Modern Imaging Techniques in the Study and Disease Diagnosis of the Mammary Glands of Animals
Abstract
Simple Summary
Abstract
1. Introduction
2. Computed Tomography
3. Positron Emission Tomography
4. Magnetic Resonance Imaging
5. Ultrasonography
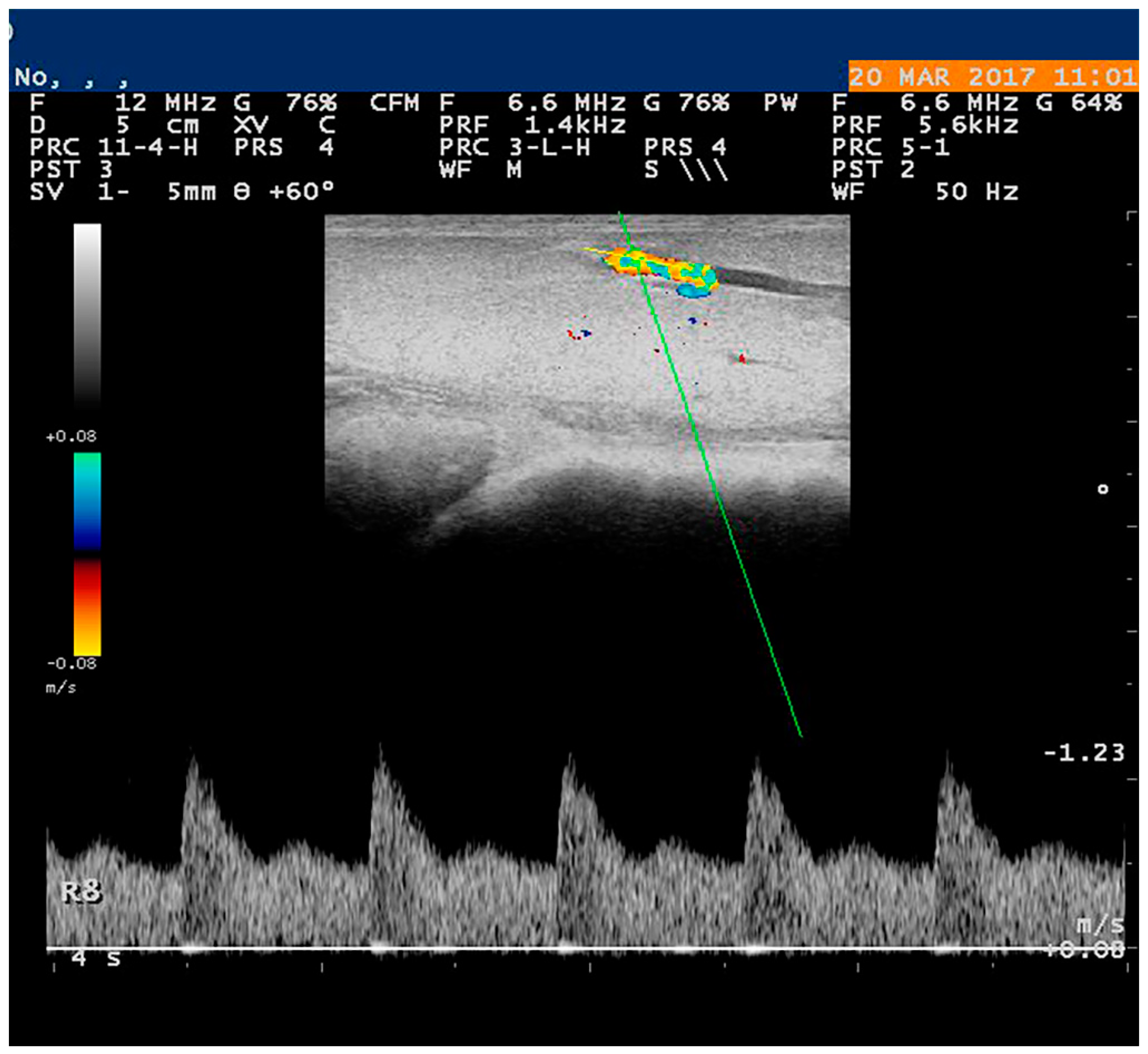
5.1. Doppler Examination
5.2. Contrast-Enhanced Examination
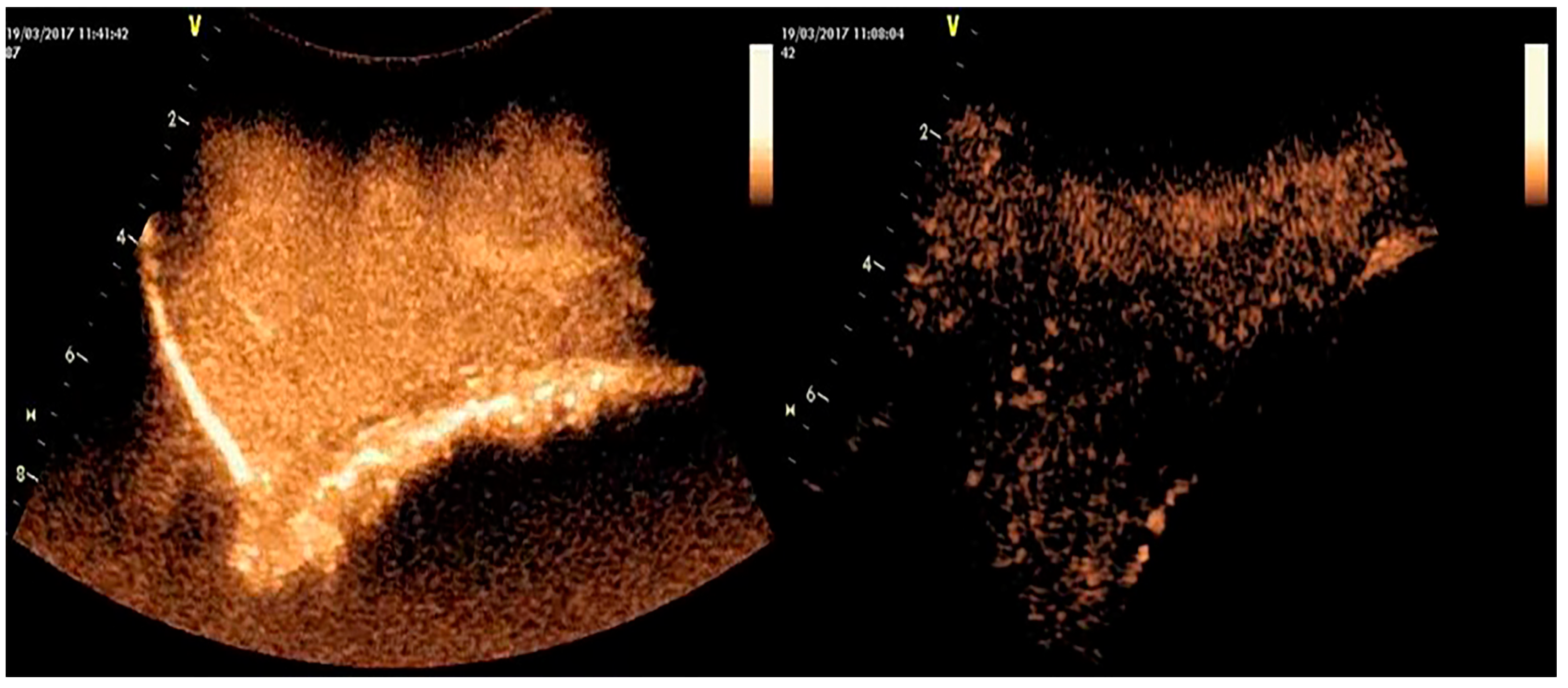
5.3. Three-Dimensional Examination
5.4. Elastography
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olsen, A.K.; Zeidler, D.; Pedersen, K.; Sørensen, M.; Jensen, S.B.; Munk, O.L. Imaging techniques: CT, MRI, and PET scanning. In Swine in the Laboratory. Surgery, Anesthesia, Imaging, and Experimental Techniques, 3rd ed.; Swindle, M.M., Smith, A.C., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 387–395. [Google Scholar]
- Otoni, C.C.; Rahal, S.C.; Vulcano, L.C.; Ribeiro, S.M.; Hette, K.; Giordano, T.; Doiche, D.P.; Amorim, R.L. Survey radiography and computerized tomography imaging of the thorax in female dogs with mammary tumors. Acta Vet. Scand. 2010, 52, 20. [Google Scholar] [CrossRef] [PubMed]
- Alstrup, A.K.O.; Winterdahl, M. Imaging Techniques in Large Animals. Scand. J. Lab. Anim. Sci. 2009, 36, 55–66. [Google Scholar] [CrossRef]
- Garamvoglyi, R.; Petrasi, Z.S.; Hevesi, A.; Jakab, C.S.; Vajda, Z.S.; Bogner, P.; Repa, I. Magnetic resonance imaging technique for the examination of canine mammary tumours. Acta Vet. Hung. 2006, 54, 143–159. [Google Scholar] [CrossRef]
- Feliciano, M.A.R.; Uscategui, R.A.R.; Maronezi, M.C.; Simões, A.P.R.; Silva, P.; Gasser, B.; Pavan, L.; Carvalho, C.F.; Canola, J.C.; Vicente, W.R.R. Ultrasonography methods for predicting malignancy in canine mammary tumors. PLoS ONE 2017, 12, e0178143. [Google Scholar] [CrossRef] [PubMed]
- Prokop, M. Principles of CT, Spiral CT, and Multislice CT. In Spiral and Multislice Computed Tomography of the Body, 1st ed.; Prokop, M., Galanski, M., Eds.; Thieme: Stuttgart, Germany, 2001; pp. 2–7. [Google Scholar]
- Maisey, M.N. Positron Emission Tomography in Clinical Medicine. In Positron Emission Tomography: Basic Sciences, 1st ed.; Bailey, D.L., Townsend, D.W., Valk, P.E., Maisey, M.N., Eds.; Springer: London, UK, 2005; pp. 1–12. [Google Scholar]
- Sánchez, D.; Romero, L.; López, S.; Campuzano, M.; Ortega, R.; Morales, A.; Guadarrama, M.; Cesarman-Maus, G.; García-Pérez, O.; Lizano, M. 18F-FDG—PET/CT in Canine Mammary Gland Tumors. Front. Vet. Sci. 2019, 6, 280. [Google Scholar] [CrossRef]
- Grover, H.; Grover, S.B.; Goyal, P.; Hedge, R.; Gupta, S.; Malhotra, S.; Li, S.; Gupta, N. Clinical and imaging features of idiopathic granulomatous mastitis—The diagnostic challenges and a brief review. Clin. Imaging 2021, 69, 126–132. [Google Scholar] [CrossRef]
- Stan, F.; Gudea, A.; Damian, A.; Gal, A.F.; Papuc, I.; Pop, A.R.; Martonos, C. Ultrasonographic Algorithm for the Assessment of Sentinel Lymph Nodes That Drain the Mammary Carcinomas in Female Dogs. Animals 2020, 10, 2366. [Google Scholar] [CrossRef]
- Sørensen, M.T.; Sejrsen, K.; Foldager, J. Estimation of Pubertal Mammary Development in Heifers by Computed Tomography. J. Dairy Sci. 1987, 70, 265–270. [Google Scholar] [CrossRef]
