Ex Vivo Biomechanical Assessment of Various Repair Techniques in a Rabbit Calcaneal Tendon Avulsion Model: Application of Polycaprolactone Plate
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
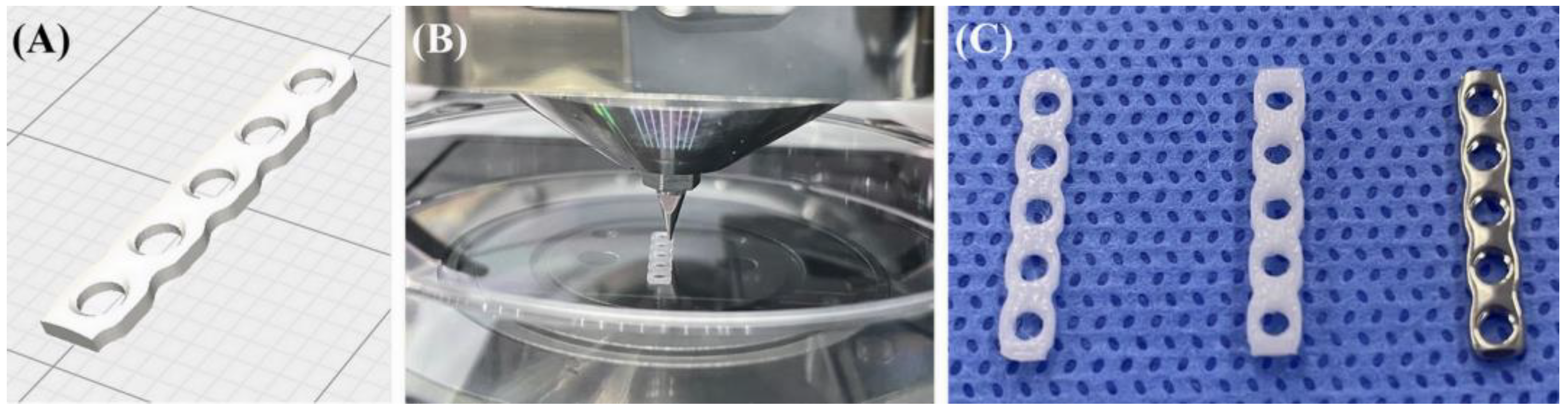
2.2. PCL Plate Production
2.3. Tendon Repair Groups
2.4. Biomechanical Evaluation
2.5. Statistical Analysis
3. Results
3.1. Load Data
3.2. Gap Formation Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- King, M.; Jerram, R. Achilles tendon rupture in dogs. Compend. Contin. Educ. Pract. Vet. 2003, 25, 613–620. [Google Scholar]
- Hale, M.J.; Zellner, E.M.; Naiman, J.H.; Kraus, K.H. Ex vivo mechanical testing of various suture patterns for use in tendon plating. Vet. Surg. 2021, 50, 1296–1303. [Google Scholar] [CrossRef] [PubMed]
- Evans, H.E.; Christensen, G.C. Ligaments and Joints of the Pelvic Limb. In Miller’s Anatomy of the Dog, 5th ed.; Hermanson, J.W., De Lahunta, A., Evans, H.E., Eds.; Elsevier: St. Louis, MO, USA, 2020; pp. 418–438. [Google Scholar]
- Nielsen, C.; Pluhar, G.E. Outcome following surgical repair of achilles tendon rupture and comparison between postoperative tibiotarsal immobilization methods in dogs: 28 cases (1997–2004). Vet. Comp. Orthop. Traumatol. 2006, 19, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Corr, S.A.; Draffan, D.; Kulendra, E.; Carmichael, S.; Brodbelt, D. Retrospective study of Achilles mechanism disruption in 45 dogs. Vet. Rec. 2010, 167, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Moores, A.P.; Owen, M.R.; Tarlton, J.F. The three-loop pulley suture versus two locking-loop sutures for the repair of canine achilles tendons. Vet. Surg. 2004, 33, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Berg, R.J.; Egger, E.L. In Vitro comparison of the three loop pulley and locking loop suture patterns for repair of canine weightbearing tendons and collateral ligaments. Vet. Surg. 1986, 15, 107–110. [Google Scholar] [CrossRef]
- Pijanowski, G.J.; Stein, L.E.; Turner, T.A. Strength characteristics and failure modes of suture patterns in severed goat tendons. Vet. Surg. 1989, 18, 335–339. [Google Scholar] [CrossRef]
- Easley, K.J.; Stashak, T.S.; Smith, F.W.; Slyke, G.V. Mechanical properties of four suture patterns for transected equine tendon repair. Vet. Surg. 1990, 19, 102–106. [Google Scholar] [CrossRef]
- Moores, A.P.; Comerford, E.J.; Tarlton, J.F.; Owen, M.R. Biomechanical and clinical evaluation of a modified 3-loop pulley suture pattern for reattachment of canine tendons to bone. Vet. Surg. 2004, 33, 391–397. [Google Scholar] [CrossRef]
- Wilson, L.; Banks, T.; Luckman, P.; Smith, B. Biomechanical evaluation of double Krackow sutures versus the three-loop pulley suture in a canine gastrocnemius tendon avulsion model. Aust. Vet. J. 2014, 92, 427–432. [Google Scholar] [CrossRef]
