An Unusual Case of Collision Testicular Tumor in a Female DSD Dog
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
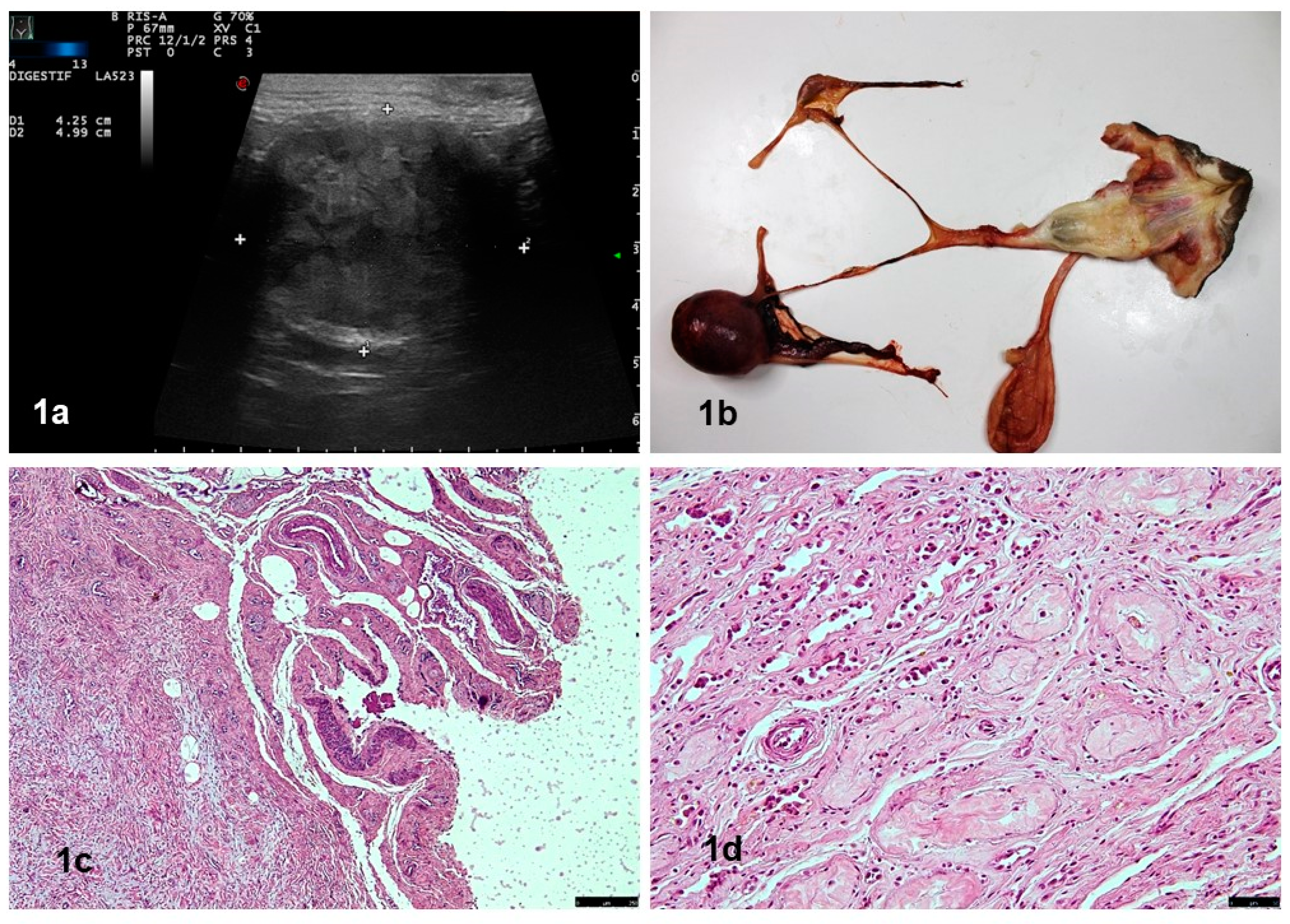
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jakab, C.; Balka, G. First report of a malignant collision skin tumour with malignant melanoma and anaplastic sarcoma components in a dog. Acta Vet. Hung. 2012, 60, 245–255. [Google Scholar] [CrossRef]
- Rodríguez, F.; Castro, P.; Ramírez, G.A. Collision Tumour of Squamous Cell Carcinoma and Malignant Melanoma in the Oral Cavity of a Dog. J. Comp. Pathol. 2016, 154, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Coca-Pelaz, A.; Triantafyllou, A.; Devaney, K.O.; Rinaldo, A.; Takes, R.P.; Ferlito, A. Collision tumors of the larynx: A critical review. Am. J. Otolaryngol. 2016, 37, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.C.; Gu, M.J.; Kim, S.W.; Kim, J.W.; Choi, J.H. Coexistence of gastrointestinal stromal tumor and inflammatory myofibroblastic tumor of the stomach presenting as a collision tumor: First case report and literature review. Diagn. Pathol. 2015, 10, 181. [Google Scholar] [CrossRef]
- Favetta, L.A.; Villagómez, D.A.F.; Iannuzzi, L.; Di Meo, G.; Webb, A.; Crain, S.; King, W.A. Disorders of sexual development and abnormal early development in domestic food-producing mammals: The role of chromosome abnormalities, environment and stress factors. Sex Dev. 2012, 6, 18–32. [Google Scholar] [CrossRef]
- Poth, T.; Breuer, W.; Walter, B.; Hecht, W.; Hermanns, W. Disorders of sex development in the dog-Adoption of a new nomenclature and reclassification of reported cases. Anim. Reprod. Sci. 2010, 121, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Marcinkowska-Swojak, M.; Szczerbal, I.; Pausch, H.; Nowacka-Woszuk, J.; Flisikowski, K.; Dzimira, S.; Nizanski, W.; Payan-Carreira, R.; Fries, R.; Kozlowski, P.; et al. Copy number variation in the region harboring SOX9 gene in dogs with testicular/ovotesticular disorder of sex development (78, XX; SRY-negative). Sci. Rep. 2015, 1, 14696. [Google Scholar] [CrossRef] [PubMed]