- Keane, M.; Paul, E.; Sturrock, C.J.; Rauch, C.; Rutland, C.S. Computed Tomography in Veterinary Medicine: Currently Published and Tomorrow’s Vision. In Computed Tomography—Advanced Applications, 1st ed.; Halefoglu, A.M., Ed.; IntechOpen: London, UK, 2017; pp. 271–283. [Google Scholar]
- Sejrsen, K.; Foldager, J.; Sørensen, M.T.; Akers, R.M.; Bauman, D.E. Effect of exogenous bovine somatotropin on pubertal mammary development in heifers. J. Dairy Sci. 1986, 69, 1528–1535. [Google Scholar] [CrossRef]
- Akers, M.R. A 100-Year Review: Mammary development and lactation. J. Dairy Sci. 2017, 100, 10332–10352. [Google Scholar] [CrossRef]
- Kim, S.; Kwon, K.; Choi, H.; Lee, Y. Evaluation of Mammary Gland Calcification in Dogs; Radiography and Computed Tomography. J. Emb. Trans. 2017, 32, 183–192. [Google Scholar] [CrossRef]
- Boone, J.M.; Kwan, A.L.; Yang, K.; Burkett, G.W.; Lindfors, K.K.; Nelson, T.R. Computed tomography for imaging the breast. J. Mammary Gland Biol. Neoplasia 2006, 11, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Soultani, C.; Patsikas, M.N.; Karayannopoulou, M.; Jakovljevic, S.; Chryssogonidis, I.; Papazoglou, L.; Papaioannou, N.; Papadopoulou, P.; Pavlidou, K.; Ilia, G.M.; et al. Assessment of sentinel lymph node metastasis in canine mammary gland tumors using computed tomographic indirect lymphography. Vet. Radiol. Ultrasound. 2017, 58, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Rangarajan, V. Appropriateness criteria of FDG PET/CT in oncology. Indian J. Radiol. Imaging 2015, 25, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy, V.; Thangaraju, M.; Prasad, P.D. Nutrient transporters in cancer: Relevance to Warburg hypothesis and beyond. Pharmacol. Ther. 2009, 121, 29–40. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Huang, T.; Sun, Y.; Jin, Z.; Li, X.F. Revisit 18F-fluorodeoxyglucose oncology positron emission tomography: “Systems molecular imaging” of glucose metabolism. Oncotarget 2017, 8, 43536–43542. [Google Scholar] [CrossRef] [PubMed]
- Kasem, J.; Wazir, U.; Mokbel, K. Sensitivity, Specificity and the Diagnostic Accuracy of PET/CT for Axillary Staging in Patients with Stage I-III Cancer: A Systematic Review of the Literature. In Vivo 2021, 35, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Meyer, P.T.; Circiumaru, V.; Cardi, C.A.; Thomas, D.H.; Bal, H.; Acton, P.D. Simplified quantification of small animal [18F] FDG PET studies using a standard arterial input function. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 948–954. [Google Scholar] [CrossRef]
- Danielsen, E.; Smith, D.F.; Poulsen, P.H.; Østergaard, L.; Gee, A.; Ishizu, K.; Venkatachalam, T.K.; Benders, D.; Hansen, S.; Gjedde, A.; et al. Positron emission tomography of living brain in minipigs and domestic pigs. Scand J. Lab. Anim. Sci. 1998, 25, 127–135. [Google Scholar]
- Olsen, A.K.; Keiding, S.; Munk, O.L. Effect of hypercapnia on cerebral blood flow and blood volume in pigs studied by positron emission tomography. Comp. Med. 2006, 56, 416–420. [Google Scholar] [PubMed]
