for binding lipids, similar to the mouse androgen-binding protein [64]. Despite the well-studied antimicrobial function, these peptides can also be crucial in inter-individual communication.
Odorant binding proteins (OBPs) belonging to the lipocalin superfamily can be found both in the main and additional olfactory systems [65], but they also were identified in the glands responsible for the secretion of chemical signals, such as the canine anal sac glands [66]. OBPs are closely homologous to the pheromone carrier proteins (such as allergen Can f 4). The primary function of the mentioned proteins is to bind the pheromone compounds and release them to the environment for manifesting the effect [67,68,69].
Some proteins of this kind were described in the nasal mucus of buffaloes and pigs, suggesting that OBP may bind the odorants for further processing [68]. Despite the apparent function in the process of odor/pheromone binding and transportation, other interesting functions of the OBP have been described. In cattle, Mitchel et al. [70] proved that OBP present in the lung and other parts of respiratory tracts might inhibit neutrophil recruitment by inflammatory mediators, having the ability to bind macrophage-derived inflammatory mediators within the airways. The study performed by Cerna et al. [71] in mice showed the co-expression of OBP with antimicrobial proteins. This confirmed that the same proteins play various roles in the organism. Thus, their presence during some specific physiological conditions could relate to particular events (can be directly involved in reproductive processes, luring, or others) but also can play another role. (Overexpression of the proteins increasing antibacterial defense mechanism is to be expected during the estrus period, which is associated with an increased abundance of microorganisms.) Thus, the presence of proteins seemingly not involved in the pheromone binding does not necessarily imply the lack of importance for the whole process. Moreover, the lack of direct function connecting their role with reproductive processes should not exclude them unequivocally in the pool of characteristics for some period compounds.
The functions of some proteins detected in our study, which are connected with sperm maturation, facilitating sperm–ovum interactions, and antibacterial activity, certainly justify their presence in the urine of females in estrus. However, they do not exclude these proteins from their implication with the compounds potentially involved in communication and attractiveness modulation. Interestingly, a phenomenon of reduced attractiveness after vaginal microflora reduction, which was observed in several species, could suggest the importance of the microflora for creating adequate semiochemical signals [61,62,63]. In dogs, sheep, and rats, reduced vaginal microflora (after antibiotic administration) decreased the sexual attractiveness of treated females [61,62,63]. However, a detailed explanation of this mechanism has not been presented. It is worth evaluating if reducing the vaginal microflora by the use of antibiotics also leads to reducing the antimicrobial peptides in female secretions. If it does, it may be that this phenomenon is involved in modifying the attractiveness of females to the males. In this context, it is worth mentioning acute phase proteins (APPs) in canine urine whose concentration remains unchanged during the reproductive cycle [72].
Despite the proteins belonging to the lipocalin family, the other proteins are also putatively involved in signaling by responding to external stimuli. These facts corroborate the hypothesis that observed proteins might be involved in chemical communication. However, conclusive confirmation of this claim requires further investigation.
5. Conclusions
In this paper, we presented the results of the proteomic examination of the urine of female canines during various stages of the ovarian cycle. We found that several proteins were significantly altered during estrus. Interestingly, some of these proteins belong to the lipocalin family; whereas, other proteins identified in the present study are involved in signaling and response to external stimuli. Some of the proteins identified herein are known to be differentially expressed concentrations in the reproductive organs or body fluids (e.g., serum, saliva, etc.) of other species. The association of specific proteins with antimicrobial properties is another promising venue for continued exploration. Furthermore, it is prudent to conduct follow-up studies to evaluate its possible involvement in the chemical communication process. Overall, proteomic evaluation is only a preliminary approach which needs additional evaluation with conspecifics to ascertain the role of proteins in canine chemical communication.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vetsci10040292/s1, Table S1a: Identified proteins using the tolerances used in the paper (precursor mass tolerance at 20 ppm, fragment mass tolerance at 0.1 Da); Table S1b: Identified proteins using the more stringent tolerances than in the paper (precursor mass tolerance at 10 ppm, fragment mass tolerance at 0.05 Da). Note that the proteins described in the paper remain deetected with high confidence.; Table S1c: Identified proteins using the stringent tolerances (precursor mass tolerance at 10 ppm, fragment mass tolerance at 0.02 Da). Note lack of detectable proteins with high coverage; Table S2: Composition of TMT 6plexes used in the experiment. Single digit identifiers refer to individual animals, while P# -to pools, in accordance with the labelling in the manuscript. Note that P1 (pool #1) uses the same reference (126) in both plexes.