- Chauhan, A.; Schimoler, P.; Miller, M.C.; Kharlamov, A.; Merrell, G.A.; Palmer, B.A. Comparing biomechanical properties, repair times, and value of common core flexor tendon repairs. Hand 2018, 13, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Strickland, J.W. Development of flexor tendon surgery: Twenty-five years of progress. J. Hand Surg. Am. 2000, 25, 214–235. [Google Scholar] [CrossRef] [PubMed]
- Klifto, C.S.; Capo, J.T.; Sapienza, A.; Yang, S.S.; Paksima, N. Flexor tendon injuries. J. Am. Acad. Orthop. Surg. 2018, 26, e26–e35. [Google Scholar] [CrossRef]
- Putterman, A.B.; Duffy, D.J.; Kersh, M.E.; Rahman, H.; Moore, G.E. Effect of a continuous epitendinous suture as adjunct to three-loop pulley and locking-loop patterns for flexor tendon repair in a canine model. Vet. Surg. 2019, 48, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Roush, J.; DeBowes, R.; Gaughan, E.M. In vitro tensile strength of transected equine flexor tendons repaired by plate fixation. Vet. Surg. 1991, 20, 345. [Google Scholar]
- Roush, J.; DeBowes, R.; Gaughan, E.M. In vivo comparison of rabbit calcanean tendon strength after repair by three-loop pulley or tendon plating methods. Vet. Surg. 1992, 21, 404. [Google Scholar]
- Shani, J.; Shahar, R. Repair of chronic complete traumatic rupture of the common calcaneal tendon in a dog, using a fascia lata graft. Vet. Comp. Orthop. Traumatol. 2000, 13, 104–108. [Google Scholar] [CrossRef]
- Jenson, P.W.; Lillich, J.D.; Roush, J.K.; Gaughan, E.M. Ex vivo strength comparison of bioabsorbable tendon plates and bioabsorbable suture in a 3-loop pulley pattern for repair of transected flexor tendons from horse cadavers. Vet. Surg. 2005, 34, 565–570. [Google Scholar] [CrossRef]
- Swiderski, J.; Fitch, R.; Staatz, A.; Lowery, J. Sonographic assisted diagnosis and treatment of bilateral gastrocnemius tendon rupture in a Labrador retriever repaired with fascia lata and polypropylene mesh. Vet. Comp. Orthop. Traumatol. 2005, 18, 258–263. [Google Scholar]
- Baltzer, W.I.; Rist, P. Achilles tendon repair in dogs using the semitendinosus muscle: Surgical technique and short-term outcome in five dogs. Vet. Surg. 2009, 38, 770–779. [Google Scholar] [CrossRef]
- Gall, T.T.; Santoni, B.G.; Egger, E.L.; Puttlitz, C.M.; Rooney, M.B. In vitro biomechanical comparison of polypropylene mesh, modified three-loop pulley suture pattern, and a combi-nation for repair of distal canine achilles’ tendon injuries. Vet. Surg. 2009, 38, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Zellner, E.M.; Hale, M.J.; Kraus, K.H. Application of tendon plating to manage failed calcaneal tendon repairs in a dog. Vet. Surg. 2018, 47, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Cocca, C.J.; Duffy, D.J.; Kersh, M.E.; Kim, W.; Groenewold, A.; Moore, G.E. Biomechanical comparison of three epitendinous suture patterns as adjuncts to a core locking loop suture for repair of canine flexor tendon injuries. Vet. Surg. 2019, 48, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Duffy, D.J.; Chang, Y.