- Black, N.F.; McJames, S.; Rust, T.C.; Kardmas, D.J. Evaluation of rapid dual-tracer 62Cu-PTSM + 62Cu-ATSM PET in dogs with spontaneously occurring tumors. Phys. Med. Biol. 2008, 53, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.W.; Cheng, Y.-C.N.; Haacke, E.M.; Thompson, M.R.; Venkatesan, R. Classical Response of a Single Nucleus to a Magnetic Field. In Magnetic Resonance Imaging: Physical Principles and Sequence Design, 2nd ed.; Brown, R.W., Cheng, Y.-C.N., Haacke, E.M., Thompson, M.R., Venkatesan, R., Eds.; Wiley-Blackwell: New York, NY, USA, 2014; pp. 19–36. [Google Scholar]
- Müller-Schimpfle, M.; Stoll, P.; Stern, W.; Kurz, S.; Dammann, F.; Claussen, C.D. Do mammography, sonography and MR mammography have a diagnostic benefit compared with mammography and sonography? AJR Am. J. Roentgenol. 1997, 168, 1323–1329. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M. MRI of the breast: State of the art. Eur. Radiol. 1998, 8, 707–725. [Google Scholar] [CrossRef]
- Lepori, D. Inflammatory breast disease: The radiologist’s role. Diagn. Interv. Imaging 2015, 96, 1045–1064. [Google Scholar] [CrossRef]
- Fowler, P.A.; Knight, C.H.; Cameron, G.G.; Foster, M.A. Use of magnetic resonance imaging in the study of goat mammary glands in vivo. J. Reprod. Fert. 1990, 89, 359–366. [Google Scholar] [CrossRef]
- Stelwagen, K.; McBride, B.W.; Grieve, D.G.; Towner, R.A. Nuclear magnetic resonance imaging and proton spectroscopy used as a technique to assess mammary gland composition in Holstein heifers. Can. J. Anim. Sci. 1990, 70, 1151–1154. [Google Scholar] [CrossRef]
- Jaramillo-Chaustre, X.L.; Bustamante-Cano, J.J.; Gómez-Parra, F. Lymphography by MRI for animal model canine. Iteckne 2017, 14, 46–61. [Google Scholar] [CrossRef]
- Aristokli, N.; Polycarpou, I.; Themistocleous, S.C.; Sophocleous, D.; Mamais, I. Comparison of the diagnostic performance of Magnetic Resonance Imaging (MRI), ultrasound and mammography for detection of breast cancer based on tumor type, breast density and patient’s history: A review. Radiography 2022, 28, 848–856. [Google Scholar] [CrossRef]
- Hamper, U.M.; DeJong, M.R.; Caskey, C.I.; Sheth, S. Power Doppler imaging: Clinical experience and correlations with color Doppler US and other imaging modalities. RadioGraphics 1997, 17, 499–513. [Google Scholar] [CrossRef]
- Trasch, K.; Wehrend, A.; Bostedt, H. Ultrasonographic description of canine mastitis. Vet. Radiol. Ultrasound 2007, 48, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Flöck, M.; Winter, P. Diagnostic ultrasonography in cattle with diseases of the mammary gland. Vet. J. 2006, 171, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Banting, A. Ultrasonographic examination of the mammary gland in cows with induced S. aureus mastitis: A criteria for prognosis and evaluation of therapy. Cattle Pract. 1998, 6, 121–124. [Google Scholar]
- Kaszak, I.; Ruszczak, A.; Kanafa, S.; Witkowska-Piłaszewicz, O.; Sacharczuk, M.; Jurka, P. New insights of canine mastitis—A review. Anim. Sci. Pap. Rep. 2018, 36, 33–44. [Google Scholar]
- Guirguis, M.S.; Adrada, B.; Santiago, L.; Candelaria, R.; Arribas, E. Mimickers of breast malignancy: Imaging findings, pathologic concordance and clinical management. Insights Imaging 2021, 12, 53. [Google Scholar] [CrossRef] [PubMed]
- Petridis, I.G.; Barbagianni, M.S.; Ioannidi, K.S.; Samaras, E.; Fthenakis, G.C.; Vloumidi, E.I. Doppler ultrasonographic examination in sheep. Small Rumin. Res. 2017, 152, 22–32. [Google Scholar] [CrossRef]