Author Contributions
M.W. and M.D. conceived the original idea; M.W. and M.D. designed and performed the experiments; M.W. carried out the experiment; M.W. and P.P. contributed to sample preparation; W.N., A.S. and S.D. helped supervise the project; M.W., P.P., W.N., M.D., A.K. and M.J.S. performed collection and analysis of data; M.W., M.J.S. and M.D. took the lead in writing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The National Science Centre (Poland), grant No. UMO-2015/17/B/NZ8/02411. The APC/BPC is financed/co-financed by Wroclaw University of Environmental and Life Sciences and the University of Life Sciences, Lublin. The cost of Open Access publication was covered/partially covered by the Society for Biology of Reproduction in Poland. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
The study was conducted following the regulations on animal experimentation and guidelines for the use of animals in research. The experimental protocol was approved by the 2nd Local Commission for Animal Experimentation in Wrocław, Poland (permission no. 17/2017).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (M.D.) upon reasonable request.
Acknowledgments
Authors would like to thank Amelia Harvey, Sarah O’Keeffe, and Sadhbh Moran for the language correction of the text. The mass spectrometry proteomics data were deposited to the ProteomeXchange Consortium via the PRIDE [73] partner repository with the dataset identifier PXD040418.
Conflicts of Interest
M.J.S. is an employee of InstaDeep Ltd. and has securities from InstaDeep Ltd. InstaDeep had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The remaining authors have no conflicts of interest to declare.
References
- Wyatt, T.D. Pheromones and Animal Behavior: Chemical Signals and Signatures, 2nd ed.; Cambridge University Press, Ed.; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Kokocińska-Kusiak, A.; Woszczyło, M.; Zybala, M.; Maciocha, J.; Barłowska, K.; Dzięcioł, M. Canine Olfaction: Physiology, Behavior, and Possibilities for Practical Applications. Animals 2021, 11, 2463. [Google Scholar] [CrossRef] [PubMed]
- Anisko, J.J. Hormonal Substrate of Estrus Odor Preference in Beagles. Physiol. Behav. 1977, 18, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Kruse, S.M.; Howard, W.E. Canid Sex Attractant Studies. J. Chem. Ecol. 1983, 9, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Kustritz, M.V.R. Collection of Tissue and Culture Samples from the Canine Reproductive Tract. Theriogenology 2006, 66, 567–574. [Google Scholar] [CrossRef]
- Dzięcioł, M.; Stańczyk, E.; Noszczyk-Nowak, A.; Niżański, W.; Ochota, M.; Kozdrowski, R. Influence of Bitches Sex Pheromones on the Heart Rate and Other Chosen Parameters of Blood Flow in Stud Dogs (Canis Familiaris). Res. Vet. Sci. 2012, 93, 1241–1247. [Google Scholar] [CrossRef]
- Jezierski, T.; Dzięcioł, M.; Szumny, A.; Niżański, W.; Woszczyło, M.; Pieczewska, B.; Godzińska, E.J. Discrimination of Estrus Odor in Urine by Male Dogs in Different Experimental Settings. J. Vet. Behav. 2019, 29, 25–30. [Google Scholar] [CrossRef]
