Effect of Prepartum Magnesium Butyrate Supplementation on Rumination Time, Milk Yield and Composition, and Blood Parameters in Dairy Cows
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Animals, Sampling, and Ethical Approval
2.2. Animal Management, Housing and Diets
2.3. Diet Analysis
2.4. Milk Sampling and Composition Analysis
2.5. Plasma BHB, NEFA, and Glucose
2.6. Serum Haptoglobin
2.7. Ionized Calcium
2.8. Rumination Time
2.9. Statistical Analysis
3. Results
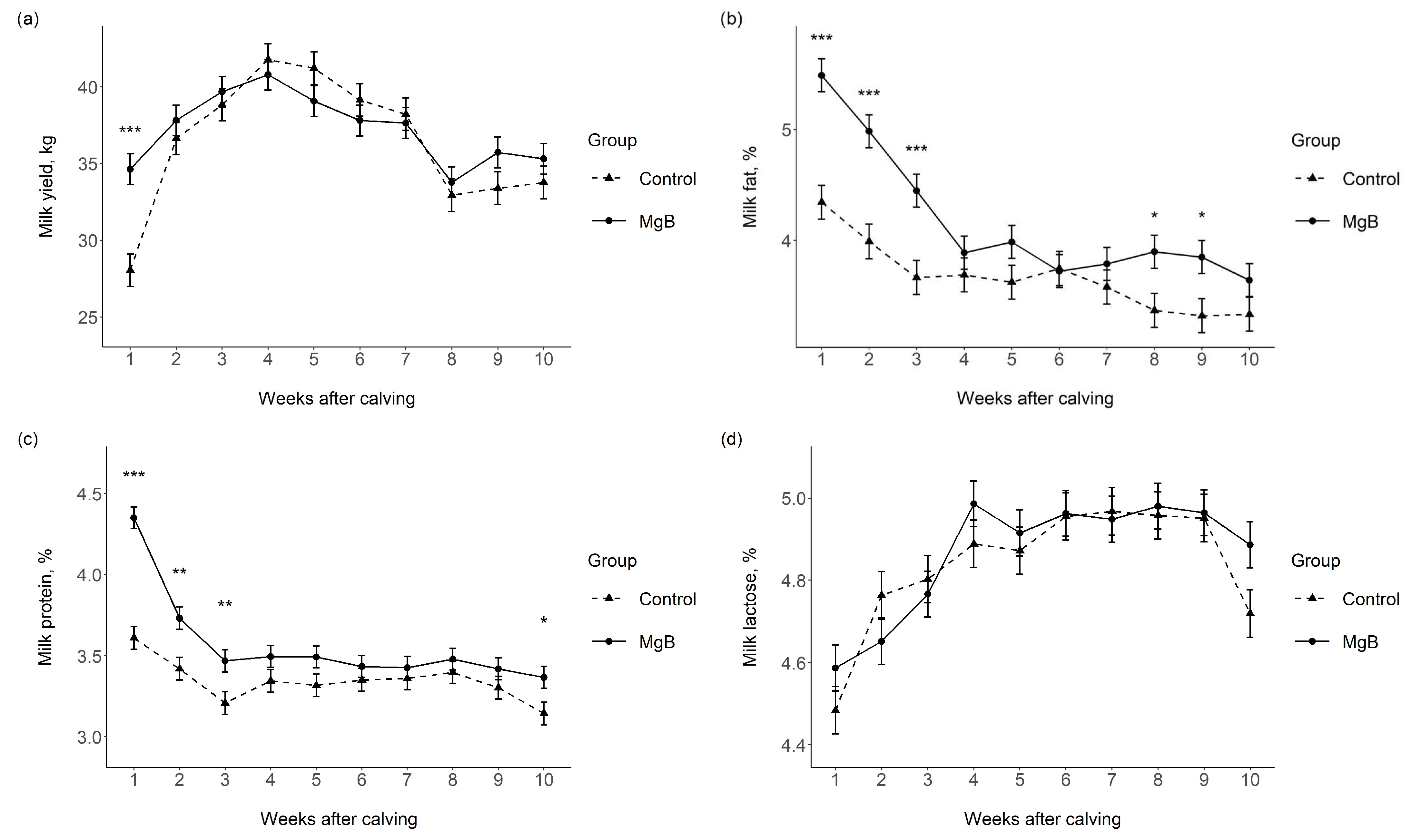
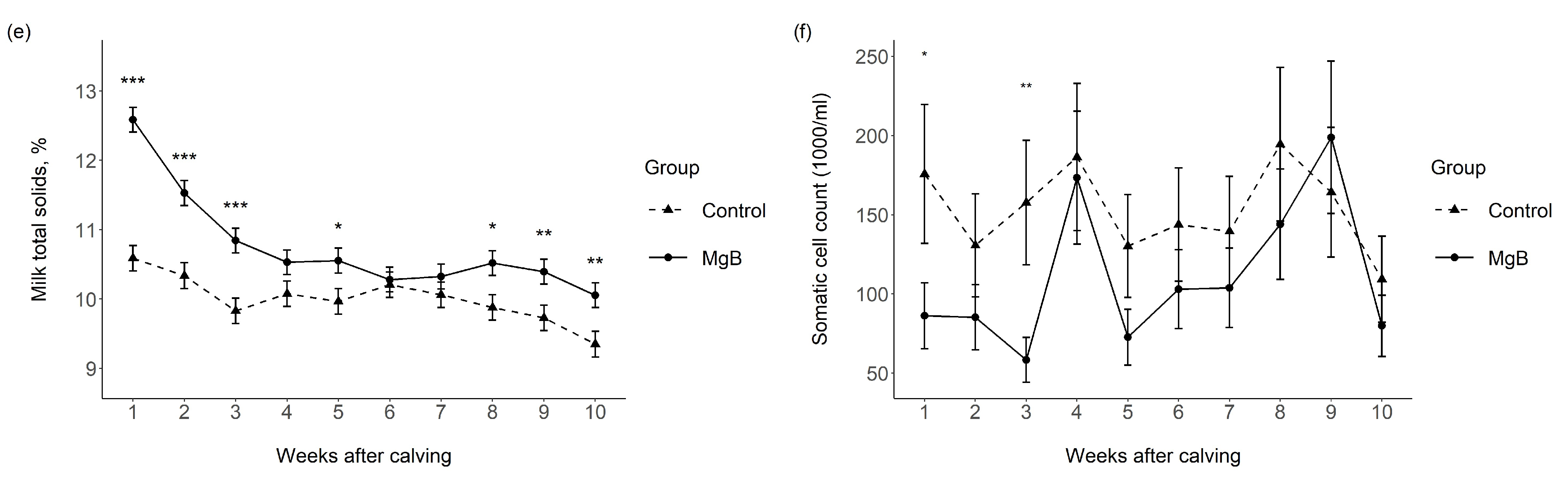
3.1. Milk Yield and Milk Chemical Composition
3.2. Blood Analytes
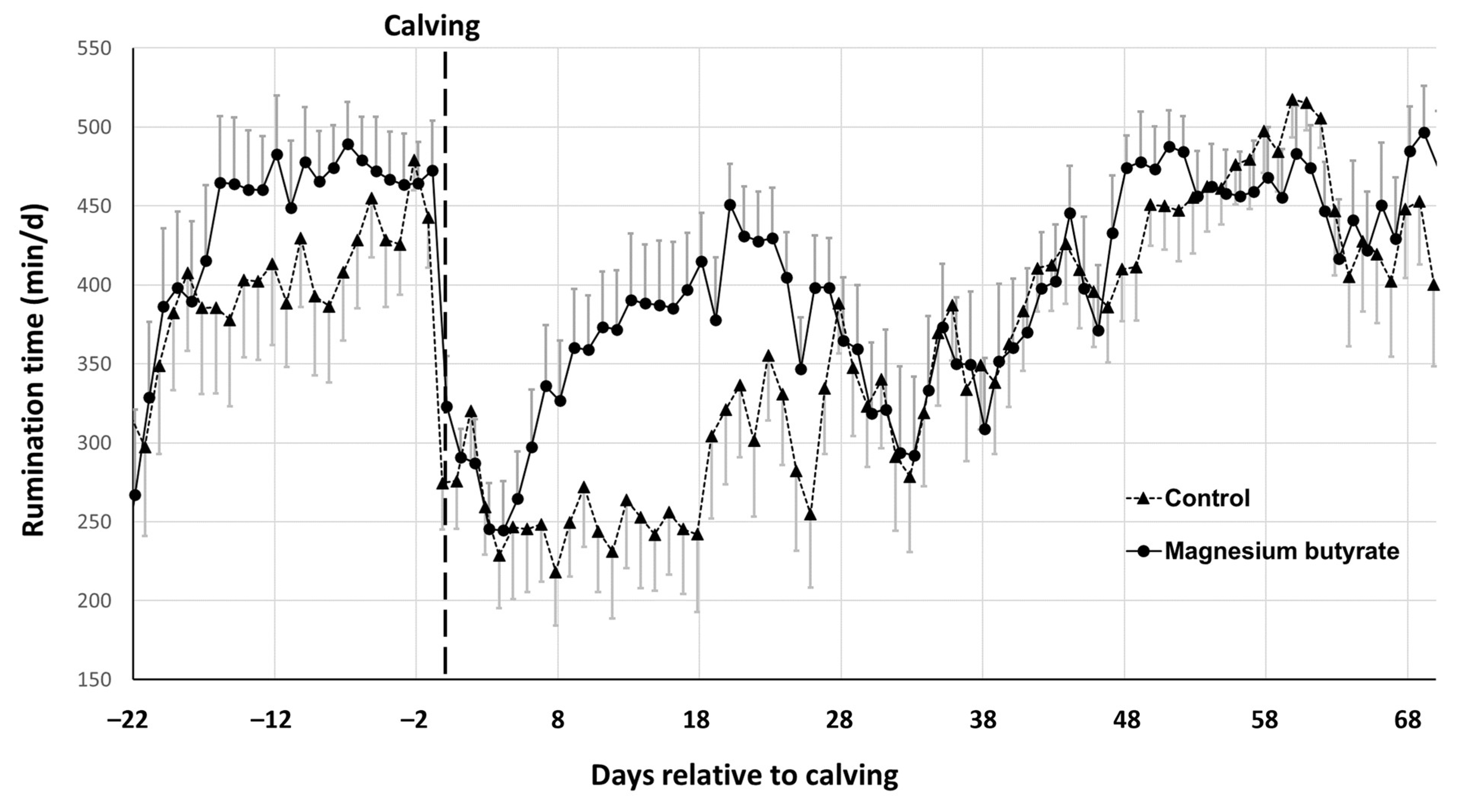
3.3. Rumination Time
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jenkins, T.C.; McGuire, M.A. Major advances in nutrition: Impact on milk composition. J. Dairy Sci. 2006, 89, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Tyasi, T.L.; Gxasheka, M.; Tlabela, C.P. Assessing the effect of nutrition on milk composition of dairy cows: A review. Int. J. Curr. Sci. 2015, 17, 56–63. [Google Scholar]
- O’Callaghan, T.F.; Hennessy, D.; McAuliffe, S.; Kilcawley, K.N.; O’Donovan, M.; Dillon, P.; Ross, R.P.; Stanton, C. Effect of pasture versus indoor feeding systems on raw milk composition and quality over an entire lactation. J. Dairy Sci. 2016, 99, 9424–9440. [Google Scholar] [CrossRef] [PubMed]
- Sakata, T.; Tamate, H. Rumen epithelial cell proliferation accelerated by rapid increase in intraruminal butyrate. J. Dairy Sci. 1987, 61, 1109–1113. [Google Scholar] [CrossRef]
- Dieho, K.; Bannink, A.; Geurts, I.A.L.; Schonewille, J.T.; Gort, G.; Dijkstra, J. Morphological adaptation of rumen papillae during the dry period and early lactation as affected by rate of increase of concentrate allowance. J. Dairy Sci. 2016, 99, 2339–2352. [Google Scholar] [CrossRef]