- Risvanli, A.; Dogan, H.; Safak, T.; Kilic, M.A.; Seker, I. The relationship between mastitis and the B-mode, colour Doppler ultrasonography measurements of supramammary lymph nodes in cows. J. Dairy Res. 2019, 86, 315–318. [Google Scholar] [CrossRef]
- Feliciano, M.A.; Vicente, W.R.; Silva, M.A. Conventional and Doppler ultrasound for the differentiation of benign and malignant canine mammary tumours. J. Small Anim. Pract. 2012, 53, 332–337. [Google Scholar] [CrossRef]
- McDonald, D.M.; Choyke, P.L. Imaging of angiogenesis: From microscope to clinic. Nat. Med. 2003, 9, 713–725. [Google Scholar] [CrossRef]
- Abma, E.; Stock, E.; De Spiegelaere, W.; Brantegem, L.V.; Vanderperren, K.; Ni, Y.; Vynck, M.; Daminet, S.; Clercq, K.D.; de Rooster, H. Power Doppler ultrasound and contrast-enhanced ultrasound demonstrate non-invasive tumour vascular response to anti-vascular therapy in canine cancer patients. Sci. Rep. 2019, 9, 9262. [Google Scholar] [CrossRef]
- Piccione, G.; Arcigli, A.; Fazio, F.; Giudice, E.; Caola, G. Pulsed wave-Doppler ultrasonographic evaluation of mammary blood flow speed in cows during different productive periods. Acta Sci. Vet. 2004, 32, 171–175. [Google Scholar] [CrossRef]
- Braun, U.; Forster, E. B-mode and colour Doppler sonographic examination of the milk vein and musculophrenic vein in dry cows and cows with a milk yield of 10 and 20 kg. Acta Vet. Scand. 2012, 54, 15. [Google Scholar] [CrossRef] [PubMed]
- Esselburn, K.M.; Hill, T.M.; Bateman, H.G., 2nd; Fluharty, F.L.; Moeller, S.J.; O’Diam, K.M.; Daniels, K.M. Examination of weekly parenchymal area by ultrasound, mammary mass, and composition in Holstein heifers reared on 1 or 3 diets from birth to 2 months of age. J. Dairy Sci. 2015, 98, 5280–5293. [Google Scholar] [CrossRef] [PubMed]
- Piccione, G.; Arcigli, A.; Assenza, A.; Percipalle, M.; Caola, G. Pulsed wave-Doppler ultrasonographic evaluation of the mammary blood flow in the ewe. Acta Vet. Brno 2004, 73, 23–27. [Google Scholar] [CrossRef]
- Barbagianni, M.S.; Mavrogianni, V.S.; Vasileiou, N.G.C.; Fthenakis, G.C.; Petridis, I.G. Ultrasonographic examination of the udder in sheep. Small Rumin. Res. 2017, 152, 86–99. [Google Scholar] [CrossRef]
- Nielsen, M.O.; Jakobsen, K.; Jørgensen, N.J. Changes in mammary blood flow during the lactation period in goats measured by the ultrasound Doppler principle. Comp. Biochem. Physiol. A Comp. Physiol. 1990, 97, 519–524. [Google Scholar] [CrossRef]
- Christensen, K.; Nielsen, M.O.; Bauer, R.; Hilden, K. Evaluation of mammary blood flow measurements in lactation goats using the ultrasound Doppler principle. Comp. Biochem. Physiol. A Comp. Physiol. 1989, 92, 385–392. [Google Scholar] [CrossRef]
- Porcionato, M.A.; Soares, W.V.; Reis, C.B.; Cortinhas, C.S.; Mestieri, L.; Santos, M.V. Milk flow, teat morphology and subclinical mastitis prevalence in Gir cows. Pesqui. Agropecu. Bras. 2010, 45, 1507–1512. [Google Scholar] [CrossRef]
- Rambabu, K.; Sreenu, M.; Kumar, R.V.S.; Rao, T.S.C. Ultrasonography of the udder and teat in buffaloes. Buffalo Bull. 2009, 28, 5–10. Available online: http://ibic.lib.ku.ac.th/.../2009-5.htm (accessed on 12 May 2022).