- Goodwin, M.; Gooding, K.M.; Regnier, F. Sex Pheromone in the Dog. Science 1979, 203, 559–561. [Google Scholar] [CrossRef]
- Dzięcioł, M.; Niżański, W.; Jezierski, T.; Szumny, A.; Godzińska, E.J.; Ochota, M.; Stańczyk, E.; Najder-Kozdrowska, L.; Woszczyło, M.; Pieczewska, B. The Efficiency of Synthetic Sex Pheromones in Sexual Arousal Stimulation in Domestic Dogs. Pol. J. Vet. Sci. 2017, 20, 429–437. [Google Scholar] [CrossRef]
- Dzięcioł, M.; Woszczylo, M.; Szumny, A.; Jezierski, T.; Kupczyński, R.; Godzińska, E.J.; Pieczewska, B.; Niżański, W. Identification of Putative Volatile Sex Pheromones in Female Domestic Dogs (Canis Familiaris). Anim. Reprod. Sci. 2018, 197, 87–92. [Google Scholar] [CrossRef]
- Woszczyło, M.; Jezierski, T.; Szumny, A.; Dzięcioł, M.; Niżański, W. The Role of Urine in Semiochemical Communication between Females and Males of Domestic Dog (Canis Familiaris) during Estrus. Animals 2020, 10, 2112. [Google Scholar] [CrossRef]
- Dzięcioł, M.; Podgórski, P.; Stańczyk, E.; Szumny, A.; Woszczyło, M.; Pieczewska, B.; Niżański, W.; Nicpoń, J.; Wrzosek, M.A. MRI Features of the Vomeronasal Organ in Dogs (Canis Familiaris). Front. Vet. 2020, 7, 159. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Stowers, L. Bespoke Behavior: Mechanisms That Modulate Pheromone-Triggered Behavior. Curr. Opin. Neurobiol. 2020, 64, 143–150. [Google Scholar] [CrossRef] [PubMed]
- McGlone, J.J.; Archer, C.; Henderson, M. Interpretive Review: Semiochemicals in Domestic Pigs and Dogs. Front. Vet. Sci. 2022, 9, 967980. [Google Scholar] [CrossRef] [PubMed]
- Marchlewska-Koj, A. Pheromonal Regulation of Bank Vole (Clethrionomys Glareolus) Reproduction. In Chemical Signals in Vertebrates 9; Springer: Boston, MA, USA, 2001; pp. 391–396. ISBN 9781461351870. [Google Scholar]
- Rasmussen; Schulte, B.; Goodwin, T.; Whitehouse, A.; Loizi, H. Sexual Dimorphism in the Performance of Chemosensory Investigatory Behaviours by African Elephants (Loxodonta Africana). Behaviour 2009, 146, 373–392. [Google Scholar] [CrossRef]
- Beynon, R.J.; Hurst, J.L. Multiple Roles of Major Urinary Proteins in the House Mouse, Mus Domesticus. Biochem. Soc. Trans. 2003, 31, 142–146. [Google Scholar] [CrossRef]
- Novotny, M.; Harvey, S.; Jemiolo, B.; Alberts, J. Synthetic Pheromones That Promote Inter-Male Aggression in Mice. Proc. Natl. Acad. Sci. USA 1985, 82, 2059–2061. [Google Scholar] [CrossRef]
- Mucignat, C.; Benati, D.; Righetti, C.; Zancanaro, C. High-Resolution Magnetic Resonance Spectroscopy of the Mouse Vomeronasal Organ. Chem. Senses 2004, 29, 693–696. [Google Scholar] [CrossRef]
- Logan, D.W.; Marton, T.F.; Stowers, L. Species Specificity in Major Urinary Proteins by Parallel Evolution. PLoS ONE 2008, 3, e3280. [Google Scholar] [CrossRef]
- Rajkumar, R.; Ilayaraja, R.; Liao, C.C.; Archunan, G.; Achiraman, S. Detection of Alpha (2u)-Globulin and Its Bound Putative Pheromones in the Preputial Gland of the Indian Commensal Rat (Rattus Rattus) Using Mass Spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 721–728. [Google Scholar] [CrossRef]