- Martens, H.; Rabbani, I.; Shen, Z.; Stumpff, F.; Deiner, C. Changes in rumen absorption processes during transition. Anim. Feed Sci. Technol. 2012, 172, 95–102. [Google Scholar] [CrossRef]
- Mentschel, J.; Leiser, R.; Mülling, C.; Pfarrer, C.; Claus, R. Butyric acid stimulates rumen mucosa development in the calf mainly by a reduction of apoptosis. Arch. Anim. Nutr. 2001, 55, 85–102. [Google Scholar] [CrossRef]
- Storm, A.C.; Hanigan, M.D.; Kristensen, N.B. Effects of ruminal ammonia and butyrate concentrations on reticuloruminal epithelial blood flow and volatile fatty acid absorption kinetics under washed reticulorumen conditions in lactating dairy cows. J. Dairy Sci. 2011, 94, 3980–3994. [Google Scholar] [CrossRef]
- Laarman, A.H.; Dionissopoulos, L.; AlZahal, O.; Greenwood, S.L.; Steele, M.A.; McBride, B.W. Butyrate and subacute ruminal acidosis affect abundance of membrane proteins involved with proton and short chain fatty acid transport in the rumen epithelium of dairy cows. Am. J. Anim. Vet. Sci. 2013, 8, 220–229. [Google Scholar] [CrossRef]
- Bergman, E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990, 70, 567–590. [Google Scholar] [CrossRef]
- Baumgard, L.H.; Collier, R.J.; Bauman, D.E. A 100-year review: Regulation of nutrient partitioning to support lactation. J. Dairy Sci. 2017, 100, 10353–10366. [Google Scholar] [CrossRef] [PubMed]
- Wankhade, P.R.; Manimaran, A.; Kumaresan, A.; Jeyakumar, S.; Ramesha, K.; Sejian, V.; Rajendran, D.; Varghese, M.R. Metabolic and immunological changes in transition dairy cows: A review. Vet. World 2017, 10, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Trevisi, E.; Minuti, A. Assessment of the innate immune response in the periparturient cow. Res. Vet. Sci. 2018, 116, 47–54. [Google Scholar] [CrossRef]