- Santos, V.J.C.; Simplício, K.M.M.G.; Sanchez, D.C.C.; Almeida, V.T.; Teixeira, P.P.M.; Coutinho, L.N.; Rodrigues, L.F.S.; Oliveira, M.E.F.; Feliciano, M.A.R.; Vicente, W.R.R. Conventional and Doppler ultrasonography on a goat with gangrenous mastitis. Vicente. Arq. Bras. Med. Vet. Zootec. 2014, 66, 1931–1935. [Google Scholar] [CrossRef]
- Mantziaras, G.; Luvoni, G.C. Advanced ultrasound techniques in small animal reproduction imaging. Reprod. Domest. Anim. 2020, 2, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Soler, M.; Dominguez, E.; Lucas, X.; Novellas, R.; Gomes-Coelho, K.V.; Espada, Y.; Agut, A. Comparison between ultrasonographic findings of benign and malignant canine mammary gland tumours using B-mode, colour Doppler, power Doppler and spectral Doppler. Res. Vet. Sci. 2016, 107, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Balaci, I.; Ciupe, S.; Pop, A.; Parlapan, L.; Arion, A.; Vasiu, I.; Purdoiu, R.; Papuc, I.; Groza, I. Ultrasonographic Findings of Mastitic and Normal Mammary Gland in Bitches. Vet. Med. 2015, 72, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.F.; Chammas, M.C.; Cerri, G.G. Physical principles of Doppler ultrasonography. Cienc. Rural 2008, 38, 872–879. [Google Scholar] [CrossRef]
- Lana, S.E.; Rutteman, G.R.; Withrow, S.J. Tumors of the Mammary Gland. In Withrow and MacEwen’s Small Animal Clinical Oncology, 4th ed.; Withrow, S.J., Vail, D.M., Eds.; Saunders Elsevier: St. Louis, MO, USA, 2007; pp. 619–638. [Google Scholar] [CrossRef]
- Wang, M.; Feng, H.L.; Liu, Y.Q.; Liu, H.; Jiang, Y.X.; Zhu, Q.L.; Dai, Q.; Li, J.C. Angiogenesis Research in Mouse Mammary Cancer Based on Contrast-enhanced Ultrasonography: Exploratory Study. Acad. Radiol. 2018, 25, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Mantziaras, G.; Vasileiou, N.G.; Ioannidi, K.S.; Mavrogianni, V.S.; Gougoulis, D.A.; Fthenakis, G.C.; Petridis, I.G.; Barbagianni, M.S. Use of contrast-enhanced ultrasonographic examination to evaluate health status of mammary glands of ewes at the end of a lactation period. J. Dairy Res. 2018, 85, 39–43. [Google Scholar] [CrossRef]
- Bouakaz, A.; De Jong, N.; Cachard, C. Standard properties of ultrasound contrast agents. Ultrasound Med. Biol. 1998, 24, 469–472. [Google Scholar] [CrossRef]
- Cosgrove, D.; Harvey, C. Clinical uses of microbubbles in diagnosis and treatment. Med. Biol. Eng. Comput. 2009, 47, 813–826. [Google Scholar] [CrossRef]
- Appis, A.W.; Tracy, M.J.; Feinstein, S.B. Update on the safety and efficacy of commercial ultrasound contrast agents in cardiac applications. Echo Res. Pract. 2015, 2, 55–62. [Google Scholar] [CrossRef]
- Vanderperren, K.; Saunders, J.H.; Van der Vekens, E.; Wydooghe, E.; de Rooster, H.; Duchateau, L.; Stock, E. B-mode and contrast-enhanced ultrasonography of the mammary gland during the estrous cycle of dogs. Anim. Reprod. Sci. 2018, 199, 15–23. [Google Scholar] [CrossRef]
- Lee, S.C.; Tchelepi, H.; Grant, E.; Desai, B.; Luo, C.; Groshen, S.; Hovanessian-Larsen, L. Contrast-Enhanced Ultrasound Imaging of Breast Masses: Adjunct Tool to Decrease the Number of False-Positive Biopsy Results. J. Ultrasound Med. 2019, 38, 2259–2273. [Google Scholar] [CrossRef] [PubMed]
- Caproni, N.; Marchisio, F.; Pecchi, A.; Canossi, B.; Battista, R.; D’Alimonte, P.; Torricelli, P. Contrast-enhanced ultrasound in the characterisation of breast masses: Utility of quantitative analysis in comparison with MRI. Eur. Radiol. 2010, 20, 1384–1395. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Feng, L.; Zhou, Q.; Chen, Q.; Liu, J.; Wu, C.; Luo, J.; Chen, J.; Wu, H.; Deng, W. The value of contrast-enhanced ultrasound in determining the location of sentinel lymph nodes in breast cancer. Cancer Imaging 2021, 21, 28. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, Y.X.; Liu, J.B.; Zhu, Q.L.; Sun, Q. Evaluation of breast lesions with contrast-enhanced ultrasound using the microvascular imaging technique: Initial observations. Breast 2008, 17, 532–539. [Google Scholar] [CrossRef]
- Wan, C.; Du, J.; Fang, H.; Li, F.; Wang, L. Evaluation of breast lesions by contrast enhanced ultrasound: Qualitative and quantitative analysis. Eur. J. Radiol. 2012, 81, e444–e450. [Google Scholar] [CrossRef]