-J.; Gaffney, L.S.; Fisher, M.B.; Moore, G.E. Effect of bite depth of an epitendinous suture on the biomechanical strength of repaired canine flexor tendons. Am. J. Vet. Res. 2019, 80, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Duffy, D.J.; Curcillo, C.P.; Chang, Y.-J.; Gaffney, L.; Fisher, M.B.; Moore, G.E. Biomechanical evaluation of an autologous flexor digitorum lateralis graft to augment the surgical repair of gastrocnemius tendon laceration in a canine ex vivo model. Vet. Surg. 2020, 49, 1545–1554. [Google Scholar] [CrossRef]
- Kim, S.H.; Choi, J.Y. Surgical outcomes and complications of septal extension graft supported by 3D printed polycaprolactone plate. Laryngoscope 2020, 130, 1680–1685. [Google Scholar] [CrossRef]
- Boonsirijarungradh, S.; Udomsom, S.; Manaspon, C.; Paengnakorn, P. Development of Customized Biodegradable Mesh Membrane for Dental Bone Graft Using Three-dimensional Printing Technique. Mater. Today Proc. 2022, 65, 2351–2357. [Google Scholar] [CrossRef]
- Lui, P.; Maffulli, N.; Rolf, C.; Smith, R. What are the validated animal models for tendinopathy? Scand. J. Med. Sci. Sport. 2011, 21, 3–17. [Google Scholar] [CrossRef]
- Hast, M.; Zuskov, A.; Soslowsky, L. The role of animal models in tendon research. Bone Jt. J. 2014, 3, 193–202. [Google Scholar] [CrossRef]
- Bottagisio, M.; Lovati, A.B. A review on animal models and treatments for the reconstruction of Achilles and flexor tendons. J. Mater. Sci. Mater. Med. 2017, 28, 45. [Google Scholar] [CrossRef]
- Gelberman, R.H.; Boyer, M.I.; Brodt, M.D.; Winters, S.C.; Silva, M.J. The effect of gap formation at the repair site on the strength and excursion of intrasynovial flexor tendons. An experimental study on the early stages of tendon-healing in dogs. J. Bone Jt. Surg. Am. 1999, 81, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Guerin, S.; Burbidge, H.; Firth, E.; Fox, S. Achilles tenorrhaphy in five dogs: A modified surgical technique and evaluation of a cranial half cast. Vet. Comp. Orthop. Traumatol. 1998, 11, 205–210. [Google Scholar]
- Fahie, M.A. Healing, diagnosis, repair, and rehabilitation of tendon conditions. Vet. Clin. N. Am. Small Anim. Pract. 2005, 35, 1195–1211. [Google Scholar] [CrossRef] [PubMed]
- Savage, R.; Risitano, G. Flexor tendon repair using a “six strand” method of repair and early active mobilisation. J. Hand Surg. Eur. Vol. 1989, 14, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Meeson, R.; Davidson, C.; Arthurs, G. Soft-tissue injuries associated with cast application for distal limb orthopaedic conditions. Vet. Comp. Orthop. Traumatol. 2011, 24, 126–131. [Google Scholar]