- Cray, C.; Zaias, J.; Altman, N.H. Acute phase response in animals: A review. Comp. Med. 2009, 59, 517–526. [Google Scholar]
- Kent, J. Acute phase proteins: Their use in veterinary diagnosis. Br. Vet. J. 1992, 148, 279–282. [Google Scholar] [CrossRef]
- Murata, H.; Shimada, N.; Yoshioka, M. Current research on acute phase proteins in veterinary diagnosis: An overview. Vet. J. 2004, 168, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Lomborg, S.R.; Nielsen, L.R.; Heegaard, P.M.; Jacobsen, S. Acute phase proteins in cattle after exposure to complex stress. Vet. Res. Commun. 2008, 32, 575–582. [Google Scholar] [CrossRef]
- Bannikov, G.; Hinds, C.; Rajala-Schultz, P.; Premanandan, C.; Rings, D.; Lakritz, J. Serum haptoglobin-matrix metalloproteinase 9 (Hp-MMP 9) complex as a biomarker of systemic inflammation in cattle. Vet. Immunol. Immunopathol. 2011, 139, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Conner, J.G.; Eckersall, P.D.; Doherty, M.; Douglas, T.A. Acute phase response and mastitis in the cow. Res. Vet. Sci. 1986, 41, 126–128. [Google Scholar] [CrossRef]
- Salvi, P.S.; Cowles, R.A. Butyrate and the Intestinal Epithelium: Modulation of Proliferation and Inflammation in Homeostasis and Disease. Cells 2021, 14, 1775. [Google Scholar] [CrossRef]
- Engelking, L.E.; Ambrose, D.J.; Oba, M. Effects of dietary butyrate supplementation and oral nonsteroidal anti-inflammatory drug administration on serum inflammatory markers and productivity of dairy cows during the calving transition. J. Dairy Sci. 2022, 105, 4144–4155. [Google Scholar] [CrossRef]