- Gelb, H.R.; Freeman, L.J.; Rohleder, J.J.; Snyder, P.W. Feasibility of contrast-enhanced ultrasound-guided biopsy of sentinel lymph nodes in dogs. Vet. Radiol. Ultrasound. 2010, 6, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Favril, S.; Stock, E.; Hernot, S.; Hesta, M.; Polis, I.; Vanderperren, K.; de Rooster, H. Sentinel lymph node mapping by near-infrared fluorescence imaging and contrast-enhanced ultrasound in healthy dogs. Vet. Comp. Oncol. 2019, 17, 89–98. [Google Scholar] [CrossRef]
- Gasser, B.; Rodriguez, M.G.K.; Uscategui, R.A.R.; Silva, P.A.; Maronezi, M.C.; Pavan, L.; Feliciano, M.A.R.; Vicente, W.R.R. Ultrasonographic characteristics of benign mammary lesions in bitches. Vet. Med. 2018, 63, 216–224. [Google Scholar] [CrossRef]
- Lamuraglia, M.; Bridal, S.L.; Santin, M.; Izzi, G.; Rixe, O.; Paradiso, A.; Lucidarme, O. Clinical relevance of contrast-enhanced ultrasound in monitoring anti-angiogenic therapy of cancer: Current status and perspectives. Crit. Rev. Oncol. Hematol. 2010, 73, 202–212. [Google Scholar] [CrossRef]
- Leoni, S.; Piscaglia, F.; Golfieri, R.; Camaggi, V.; Vidili, G.; Pini, P.; Bolondi, L. The impact of vascular and nonvascular findings on the noninvasive diagnosis of small hepatocellular carcinoma based on the EASL and AASLD criteria. Am. J. Gastroenterol. 2010, 105, 599–609. [Google Scholar] [CrossRef]
- Franz, S.; Hofmann-Parisot, M.M.; Baumgartner, W. Evaluation of three-dimensional ultrasonography of the bovine mammary gland. Am. J. Vet. Res. 2004, 65, 1159–1163. [Google Scholar] [CrossRef] [PubMed]
- Fasulkov, I.; Karadaev, M.; Vasilev, N.; Nikolov, M.; Nonov, T. Three-dimensional ultrasonography of the mammary gland in lactating cows. Tradit. Mod. Vet. Med. 2018, 3, 109–113. [Google Scholar] [CrossRef]
- Kotsianos, D.; Wirth, S.; Fischer, T.; Hiltawsky, K.; Sittek, H.; Reiser, M. 3D-Ultraschall (3D-US) in der Diagnostik von Mammaherdbefunden [3D ultrasound (3D US) in the diagnosis of focal breast lesions]. Radiologe 2005, 45, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Weismann, C.; Mayr, C.; Egger, H.; Auer, A. Breast Sonography—2D, 3D, 4D Ultrasound or Elastography? Breast Care 2011, 6, 98–103. [Google Scholar] [CrossRef]
- Pretorius, D.H.; Nelson, T.R. Three-dimensional ultrasound. Ultrasound Obstet. Gynecol. 1995, 5, 219–221. [Google Scholar] [CrossRef]
- Riccabona, M.; Nelson, T.R.; Pretorius, D.H. Three-dimensional ultrasound: Accuracy of distance and volume measurements. Ultrasound Obstet Gynecol. 1996, 7, 429–434. [Google Scholar] [CrossRef]
- Nelson, T.R.; Pretorius, D.H.; Hull, A.; Riccabona, M.; Sklansky, M.S.; James, G. Sources and impact of artifacts on clinical three-dimensional ultrasound imaging. Ultrasound Obstet Gynecol. 2000, 16, 374–383. [Google Scholar] [CrossRef]
- Dimitrova, V.; Markov, D.; Dimitrov, R. 3D and 4D ultrasonography in obstetrics. Akush. Ginekol. (Sofiia) 2007, 46, 31–40. (In Bulgarian) [Google Scholar]
- Pretorius, D.H.; Nelson, T.R.; James, G. Three-dimensional ultrasound in obstetrics. In Diagnostic Imaging of Fetal Anomalies; Nyberg, D.A., McGahan, J.P., Pretorius, D.H., Pilu, G., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2003; pp. 969–988. ISBN 0-7817-3211-5. [Google Scholar]
- Chen, D.R.; Lai, H.W. Three-dimensional ultrasonography for breast malignancy detection. Expert Opin. Med. Diagn. 2011, 5, 253–261. [Google Scholar] [CrossRef]
- Hildebrandt, T.B.; Drews, B.; Kurz, J.; Hermes, R.; Yang, S.; Göritz, F. Pregnancy monitoring in dogs and cats using 3D and 4D ultrasonography. Reprod. Domest. Anim. 2009, 44, 125–128. [Google Scholar] [CrossRef]
- Ophir, J.; Céspedes, I.; Ponnekanti, H.; Yazdi, Y.; Li, X. Elastography: A quantitative method for imaging the elasticity of biological tissues. Ultrason. Imaging 1991, 13, 111–134. [Google Scholar] [CrossRef]
- Dobruch-Sobczak, K.; Sudoł-Szopińska, I. The usefulness of sonoelastography in the differential diagnosis of solid breast lesions. Ultrasound Med. Biol. 2011, 37, 100. [Google Scholar] [CrossRef]
- Li, Y.; Snedeker, J.G. Elastography: Modality-specific approaches, clinical applications, and research horizons. Skeletal Radiol. 2011, 40, 389–397. [Google Scholar] [CrossRef]