- Chang, Y.J.; Duffy, D.J.; Beamon, W.; Moore, G.E. Ex vivo biomechanical characteristics and effects on gap formation of using an internal fixation plate to augment primary three-loop pulley repair of canine gastrocnemius tendons. Am. J. Vet. Res. 2022, 83, 305–311. [Google Scholar] [CrossRef]
- Tang, J.B.; Zhang, Y.; Cao, Y.; Xie, R.G. Core suture purchase affects strength of tendon repairs. J. Hand Surg. Am. 2005, 30, 1262–1266. [Google Scholar] [CrossRef]
- Dy, C.J.; Hernandez-Soria, A.; Ma, Y.; Roberts, T.R.; Daluiski, A. Complications after flexor tendon repair: A systematic review and meta-analysis. J. Hand Surg. Am. 2012, 37, 543–551. [Google Scholar] [CrossRef]
- Kamal, R.N.; Yao, J. Evidence-based medicine: Surgical management of flexor tendon lacerations. Plast. Reconstr. Surg. 2017, 140, 130e. [Google Scholar] [CrossRef]
- Schulz, K.S.; Hayashi, K.; Fossum, T.W. Management of muscle and tendon injury or disease. In Small Animal Surgery, 5th ed.; Fossum, T.W., Ed.; Elsevier: Philadelphia, PA, USA, 2019; pp. 1280–1294. [Google Scholar]
- Stein, L.E.; Pijanowski, G.J.; Johnson, A.L. A histological evaluation of rabbit tendons sutured using the Bunnell pattern. Vet. Surg. 1985, 14, 145–148. [Google Scholar] [CrossRef]
- Chanchareonsook, N.; Tideman, H.; Feinberg, S.E.; Hollister, S.J.; Jongpaiboonkit, L.; Kin, L.; Jansen, J.A. Subcutaneous tissue response to titanium, poly (ϵ-caprolactone), and carbonate-substituted hydroxyapatite-coated poly (ϵ-caprolactone) plates: A rabbit study. J. Biomed. Mater. Res. A 2013, 101, 2258–2266. [Google Scholar] [CrossRef] [PubMed]
- McKeen, L.W. 13—Environmentally friendly polymers. In Permeability Properties of Plastics and Elastomers, 4th ed.; McKeen, L.W., Ed.; William Andrew: Norwich, NY, USA, 2017; pp. 305–323. [Google Scholar]
- Deshmukh, K.; Ahamed, M.B.; Deshmukh, R.R.; Pasha, S.K.; Bhagat, P.R.; Chidambaram, K. Biopolymer composites with high dielectric performance: Interface engineering. In Biopolymer Composites in Electronics, 1st ed.; Sadasivuni, K.K., Ponnamma, D., Kim, J., Cabibihan, J.J., Al Maadeed, M.A., Eds.; Elsevier: Cambridge, MA, USA, 2017; pp. 27–128. [Google Scholar]
- Fu, X.; Sammons, R.L.; Bertóti, I.; Jenkins, M.J.; Dong, H. Active screen plasma surface modification of polycaprolactone to improve cell attachment. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 314–320. [Google Scholar] [CrossRef]
- Aspenberg, P.; Virchenko, O. Platelet concentrate injection improves Achilles tendon repair in rats. Acta Orthop. Scand. 2004, 75, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Bosch, G.; van Schie, H.T.; de Groot, M.W.; Cadby, J.A.; van de Lest, C.H.; Barneveld, A.; van Weeren, P.R. Effects of platelet-rich plasma on the quality of repair of mechanically induced core lesions in equine superficial digital flexor tendons: A placebo-controlled experimental study. J. Orthop. Res. 2010, 28, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, D.; de Souza, R.A.; de Carvalho, W.R.G.; Xavier, M.; de Carvalho, P.K.; Cunha, T.C.R.; Villaverde, A.B. Low-level laser therapy combined with platelet-rich plasma on the healing calcaneal tendon: A histological study in a rat model. Lasers Med. Sci. 2013, 28, 1489–1494. [Google Scholar] [CrossRef] [PubMed]