- Sprecher, D.J.; Hostetler, D.E.; Kanneene, J.B. A lameness scoring system that uses posture and gait to predict dairy cattle reproductive performance. Theriogenology 1997, 47, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis, 17th ed.; Horwitz, W., Ed.; Association of Analytical Chemists: Washington, DC, USA, 2000. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Bach Knudsen, K.E.; Aaman, P.; Eggum, B.O. Nutritive value of Danish-grown barley varieties, I. Carbohydrates and other major constituents. J. Cereal Sci. 1987, 6, 173–186. [Google Scholar] [CrossRef]
- Larsson, K.; Bengtsson, S. Metodbeskrivning Nr. 22. Statens Lantbrukskemiske Laboratorium; Swedish University of Agricultural Sciences: Uppsala, Sweden, 1983. [Google Scholar]
- Schirmann, K.; von Keyserlingk, M.A.G.; Weary, D.M.; Veira, D.M.; Heuwieser, W. Technical note: Validation of a system for monitoring rumination in dairy cows. J. Dairy Sci. 2009, 92, 6052–6055. [Google Scholar] [CrossRef]
- Clark, C.E.F.; Lyons, N.A.; Millapan, L.; Talukder, S.; Cronin, G.M.; Kerrisk, K.L.; Garcia, S.C. Rumination and activity levels as predictors of calving for dairy cows. Animal 2015, 9, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Kovács, L.; Kézér, F.L.; Ruff, F.; Szenci, O. Rumination time and reticuloruminal temperature as possible predictors of dystocia in dairy cows. J. Dairy Sci. 2017, 100, 1568–1579. [Google Scholar] [CrossRef]
- Horváth, A.; Lénárt, L.; Csepreghy, A.; Madar, M.; Pálffy, M.; Szenci, O. A field study using different technologies to detect calving at a large-scale Hungarian dairy farm. Reprod. Domest. Anim. 2021, 56, 673–679. [Google Scholar] [CrossRef]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org (accessed on 24 February 2023).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lenth, R.V. Emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.7.2. 2022. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 24 February 2023).