- Lyle, S.K. Disorders of sexual development in the dog and cat. Theriogenology 2007, 68, 338–343. [Google Scholar] [CrossRef]
- Meyers-Wallen, V.N. Gonadal and sex differentiation abnormalities of dogs and cats. Sex Dev. 2012, 6, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Krzemińska, P.; D’Anza, E.; Ciotola, F.; Paciello, O.; Restucci, B.; Peretti, V.; Albarella, S.; Switonski, M. Polymorphisms of MAMLD1, SRD5A2, and AR Candidate Genes in Seven Dogs (78, XY; SRY-Positive) Affected by Hypospadias or Cryptorchidism. Sex Dev. 2019, 13, 92–98. [Google Scholar] [CrossRef]
- Herndon, A.M.; Casal, M.L.; Jaques, J.T. Testicular neoplasia in the retained testicles of an intersex male dog. J. Am. Anim. Hosp. Assoc. 2012, 48, 118–124. [Google Scholar] [CrossRef]
- Dzimira, S.; Nizanski, W.; Ochota, M.; Madej, J.A. Histopathological pattern of gonads in cases of sex abnormalities in dogs: An attempt of morphological evaluation involving potential for neoplasia. Pathol. Res. Pract. 2015, 211, 772–775. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Bai, C.; Li, Y.; Li, Y.; Hou, J.; Zhao, Z.; Han, W. Sex identification of dog by PCR based on the differences in the AMELX and AMELY genes. Anim. Genet. 2013, 44, 606. [Google Scholar] [CrossRef]
- Edwards, J.F.; Cullen, J.M.; Kennedy, P.C. Histological Classification of Tumors of the Genital System of Domestic Animals, 2nd ed.; WHO Volume 4 of Second Series, International Classification of Tumors of Domestic Animals; Armed Forces Institute of Pathology: Washington, DC, USA, 1999. [Google Scholar]
- Albarella, S.; Lorenzi, L.; Rossi, E.; Prisco, F.; Riccardi, M.G.; Restucci, B.; Ciotola, F.; Parma, P. Analysis of XX SRY-Negative Sex Reversal Dogs. Animals 2020, 10, 1667. [Google Scholar] [CrossRef] [PubMed]
- Szczerbal, I.; Nizanski, W.; Dzimira, S.; Nowacka-Woszuk, J.; Stachecka, J.; Biezynski, J.; Ligocka, Z.; Jagodka, D.; Fabian-Kurzok, H.; Switonski, M. Chromosome abnormalities in dogs with disorders of sex development (DSD). Anim. Reprod. Sci. 2021, 230, 106771. [Google Scholar] [CrossRef]