- Dehnhard, M.; Hildebrandt, T.; Knauf, T.; Ochs, A.; Ringleb, J.; Göritz, F. Chemical Signals in Giant Panda Urine (Ailuropoda Melanoleuca). In Chemical Signals in Vertebrates 10; Springer: Boston, MA, USA, 2006; pp. 110–117. ISBN 9780387251592. [Google Scholar]
- Rajanarayanan, S.; Archunan, G. Identification of Urinary Sex Pheromones in Female Buffaloes and Their Influence on Bull Reproductive Behaviour. Res. Vet. Sci. 2011, 91, 301–305. [Google Scholar] [CrossRef]
- Dehnhard, M.; Hildebrandt, T.B.; Meerheim, C.; Valentine, I.; Göritz, F. Chemical Signals in Giant Panda Urine (Ailuropoda Melanoleuca). In Chemical Signals in Vertebrates 13; Springer International Publishing: Cham, Switzerland, 2016; pp. 363–379. ISBN 9783319220253. [Google Scholar]
- Ramachandran, R.; Vinothkumar, A.; Sankarganesh, D.; Suriyakalaa, U.; Aathmanathan, V.S.; Kamalakkannan, S.; Nithya, V.; Angayarkanni, J.; Archunan, G.; Akbarsha, M.A.; et al. Detection of Estrus Biomarkers in the Body Exudates of Kangayam Cattle (Bos Indicus) from Interplay of Hormones and Behavioral Expressions. Domest. Anim. Endocrinol. 2020, 72, 106392. [Google Scholar] [CrossRef]
- Nagnan-Le Meillour, P.; Descamps, A.; Le Danvic, C.; Grandmougin, M.; Saliou, J.-M.; Klopp, C.; Milhes, M.; Bompard, C.; Chesneau, D.; Poissenot, K.; et al. Identification of Potential Chemosignals in the European Water Vole Arvicola Terrestris. Sci. Rep. 2019, 9, 18378. [Google Scholar] [CrossRef]
- Sankarganesh, D.; Suriyakalaa, U.; Ramachandran, R.; Achiraman, S.; Arunachalam, S.; Angayarkanni, J. Urinary Volatile Metabolomics as a Viable Alternative Diagnostic Tool for Polycystic Ovary Syndrome: An Exploratory Hypothesis. Med. Hypotheses 2019, 124, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Winiarczyk, D.; Michalak, K.; Adaszek, Ł.; Winiarczyk, M.; Winiarczyk, S. Urinary Proteome of Dogs with Kidney Injury during Babesiosis. BMC Vet. Res. 2019, 15, 439. [Google Scholar] [CrossRef]
- Ajayi, A.F.; Akhigbe, R.E. Staging of the Estrus Cycle and Induction of Estrus in Experimental Rodents: An Update. Fertil. Res. Pract. 2020, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Ferlizza, E.; Isani, G.; Dondi, F.; Andreani, G.; Vasylyeva, K.; Bellei, E.; Almeida, A.M.; Matzapetakis, M. Urinary Proteome and Metabolome in Dogs (Canis Lupus Familiaris): The Effect of Chronic Kidney Disease. J. Proteom. 2020, 222, 103795. [Google Scholar] [CrossRef] [PubMed]
- Dubin, R.F.; Rhee, E.P. Proteomics and Metabolomics in Kidney Disease, Including Insights into Etiology, Treatment, and Prevention. Clin. J. Am. Soc. Nephrol. 2020, 15, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Miller, I.; Schlosser, S.; Palazzolo, L.; Veronesi, M.C.; Eberini, I.; Gianazza, E. Some More about Dogs: Proteomics of Neglected Biological Fluids. J. Proteom. 2020, 218, 103724. [Google Scholar] [CrossRef] [PubMed]