- Gastwirth, J.L.; Gel, Y.R.; Wallace, W.L.; Lyubchich, V.; Miao, W.; Noguchi, K. Lawstat: Tools for Biostatistics, Public Policy, and Law. R Package Version 3.4. 2020. Available online: https://CRAN.R-project.org/package=lawstat (accessed on 24 February 2023).
- Jurkovich, V.; Bakony, M.; Laky, E.; Ruff, F.; Kézér, F.L.; Bende, A.; Kovács, L. Cardiac vagal tone, plasma cortisol, and dehydroepiandrosterone response to an ACTH challenge in lame and nonlame dairy cows. Domest. Anim. Endocrinol. 2020, 71, 106388. [Google Scholar] [CrossRef]
- Puppel, K.; Gołębiewski, M.; Grodkowski, G.; Slósarz, J.; Kunowska-Slósarz, M.; Solarczyk, P.; Łukasiewicz, M.; Balcerak, M.; Przysucha, T. Composition and Factors Affecting Quality of Bovine Colostrum: A Review. Animals 2019, 9, 1070. [Google Scholar] [CrossRef] [PubMed]
- Izumi, K.; Fukumori, R.; Oikawa, S.; Oba, M. Short communication: Effects of butyrate supplementation on the productivity of lactating dairy cows fed diets differing in starch content. J. Dairy Sci. 2019, 102, 11051–11056. [Google Scholar] [CrossRef]
- Kowalski, Z.M.; Górka, P.; Flaga, J.; Barteczko, A.; Burakowska, K.; Oprządek, J.; Zabielski, R. Effect of microencapsulated sodium butyrate in the close-up diet on performance of dairy cows in the early lactation period. J. Dairy Sci. 2015, 98, 3284–3291. [Google Scholar] [CrossRef]
- De Groot, B.; Edwards, J.; Schonewille, T. Magnesium butyrate is a readily available magnesium source in dairy cow nutrition. Preprints 2022, 2022030120. [Google Scholar] [CrossRef]
- National Academy of Sciences, Engineering and Medicine (NASEM). Nutrient Requirements of Dairy Cattle, 8th ed.; The National Academies Press: Washington, DC, USA, 2021. [Google Scholar]
- Nyman, A.K.; Emanuelson, U.; Holtenius, K.; Ingvartsen, K.L.; Larsen, T.; Persson Waller, K. Metabolites and immune variables associated with somatic cell counts of primiparous dairy cows. J. Dairy Sci. 2008, 91, 2996–3009. [Google Scholar] [CrossRef]
- Aabdin, Z.U.; Bilal, M.S.; Dai, H.; Abaker, J.A.; Liu, X.; Benazir, S.; Yan, J.; Shen, X. NOD1/NF-κB signaling pathway inhibited by sodium butyrate in the mammary gland of lactating goats during sub-acute ruminal acidosis. Microb. Pathog. 2018, 122, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Davie, J.R. Inhibition of histone deacetylase activity by butyrate. J. Nutr. 2003, 133, 2485–2493. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.T.; Cresci, G.A.M. The Immunomodulatory functions of butyrate. J. Inflamm. Res. 2021, 18, 6025–6041. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, J.; Huang, J.; Chang, G.; Roy, A.C.; Gao, Q.; Cheng, X.; Shen, X. Sodium butyrate attenuated iE-DAP induced inflammatory response in the mammary glands of dairy goats fed high-concentrate diet. J. Sci. Food Agric. 2020, 101, 1218–1227. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, Y.; Zhang, R.; He, T.; Huang, G.; Tian, K.; Liu, J.; Chen, J.; Dong, G. Sodium butyrate more effectively mitigates the negative effects of high-concentrate diet in dairy cows than sodium β-hydroxybutyrate via reducing free bacterial cell wall components in rumen fluid and plasma. Toxins 2021, 13, 352. [Google Scholar] [CrossRef] [PubMed]