- Capel, B. Women in reproductive science: To be or not to be a testis. Reproduction. 2019, 158, F101–F111. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.F.; Long, S.E.; Strohmenger, G.D. Testicular neoplasia in an intersex dog. J. Small Anim. Pract. 1976, 17, 247–253. [Google Scholar] [CrossRef]
- Park, E.J.; Lee, S.H.; Jo, Y.K.; Hahn, S.E.; Go, D.M.; Lee, S.H.; Lee, B.C.; Jang, G. Coincidence of Persistent Müllerian duct syndrome and testicular tumors in dogs. BMC Vet. Res. 2017, 13, 156. [Google Scholar] [CrossRef]
- Dzimira, S.; Wydooghe, E.; Van Soom, A.; Van Brantegem, L.; Nowacka-Woszuk, J.; Szczerbal, I.; Switonski, M. Sertoli Cell Tumour and Uterine Leiomyoma in Miniature Schnauzer Dogs with Persistent Müllerian Duct Syndrome Caused by Mutation in the AMHR2 Gene. J. Comp. Pathol. 2018, 161, 20–24. [Google Scholar] [CrossRef]
- Schwartz, R.; Sugai, N.J.; Eden, K.; Castaneda, C.; Jevit, M.; Raudsepp, T.; Cecere, J.T. Case Report: Disorder of Sexual Development in a Chinese Crested Dog With XX/XY Leukocyte Chimerism and Mixed Cell Testicular Tumors. Front. Vet. Sci. 2022, 9, 937991. [Google Scholar] [CrossRef]
- Sanpera, N.; Masot, N.; Janer, M.; Romeo, C.; de Pedro, R. Oestrogen-induced bone marrow aplasia in a dog with a Sertoli cell tumour. J. Small Anim. Pract. 2002, 43, 365–369. [Google Scholar] [CrossRef]
- Quartuccio, M.; Marino, G.; Garufi, G.; Cristarella, S.; Zanghì, A. Sertoli cell tumors associated with feminizing syndrome and spermatic cord torsion in two cryptorchid dogs. J. Vet. Sci. 2012, 13, 207–209. [Google Scholar] [CrossRef]
- Ortega-Pacheco, A.; Rodríguez-Buenfil, J.C.; Segura-Correa, J.C.; Bolio-Gonzalez, M.E.; Jiménez-Coello, M.; Linde Forsberg, C. Pathological conditions of the reproductive organs of male stray dogs in the tropics: Prevalence, risk factors, morphological findings and testosterone concentrations. Reprod. Domest. Anim. 2006, 41, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Grieco, V.; Riccardi, E.; Greppi, G.F.; Teruzzi, F.; Iermanò, V.; Finazzi, M. Canine testicular tumours: A study on 232 dogs. J. Comp. Pathol. 2008, 138, 86–89. [Google Scholar] [CrossRef]