- Allahverdi, A.; Sharifi, D.; Takhtfooladi, M.A.; Hesaraki, S.; Khansari, M.; Dorbeh, S.S. Evaluation of low-level laser therapy, platelet-rich plasma, and their combination on the healing of Achilles tendon in rabbits. Lasers Med. Sci. 2015, 30, 1305–1313. [Google Scholar] [CrossRef] [PubMed]


| Suture Pattern | Mean Yield Force, N | Mean Peak Force, N | Mean Failure Force, N |
|---|---|---|---|
| 3LP | 14.97 ± 2.28 a | 18.03 ± 4.35 a | 16.88 ± 3.76 a |
| 3LP + ES | 31.44 ± 11.36 b | 41.01 ± 10.17 b | 38.33 ± 9.81 b |
| 3LP + 1-mm PCL plate | 19.92 ± 3.58 a,c | 28.75 ± 5.23 c | 26.47 ± 5.06 a,b |
| 3LP + 2-mm PCL plate | 30.13 ± 5.44 b,c | 39.29 ± 4.00 b | 37.30 ± 5.27 b |
| 3LP + 1.5-mm titanium plate | 52.20 ± 9.01 d | 67.55 ± 9.18 d | 60.36 ± 14.00 c |
| Suture Pattern | 1-mm Gapping | 3-mm Gapping | ||
|---|---|---|---|---|
| Force, Mean ± SD, N | Frequency (%) | Force, Mean ± SD, N | Frequency (%) | |
| 3LP | 16.02 ± 3.81 a | 10/10 (100%) | N/A | 0 |
| 3LP + ES | 30.09 ± 4.98 b,c | 10/10 (100%) | 32.97 ± 3.42 a | 2/10(20%) |
| 3LP + 1-mm PCL plate | 24.71 ± 6.38 a,b | 10/10 (100%) | N/A | 0 |
| 3LP + 2-mm PCL plate | 27.27 ± 4.45 b | 10/10 (100%) | 39.02 ± 2.13 b | 7/10(70%) |
| 3LP + 1.5-mm titanium plate | 39.35 ± 13.15 c | 10/10 (100%) | 49.46 ± 6.76 c | 9/10(90%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huidan, Z.; Kang, J.; Kim, N.; Heo, S. Ex Vivo Biomechanical Assessment of Various Repair Techniques in a Rabbit Calcaneal Tendon Avulsion Model: Application of Polycaprolactone Plate. Vet. Sci. 2023, 10, 289. https://doi.org/10.3390/vetsci10040289
Huidan Z, Kang J, Kim N, Heo S. Ex Vivo Biomechanical Assessment of Various Repair Techniques in a Rabbit Calcaneal Tendon Avulsion Model: Application of Polycaprolactone Plate. Veterinary Sciences. 2023; 10(4):289. https://doi.org/10.3390/vetsci10040289
Chicago/Turabian StyleHuidan, Zheng, Jinsu Kang, Namsoo Kim, and Suyoung Heo. 2023. "Ex Vivo Biomechanical Assessment of Various Repair Techniques in a Rabbit Calcaneal Tendon Avulsion Model: Application of Polycaprolactone Plate" Veterinary Sciences 10, no. 4: 289. https://doi.org/10.3390/vetsci10040289
APA StyleHuidan, Z., Kang, J., Kim, N., & Heo, S. (2023). Ex Vivo Biomechanical Assessment of Various Repair Techniques in a Rabbit Calcaneal Tendon Avulsion Model: Application of Polycaprolactone Plate. Veterinary Sciences, 10(4), 289. https://doi.org/10.3390/vetsci10040289