- Huzzey, J.M.; Duffield, T.F.; LeBlanc, S.J.; Veira, D.M.; Weary, D.M.; von Keyserlingk, M.A.G. Short communication: Haptoglobin as an early indicator of metritis. J. Dairy Sci. 2009, 92, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Salonen, M.; Hirvonen, J.; Pyörälä, S.; Sankari, S.; Sandholm, M. Quantitative determination of bovine serum haptoglobin in experimentally induced Escherichia coli mastitis. Res. Vet. Sci. 1996, 60, 88–91. [Google Scholar] [CrossRef]
- Godson, D.L.; Campos, M.; Attah-Poku, S.K.; Redmond, M.J.; Cordeiro, D.M.; Sethi, M.S.; Harland, R.J.; Babiuk, L.A. Serum haptoglobin as an indicator of the acute phase response in bovine respiratory disease. Vet. Immunol. Immunopathol. 1996, 51, 277–292. [Google Scholar] [CrossRef]
- Humblet, M.F.; Guyot, H.; Boudry, B.; Mbayahi, F.; Hanzen, C.; Rollin, F.; Godeau, J.M. Relationship between haptoglobin, serum amyloid A, and clinical status in a survey of dairy herds during a 6-month period. Vet. Clin. Pathol. 2006, 35, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Eckersall, P.D.; Bell, R. Acute phase proteins: Biomarkers of infection and inflammation in veterinary medicine. Vet. J. 2010, 185, 23–27. [Google Scholar] [CrossRef]
- Chan, J.P.; Chu, C.C.; Fung, H.P.; Chuang, S.T.; Lin, Y.C.; Chu, R.M.; Lee, S.L. Serum haptoglobin concentration in cattle. J. Vet. Med. Sci. 2004, 66, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Cartes, D.; Strappini, A.; Sepúlveda-Varas, P. Provision of shelter during the prepartum period: Effects on behavior, blood analytes, and health status in dairy cows in winter. J. Dairy Sci. 2021, 104, 3508–3521. [Google Scholar] [CrossRef]
- McArt, J.A.; Nydam, D.V.; Oetzel, G.R.; Overton, T.R.; Ospina, P.A. Elevated non-esterified fatty acids and β-hydroxybutyrate and their association with transition dairy cow performance. Vet. J. 2013, 198, 560–570. [Google Scholar] [CrossRef]
- Overton, T.R.; McArt, J.A.A.; Nydam, D.V. A 100-year review: Metabolic health indicators and management of dairy cattle. J. Dairy Sci. 2017, 100, 10398–10417. [Google Scholar] [CrossRef]
- González, F.D.; Muiño, R.; Pereira, V.; Campos, R.; Benedito, J.L. Relationship among blood indicators of lipomobilization and hepatic function during early lactation in high-yielding dairy cows. J. Vet Sci. 2011, 12, 251–255. [Google Scholar] [CrossRef]
- Weber, C.; Hametner, C.; Tuchscherer, A.; Losand, B.; Kanitz, E.; Otten, W.; Singh, S.P.; Bruckmaier, R.M.; Becker, F.; Kanitz, W.; et al. Variation in fat mobilization during early lactation differently affects feed intake, body condition, and lipid and glucose metabolism in high-yielding dairy cows. J. Dairy Sci. 2013, 96, 165–180. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, S.J.; Leslie, K.E.; Duffield, T.F. Metabolic predictors of displaced abomasum in dairy cattle. J. Dairy Sci. 2005, 88, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Büchel, S.; Sundrum, A. Short communication: Decrease in rumination time as an indicator of the onset of calving. J. Dairy Sci. 2014, 97, 3120–3127. [Google Scholar] [CrossRef]