- Nielsen, S.W.; Lein, D.H. Tumours of the testis. Bull. World Health Organ. 1974, 50, 71–78. [Google Scholar] [PubMed]
- Patnaik, A.K.; Mostofi, F.K. A clinicopathologic, histologic, and immunohistochemical study of mixed germ cell-stromal tumors of the testis in 16 dogs. Vet. Pathol. 1993, 30, 287–295. [Google Scholar] [CrossRef]
- Andràs, L.; Csaba, J. Collision mixed tumour of the testicle in dog. Oncological case report and literature data. Magy. Allatorv. Lapja 2010, 132, 93–100. [Google Scholar]
- Rifici, C.; Quartuccio, M.; Sfacteria, A.; Lanteri, G.; Abbate, J.M.; Cristarella, S.; Mazzullo, G. A case of neoplastic synchronism in a dog. Res. Vet. Sci. 2021, 140, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Mancebo, S.E.; Marchetti, M.A.; Hollmann, T.J.; Marghoob, A.A.; Busam, K.J.; Halpern, A.C. Melanoma in situ colonizing basal cell carcinoma: A case report and review of the literature. Dermatol. Pract. Concept. 2015, 5, 25–30. [Google Scholar] [CrossRef]
- Scott, J.E.; Liptak, J.M.; Powers, B.E. Malignant collision tumors in two dogs. J. Am. Vet. Med. Assoc. 2017, 251, 941–945. [Google Scholar] [CrossRef]
- Andras, L.; Csaba, J.; Rusvai, M.; Balka, G.; Janina, K. Claudin-5-positive perianal collision mixed skin tumour in dog. Oncological case report. Magy. Allatorv. Lapja 2010, 132, 461–465. [Google Scholar]
- Pallatto, V.A.; Bechtold, M.A. Mast cell and plasma cell collision tumor in the spleen of a dog. Vet. Clin. Pathol. 2018, 47, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.; Beck, S.; Gonzàlez-Gasch, E.; Harper, A. Collision tumour of two nodal metastases (adenocarcinoma and mast cell tumour) in a dog. Vet. Rec. 2020, 8, e001231. [Google Scholar] [CrossRef]
- Rifici, C.; Sfacteria, A.; Di Giorgio, S.; Giambrone, G.; Marino, G.; Mazzullo, G. Mast Cell Tumor and Mammary Gland Carcinoma Collision Tumor in a dog. Case report and literature review. J. Hell. Vet. Med. Soc. 2022, 73, 4675–4680. [Google Scholar] [CrossRef]