- Pieper, R.; Gatlin, C.L.; Makusky, A.J.; Russo, P.S.; Schatz, C.R.; Miller, S.S.; Su, Q.; McGrath, A.M.; Estock, M.A.; Parmar, P.P.; et al. The Human Serum Proteome: Display of Nearly 3700 Chromatographically Separated Protein Spots on Two-Dimensional Electrophoresis Gels and Identification of 325 Distinct Proteins. Proteomics 2003, 3, 1345–1364. [Google Scholar] [CrossRef]
- Miller, I.; Friedlein, A.; Tsangaris, G.; Maris, A.; Fountoulakis, M.; Gemeiner, M. The Serum Proteome of Equus Caballus. Proteomics 2004, 4, 3227–3234. [Google Scholar] [CrossRef] [PubMed]
- Presslmayer, A. Identifizierung Caniner Serumproteine Mittels Elektrophoretischer Verfahren. Master’s Thesis, University of Natural Resources and Applied Life Science Sciences, Vienna, Austria, 2002. [Google Scholar]
- Atherton, M.J.; Braceland, M.; Harvie, J.; Burchmore, R.J.; Eadie, S.; Eckersall, P.D.; Morris, J.S. Characterisation of the Normal Canine Serum Proteome Using a Novel Electrophoretic Technique Combined with Mass Spectrometry. Vet. J. 2013, 196, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Atherton, M.J.; Braceland, M.; Fontaine, S.; Waterston, M.M.; Burchmore, R.J.; Eadie, S.; Eckersall, P.D.; Morris, J.S. Changes in the Serum Proteome of Canine Lymphoma Identified by Electrophoresis and Mass Spectrometry. Vet. J. 2013, 196, 320–324. [Google Scholar] [CrossRef]
- Szczubiał, M.; Wawrzykowski, J.; Dąbrowski, R.; Krawczyk, M.; Kankofer, M. Preliminary Study on Plasma Proteins in Pregnant and Non-Pregnant Female Dogs. Theriogenology 2017, 97, 1–8. [Google Scholar] [CrossRef]
- Dąbrowski, R.; Kostro, K.; Szczubiał, M. Concentrations of C-Reactive Protein, Serum Amyloid A, and Haptoglobin in Uterine Arterial and Peripheral Blood in Bitches with Pyometra. Theriogenology 2013, 80, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Franco-Martínez, L.; Horvatić, A.; Gelemanović, A.; Samardžija, M.; Mrljak, V.; Contreras-Aguilar, M.D.; Martínez-Subiela, S.; Dabrowski, R.; Tvarijonaviciute, A. Changes in the Salivary Proteome Associated with Canine Pyometra. Front. Vet. Sci. 2020, 7, 277. [Google Scholar] [CrossRef] [PubMed]
- Miller, I.; Preßlmayer-Hartler, A.; Wait, R.; Hummel, K.; Sensi, C.; Eberini, I.; Razzazi-Fazeli, E.; Gianazza, E. In between—Proteomics of Dog Biological Fluids. J. Proteom. 2014, 106, 30–45. [Google Scholar] [CrossRef] [PubMed]
- Teinfalt, M.; Miller, I.; Loupal, G.; Thalhammer, J.G.; Gemeiner, M. Quantitative Determination of a Canine Prostate Specific Protein and Its Clinical Relevance. Tierärztliche Praxis. Ausgabe K Kleintiere/Heimtiere 2000, 28, 127–131. [Google Scholar]
- Kustritz, M.V.R. Reproductive Behavior of Small Animals. Theriogenology 2005, 64, 734–746. [Google Scholar] [CrossRef]
- Arlt, S. Canine Ovulation Timing: A Survey on Methodology and an Assessment on Reliability of Vaginal Cytology. Reprod. Domest. Anim. 2018, 53 (Suppl. S3), 53–62. [Google Scholar] [CrossRef]
- Brugger, N.; Otzdorff, C.; Walter, B.; Hoffmann, B.; Braun, J. Quantitative Determination of Progesterone (P4) in Canine Blood Serum Using an Enzyme-Linked Fluorescence Assay. Reprod. Domest. Anim. 2011, 46, 870–873. [Google Scholar] [CrossRef]