- Schirmann, K.; Chapinal, N.; Weary, D.M.; Vickers, L.; von Keyserlingk, M.A.G. Short communication: Rumination and feeding behavior before and after calving in dairy cows. J. Dairy Sci. 2013, 96, 7088–7092. [Google Scholar] [CrossRef]
- Mammi, L.M.E.; Cavallini, D.; Fustini, M.; Fusaro, I.; Giammarco, M.; Formigoni, A.; Palmonari, A. Calving difficulty influences rumination time and inflammatory profile in Holstein dairy cows. J. Dairy Sci. 2021, 104, 750–761. [Google Scholar] [CrossRef]
- Pahl, C.; Hartung, E.; Grothmann, A.; Mahlkow-Nerge, K.; Haeussermann, A. Rumination activity of dairy cows in the 24 hours before and after calving. J. Dairy Sci. 2014, 97, 6935–6941. [Google Scholar] [CrossRef]
- Yang, W.Z.; Beauchemin, K.A. Effects of physically effective fiber on chewing activity and rumen pH of dairy cows fed diets based on barley silage. J. Dairy Sci. 2006, 89, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Calamari, L.; Soriani, N.; Panella, G.; Petrera, F.; Minuti, A.; Trevisi, E. Rumination time around calving: An early signal to detect cows at greater risk of disease. J. Dairy Sci. 2014, 97, 3635–3647. [Google Scholar] [CrossRef]
- Stangaferro, M.L.; Wijma, R.; Caixeta, L.S.; Al-Abri, M.A.; Giordano, J.O. Use of rumination and activity monitoring for the identification of dairy cows with health disorders: Part III. Metritis. J. Dairy Sci. 2016, 99, 7422–7433. [Google Scholar] [CrossRef]
- Paudyal, S. Using rumination time to manage health and reproduction in dairy cattle: A review. Vet. Q. 2021, 41, 292–300. [Google Scholar] [CrossRef]
| Month | Average THI | No. of Animals Enrolled | ||
|---|---|---|---|---|
| Control | MgB | Control | MgB | |
| February | 42 | 40 | 4 | 5 |
| March | 47 | 47 | 6 | 6 |
| April | 50 | 50 | 7 | 8 |
| May | 58 | 58 | 6 | 7 |
| June | 70 | 71 | 5 | 5 |
| July | 72 | 72 | 3 | 3 |
| Item | Prepartum TMR | Postpartum TMR | |
|---|---|---|---|
| Ingredient | Control | MgB 1 | |
| Forage components (% DM) | |||
| Corn silage | 61.8 | 61.8 | 60.2 |
| Rye hay | - | - | 10.8 |
| Alfalfa baled silage | 10.9 | 10.9 | 12.9 |
| Grass hay | 7.3 | 7.3 | 5.3 |
| Straw | 12.7 | 12.7 | - |
| Brewer’s grains | 7.3 | 7.3 | 10.8 |
| Concentrate components (% DM) | |||
| Corn flour | 27.6 | 27.6 | 52.0 |
| Oats | 13.8 | 13.8 | 13.6 |
| Extracted rapeseed meal | 13.8 | 13.8 | 17.6 |
| Extracted sunflower meal | 34.5 | 34.5 | 12.0 |
| Premix Mipro Pren 250 2 | 10.3 | 10.3 | - |
| Premix Mipro RB 600 3 | - | - | 4.8 |
| Chemical composition 4 | |||
| Dry matter (DM), g/kg | 437 | 441 | 426 |
| Ash, g/kg of DM | 84 | 86 | 71 |
| Crude protein, g/kg of DM | 114 | 114 | 167 |
| Ether extract, g/kg of DM | 28 | 29 | 35 |
| Crude fiber, g/kg of DM | 241 | 232 | 143 |