- Alam, F.; Naito, K.; Horiguchi, J.; Fukuda, H.; Tachikake, T.; Ito, K. Accuracy of sonographic elastography in the differential diagnosis of enlarged cervical lymph nodes: Comparison with conventional B-mode sonography. AJR Am. J. Roentgenol. 2008, 191, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Ophir, J.; Garra, B.; Kallel, F.; Konofagou, E.; Krouskop, T.; Righetti, R.; Varghese, T. Elastographic imaging. Ultrasound Med. Biol. 2000, 26, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Bamber, J.; Cosgrove, D.; Dietrich, C.F.; Fromageau, J.; Bojunga, J.; Calliada, F.; Cantisani, V.; Correas, J.M.; D’Onofrio, M.; Drakonaki, E.E.; et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology. Ultraschall Med. 2013, 34, 169–184. [Google Scholar] [CrossRef]
- Skerl, K.; Eichhorn, B.; Poltorjanoks, R.; Cochran, S.; Evans, A. Introduction of a Measurement Setup to Monitor the Pressure Applied During Handheld Ultrasound Elastography. Ultrasound Med. Biol. 2020, 46, 2556–2559. [Google Scholar] [CrossRef]
- Glińska-Suchocka, K.; Jankowski, M.; Kubiak, K.; Spuzak, J.; Dzimira, S.; Nicpon, J. Application of shear wave elastography in the diagnosis of mammary gland neoplasm in dogs. Pol. J. Vet. Sci. 2013, 16, 477–482. [Google Scholar] [CrossRef]
- Dudea, S.M.; Lenghel, M.; Botar-Jid, C.; Vasilescu, D.; Duma, M. Ultrasonography of superficial lymph nodes: Benign vs. malignant. Med. Ultrason. 2012, 14, 294–306. [Google Scholar]
- Lenghel, L.M.; Bolboaca, S.D.; Botar-Jid, C.; Baciut, G.; Dudea, S.M. The value of a new score for sonoelastographic differentiation between benign and malignant cervical lymph nodes. Med. Ultrason. 2012, 14, 271–277. [Google Scholar]
- Feliciano, M.A.R.; Ramirez, R.A.U.; Maronezi, M.C.; Maciel, G.S.; Avante, M.L.; Senhorello, I.L.S.; Mucédola, T.; Gasser, B.; Carvalho, C.F.; Vicente, W.R.R. Accuracy of four ultrasonography techniques in predicting histopathological classification of canine mammary carcinomas. Vet. Radiol. Ultrasound 2018, 59, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Feliciano, M.A.R.; Maronezi, M.C.; Brito, M.B.S.; Simões, A.P.R.; Maciel, G.S.; Castanheira, T.L.L.; Garrido, E.; Uscategui, R.R.; Miceli, N.G.; Vicente, W.R.R. Doppler and Elastography as complementary diagnostic methods for mammary neoplasms in female cats. Arq. Bras. Med. Vet. Zootec. 2015, 67, 935–939. [Google Scholar] [CrossRef]
- Pieczewska, B.; Glińska-Suchocka, K.; Niżański, W.; Dzięcioł, M. Decreased Size of Mammary Tumors Caused by Preoperative Treatment with Aglepristone in Female Domestic Dogs (Canis familiaris) Do Not Influence the Density of the Benign Neoplastic Tissue Measured Using Shear Wave Elastography Technique. Animals 2021, 11, 527. [Google Scholar] [CrossRef] [PubMed]
- Balleyguier, C.; Ciolovan, L.; Ammari, S.; Canale, S.; Sethom, S.; Al Rouhbane, R.; Vielh, P.; Dromain, C. Breast elastography: The technical process and its applications. Diagn. Interv. Imaging 2013, 94, 503–513. [Google Scholar] [CrossRef]
- Sharun, K.; Dhama, K.; Tiwari, R.; Gugjoo, M.B.; Iqbal Yatoo, M.; Patel, S.K.; Pathak, M.; Karthik, K.; Khurana, S.K.; Singh, R.; et al. Advances in therapeutic and managemental approaches of bovine mastitis: A comprehensive review. Vet. Q. 2021, 41, 107–136. [Google Scholar] [CrossRef]
- Mørk, T.; Waage, S.; Tollersrud, T.; Kvitle, B.; Sviland, S. Clinical mastitis in ewes; bacteriology, epidemiology and clinical features. Acta Vet. Scand. 2007, 49, 23. [Google Scholar] [CrossRef]
- Valdivia, G.; Alonso-Diez, Á.; Pérez-Alenza, D.; Peña, L. From Conventional to Precision Therapy in Canine Mammary Cancer: A Comprehensive Review. Front. Vet. Sci. 2021, 8, 623800. [Google Scholar] [CrossRef]
- Giménez, F.; Hecht, S.; Craig, L.E.; Legendre, A.M. Early detection, aggressive therapy: Optimizing the management of feline mammary masses. J. Feline Med. Surg. 2010, 12, 214–224. [Google Scholar] [CrossRef]
- Pluguez-Turull, C.W.; Nanyes, J.E.; Quintero, C.J.; Alizai, H.; Mais, D.D.; Kist, K.A.; Dornbluth, N.C. Idiopathic Granulomatous Mastitis: Manifestations at Multimodality Imaging and Pitfalls. Radiographics 2018, 38, 330–356. [Google Scholar] [CrossRef]
- Bazelaire, C.; Groheux, D.; Chapellier, M.; Sabatier, F.; Scémama, A.; Pluvinage, A.; Albiter, M.; de Kerviler, E. Breast inflammation: Indications for MRI and PET-CT. Diagn. Interv. Imaging 2012, 93, 104–115. [Google Scholar] [CrossRef]