- Orsburn, B.C. Proteome Discoverer-A Community Enhanced Data Processing Suite for Protein Informatics. Proteomes 2021, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Muthukumar, S.; Rajesh, D.; Saibaba, G.; Alagesan, A.; Rengarajan, R.L.; Archunan, G. Urinary Lipocalin Protein in a Female Rodent with Correlation to Phases in the Estrus Cycle: An Experimental Study Accompanied by in Silico Analysis. PLoS ONE 2013, 8, e371357. [Google Scholar] [CrossRef]
- Virtanen, T.; Zeiler, T.; Mäntyjärvi, R. Important Animal Allergens Are Lipocalin Proteins: Why Are They Allergenic? Int. Arch. Allergy Immunol. 1999, 120, 247–258. [Google Scholar] [CrossRef]
- Rosenberg, M.E.; Silkensen, J. Clusterin: Physiologic and Pathophysiologic Considerations. Int. J. Biochem. Cell 1995, 27, 633–645. [Google Scholar] [CrossRef]
- Jones, S.E.; Jomary, C. Clusterin. Int. J. Biochem. Cell Biol. 2002, 34, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Jenne, D.E.; Tschopp, J. Clusterin: The Intriguing Guises of a Widely Expressed Glycoprotein. Trends Biochem. Sci. 1992, 17, 154–159. [Google Scholar] [CrossRef]
- Fahiminiya, S.; Reynaud, K.; Labas, V.; Batard, S.; Chastant-Maillard, S.; Gérard, N. Steroid Hormones Content and Proteomic Analysis of Canine Follicular Fluid during the Preovulatory Period. Reprod. Biol. Endocrinol. 2010, 8, 132. [Google Scholar] [CrossRef]
- Zwain, I.; Amato, P. Clusterin Protects Granulosa Cells from Apoptotic Cell Death during Follicular Atresia. Exp. Cell Res. 2000, 257, 101–110. [Google Scholar] [CrossRef]
- Jin, D.F.; Muffly, K.E.; Okulicz, W.C.; Kilpatrick, D.L. Estrus Cycle- and Pregnancy-Related Differences in Expression of the Proenkephalin and Proopiomelanocortin Genes in the Ovary and Uterus. Endocrinology 1988, 122, 1466–1471. [Google Scholar] [CrossRef]
- Muffly, K.E.; Jin, D.F.; Okulicz, W.C.; Kilpatrick, D.L. Gonadal Steroids Regulate Proenkephalin Gene Expression in a Tissue-Specific Manner within the Female Reproductive System. Mol. Endocrinol. 1988, 2, 979–985. [Google Scholar] [CrossRef]
- Konturek, S.J.; Tasler, J.; Krol, R.; Dembinski, A.; Coy, D.H.; Schally, A.V. Effect of Somatostatin Analogs on Gastric and Pancreatic Secretion. Proc. Soc. Exp. Biol. Med. 1977, 155, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Henriques, S.T.; Tan, C.C.; Craik, D.J.; Clark, R.J. Structural and Functional Analysis of Human Liver-Expressed Antimicrobial Peptide 2. Chembiochem 2010, 11, 2057–2148. [Google Scholar] [CrossRef]
- Valdez-Miramontes, C.E.; De Haro-Acosta, J.; Aréchiga-Flores, C.F.; Verdiguel-Fernández, L.; Rivas-Santiago, B. Antimicrobial Peptides in Domestic Animals and Their Applications in Veterinary Medicine. Peptides 2021, 142, 170576. [Google Scholar] [CrossRef] [PubMed]
- Allen, W.E.; Dagnall, G.J.R. Some Observations on the Aerobic Bacterial Flora of the Genital Tract of the Dog and Bitch. J. Small Anim. Pract. 1982, 23, 325–335. [Google Scholar] [CrossRef]