| NDF, g/kg of DM | 507 | 494 | 339 |
| ADF, g/kg of DM | 303 | 280 | 190 |
| ADL, g/kg of DM | 50 | 47 | 39 |
| Starch, g/kg of DM | 154 | 158 | 283 |
| Sugar, g/kg of DM | 26 | 26 | 29 |
| Days Relative to Calving 1 | p Value | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −21 | −14 | −7 | −1 | 0 | 7 | 14 | 21 | 35 | 70 | SEM | Treatment | Time | Treatment × Time | ||
| BHB (mmol/L) | Control | 0.37 | 0.38 | 0.36 | 0.36 | 0.34 | 0.71 | 0.43 | 0.60 | 0.60 | 0.51 | 0.074 | 0.825 | <0.001 | 0.891 |
| MgB | 0.38 | 0.33 | 0.33 | 0.36 | 0.33 | 0.75 | 0.51 | 0.45 | 0.54 | 0.52 | |||||
| NEFA (mmol/L) | Control | 0.24 | 0.28 | 0.33 | 0.39 | 0.80 | 0.73 | 0.55 | 0.43 | 0.28 | 0.17 | 0.075 | 0.355 | <0.001 | 0.937 |
| MgB | 0.18 | 0.23 | 0.28 | 0.30 | 0.77 | 0.66 | 0.44 | 0.40 | 0.22 | 0.14 | |||||
| Glucose (mmol/L) | Control | 3.91 | 3.82 | 3.93 | 3.77 | 6.28 | 2.98 | 3.19 | 3.39 | 3.39 | 3.76 | 0.194 | 0.586 | <0.001 | 0.111 |
| MgB | 3.94 | 3.97 | 3.94 | 3.92 | 5.81 | 3.07 | 3.22 | 3.34 | 3.40 | 3.59 | |||||
| Haptoglobin (g/L) | Control | 0.40 | 0.40 | 0.41 | 0.42 | 0.43 | 0.66 a | 0.52 a | 0.59 a | 0.60 a | 0.51 a | 0.025 | <0.001 | <0.001 | <0.001 |
| MgB | 0.38 | 0.39 | 0.38 | 0.41 | 0.42 | 0.50 b | 0.43 b | 0.43 b | 0.45 b | 0.39 b | |||||
| Ionized Ca (mmol/L) | Control | 1.21 | 1.20 | 1.20 | 1.21 | 1.08 | 1.14 | 1.15 | 1.15 | 1.15 | 1.10 | 0.01 | 0.922 | <0.001 | 0.943 |
| MgB | 1.20 | 1.21 | 1.20 | 1.20 | 1.10 | 1.14 | 1.15 | 1.15 | 1.14 | 1.10 | |||||
| Rumination Time Parameters 1 | Group | p Value 2 | ||
|---|---|---|---|---|
| Units | Control | MgB | ||
| Baseline | min/d | 351.5 ± 46.4 | 347.0 ± 45.5 | 0.850 |
| AUC PRE | min | 8582.6 ± 1210.4 | 9568.0 ± 1320.5 | 0.456 |
| AUC POST | min | 22 390.5 ± 3432.3 | 28 235.4 ± 4067.2 | 0.012 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fébel, H.; Edwards, J.; Pajor, F.; Jurkovich, V.; Bakony, M.; Kovács, L. Effect of Prepartum Magnesium Butyrate Supplementation on Rumination Time, Milk Yield and Composition, and Blood Parameters in Dairy Cows. Vet. Sci. 2023, 10, 276. https://doi.org/10.3390/vetsci10040276
Fébel H, Edwards J, Pajor F, Jurkovich V, Bakony M, Kovács L. Effect of Prepartum Magnesium Butyrate Supplementation on Rumination Time, Milk Yield and Composition, and Blood Parameters in Dairy Cows. Veterinary Sciences. 2023; 10(4):276. https://doi.org/10.3390/vetsci10040276
Chicago/Turabian StyleFébel, Hedvig, Joan Edwards, Ferenc Pajor, Viktor Jurkovich, Mikolt Bakony, and Levente Kovács. 2023. "Effect of Prepartum Magnesium Butyrate Supplementation on Rumination Time, Milk Yield and Composition, and Blood Parameters in Dairy Cows" Veterinary Sciences 10, no. 4: 276. https://doi.org/10.3390/vetsci10040276
APA StyleFébel, H., Edwards, J., Pajor, F., Jurkovich, V., Bakony, M., & Kovács, L. (2023). Effect of Prepartum Magnesium Butyrate Supplementation on Rumination Time, Milk Yield and Composition, and Blood Parameters in Dairy Cows. Veterinary Sciences, 10(4), 276. https://doi.org/10.3390/vetsci10040276