- Kathrins, M.; Kolon, T.F. Malignancy in disorders of sex development. Transl. Androl. Urol. 2016, 5, 794–798. [Google Scholar] [CrossRef]

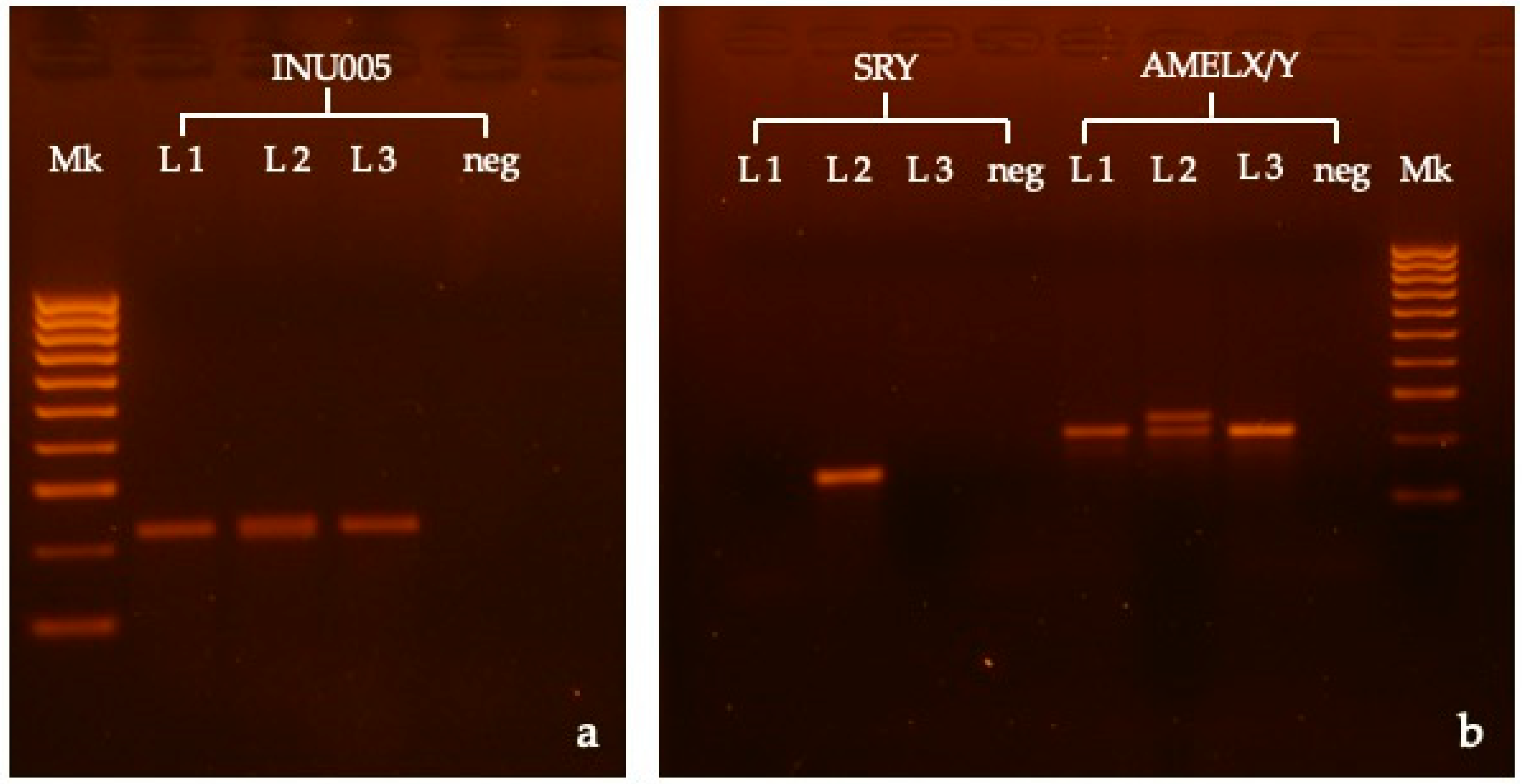
| Gene | Primers | Sequence (5′-3′) | Fragment Size (bp) | T° Annealing | Genome Pos |
|---|---|---|---|---|---|
| INU055 | U | CCAGGCGTCCCTATCCATCT | 210 | 60° | Chr10: 67,428,683–67,428,473 |
| L | GCACCACTTTGGGCTCCTTC | ||||
| Sry | CFA-SRY-F2 | GCAGGTGCACGTAGATGAGA | 142 | 57° | ChrY: 1,350,170–1,350,312 |
| CFA_SRY_Short_R3 | TGTGGTACTCCTGTTGCAG | ||||
| Amelx/y [11] | CFA_AmelxF | ATAATGACAAAGAAAACATGAC | 215/247 | 55° | ChrX: 7,828,350–7,828,136 |
| CFA_AmelxR | CTGCTGAGCTGGCACCAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rifici, C.; D’Anza, E.; Zappone, V.; Albarella, S.; Grieco, V.; Quartuccio, M.; Cristarella, S.; Mannarino, C.; Ciotola, F.; Mazzullo, G. An Unusual Case of Collision Testicular Tumor in a Female DSD Dog. Vet. Sci. 2023, 10, 251. https://doi.org/10.3390/vetsci10040251
Rifici C, D’Anza E, Zappone V, Albarella S, Grieco V, Quartuccio M, Cristarella S, Mannarino C, Ciotola F, Mazzullo G. An Unusual Case of Collision Testicular Tumor in a Female DSD Dog. Veterinary Sciences. 2023; 10(4):251. https://doi.org/10.3390/vetsci10040251
Chicago/Turabian StyleRifici, Claudia, Emanuele D’Anza, Viola Zappone, Sara Albarella, Valeria Grieco, Marco Quartuccio, Santo Cristarella, Cornelia Mannarino, Francesca Ciotola, and Giuseppe Mazzullo. 2023. "An Unusual Case of Collision Testicular Tumor in a Female DSD Dog" Veterinary Sciences 10, no. 4: 251. https://doi.org/10.3390/vetsci10040251
APA StyleRifici, C., D’Anza, E., Zappone, V., Albarella, S., Grieco, V., Quartuccio, M., Cristarella, S., Mannarino, C., Ciotola, F., & Mazzullo, G. (2023). An Unusual Case of Collision Testicular Tumor in a Female DSD Dog. Veterinary Sciences, 10(4), 251. https://doi.org/10.3390/vetsci10040251