- Maksimović, A.; Maksimović, Z.; Filipović, S.; Beširović, H.; Rifatbegović, M. Vaginal and Uterine Bacteria of Healthy Bitches during Different Stages of Their Reproductive Cycle. Vet. Rec. 2012, 171, 375. [Google Scholar] [CrossRef]
- Merkx, J.; Slob, A.K.; van der Werff ten Bosch, J.J. Vaginal Bacterial Flora Partially Determines Sexual Attractivity of Female Rats. Physiol. Behav. 1988, 44, 147–149. [Google Scholar] [CrossRef]
- Ungerfeld, R.; Silva, L. The Presence of Normal Vaginal Flora Is Necessary for Normal Sexual Attractiveness of Estrus Ewes. Appl. Anim. Behav. Sci. 2005, 93, 245–250. [Google Scholar] [CrossRef]
- Dzięcioł, M.; Niżański, W.; Stańczyk, E.; Kozdrowski, R.; Najder-Kozdrowska, L.; Twardoń, J. The Influence of Antibiotic Treatment of Bitches in Estrus on Their Attractiveness to Males during Mating. Pol. J. Vet. Sci. 2013, 16, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Bienboire-Frosini, C.; Durairaj, R.; Pelosi, P.; Pageat, P. The Major Cat Allergen Fel d 1 Binds Steroid and Fatty Acid Semiochemicals: A Combined in Silico and in Vitro Study. Int. J. Mol. Sci. 2020, 21, 1365. [Google Scholar] [CrossRef]
- Krishna, N.S.; Getchell, M.L.; Margolis, F.L.; Getchell, T.V. Differential Expression of Vomeromodulin and Odorant-Binding Protein, Putative Pheromone and Odorant Transporters, in the Developing Rat Nasal Chemosensory Mucosae. J. Neurosci. Res. 1995, 40, 54–71. [Google Scholar] [CrossRef]
- Janssenswillen, S.; Roelants, K.; Carpentier, S.; de Rooster, H.; Metzemaekers, M.; Vanschoenwinkel, B.; Proost, P.; Bossuyt, F. Odorant-Binding Proteins in Canine Anal Sac Glands Indicate an Evolutionarily Conserved Role in Mammalian Chemical Communication. BMC Ecol. Evol. 2021, 21, 182. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, P. The Role of Perireceptor Events in Vertebrate Olfaction. Cell. Mol. Life Sci. 2001, 58, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Archunan, G. Odorant Binding Proteins: A Key Player in the Sense of Smell. Bioinformation 2018, 14, 036–037. [Google Scholar] [CrossRef]
- Pelosi, P.; Knoll, W. Odorant-Binding Proteins of Mammals. Biol. Rev. Camb. Philos. Soc. 2022, 97, 20–44. [Google Scholar] [CrossRef]
- Mitchell, G.B.; Clark, M.E.; Lu, R.; Caswell, J.L. Localization and Functional Characterization of Pulmonary Bovine Odorant-Binding Protein. Vet. Pathol. 2011, 48, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Černá, M.; Kuntová, B.; Talacko, P.; Stopková, R.; Stopka, P. Differential Regulation of Vaginal Lipocalins (OBP, MUP) during the Estrus Cycle of the House Mouse. Sci. Rep. 2017, 7, 11674. [Google Scholar] [CrossRef] [PubMed]
- Ulutas, P.A.; Musal, B.; Kiral, F.; Bildik, A. Acute Phase Protein Levels in Pregnancy and Estrus Cycle in Bitches. Res. Vet. Sci. 2009, 86, 373–376. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; García-Seisdedos, D.; Hewapathirana, S.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The PRIDE Database Resources in 2022: A Hub for Mass Spectrometry-Based Proteomics Evidences. Nucleic Acids Res. 2022, 50, D543–D552. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).