- Dong, A.; Wang, Y.; Lu, J.; Zuo, C. Spectrum of the Breast Lesions with Increased 18F-FDG Uptake on PET/CT. Clin. Nucl. Med. 2016, 41, 543–557. [Google Scholar] [CrossRef]
- Kitajima, K.; Miyoshi, Y. Present and future role of FDG-PET/CT imaging in the management of breast cancer. Jpn. J. Radiol. 2016, 34, 167–180. [Google Scholar] [CrossRef]
- Jesinger, R.A.; Lattin, G.E.; Ballard, E.A.; Zelasko, S.M.; Glassman, L.M. Vascular abnormalities of the breast: Arterial and venous disorders, vascular masses, and mimic lesions with radiologic-pathologic correlation. Radiographics 2011, 31, 117–136. [Google Scholar] [CrossRef]
- Dzięcioł, M.; Scholbach, T.; Stańczyk, E.; Ostrowska, J.; Kinda, W.; Woźniak, M.; Atamaniuk, W.; Skrzypczak, P.; Niżański, W.; Wieczorek, A.; et al. Dynamic tissue perfusion measurement in the reproductive organs of the female and male dogs. Bull. Vet. Inst. Pulawy 2014, 58, 149–155. [Google Scholar] [CrossRef]
- Park, A.H.; Seo, B.K. Up-to-date Doppler techniques for breast tumor vascularity: Superb microvascular imaging and contrast-enhanced ultrasound. Ultrasonography 2018, 37, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Choi, H.Y.; Baek, S.Y.; Lim, S.M. Role of color and power Doppler imaging in differentiating between malignant and benign solid breast masses. J. Clin. Ultrasound 2002, 30, 459–464. [Google Scholar] [CrossRef]
- Fazzio, R.T.; Shah, S.S.; Sandhu, N.P.; Glazebrook, K.N. Idiopathic granulomatous mastitis: Imaging update and review. Insights Imaging 2016, 7, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, T.; Melo, M.F.V.; Musch, G.; Harris, R.S.; Venegas, J.G.; Winkler, T. Image-derived input function for assessment of 18F-FDG uptake by the inflamed lung. J. Nucl. Med. 2007, 48, 1889–1896. [Google Scholar] [CrossRef] [PubMed]
- Hussein, A.H.; EL-Khabaz Khaled, A.S.; Malek, S.S. Is udder ultrasonography a diagnostic tool for subclinical mastitis in sheep? Small Rumin. Res. 2015, 129, 121–128. [Google Scholar] [CrossRef]
- Suzuki, N.; Kurose, T.; Kaneko, S.; Haraguchi, A.; Isobe, N. Outcome prediction from the first examination in clinical mastitis using ultrasonography in dairy cows. Anim. Sci. J. 2020, 91, e13452. [Google Scholar] [CrossRef]
- Narváez-Semanate, J.L.; Daza-Bolaños, C.A.; Valencia-Hoyos, C.E.; Hurtado-Garzón, D.T.; Acosta-Jurado, D.C. Diagnostic methods of subclinical mastitis in bovine milk: An overview. Rev. Fac. Nac. Agron. 2022, 75, 10077–10088. [Google Scholar] [CrossRef]
- Ntemka, A.; Tsakmakidis, I.; Boscos, C.; Theodoridis, A.; Kiossis, E. The Role of Ewes’ Udder Health on Echotexture and Blood Flow Changes during the Dry and Lactation Periods. Animals 2022, 12, 2230. [Google Scholar] [CrossRef]
- Souza, P.M.; Mamprim, M.J.; Lopes, M.D. Mode B-Ultrasound and Doppler in Malignant Tumors of the Mammary Gland of Female Dogs. Ultrasound Med. Biol. 2013, 39, 78. [Google Scholar] [CrossRef]
- Petrucci, G.; Henriques, J.; Gregório, H.; Vicente, G.; Prada, J.; Pires, I.; Lobo, L.; Medeiros, R.; Queiroga, F. Metastatic feline mammary cancer: Prognostic factors, outcome and comparison of different treatment modalities—A retrospective multicentre study. J. Feline Med. Surg. 2021, 23, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.; Uscategui, R.A.R.; Maronezi, M.C.; Gasser, B.; Pavan, L.; Gatto, I.R.H.; de Almeida, V.T.; Vicente, W.R.R.; Feliciano, M.A.R. Ultrasonography for lymph nodes metastasis identification in bitches with mammary neoplasms. Sci. Rep. 2018, 8, 17708. [Google Scholar] [CrossRef] [PubMed]
- Hillaert, A.; Stock, E.; Duchateau, L.; de Rooster, H.; Devriendt, N.; Vanderperren, K. B-Mode and Contrast-Enhanced Ultrasonography Aspects of Benign and Malignant Superficial Neoplasms in Dogs: A Preliminary Study. Animals 2022, 12, 2765. [Google Scholar] [CrossRef]
- Marquardt, C.; Burkhardt, E.; Failing, K.; Wehrend, A. Sonographic examination of mammary tumors in bitches. Part 1: Individual criteria detectable by sonography and their correlation with tumor dignity. Tierärztl. Prax. 2003, 31, 275–283. [Google Scholar] [CrossRef]
- Nyman, H.T.; Nielsen, O.L.; McEvoy, F.J.; Lee, M.H.; Martinussen, T.; Hellmén, E.; Kristensen, A.T. Comparison of B-mode and Doppler ultrasonographic findings with histologic features of benign and malignant mammary tumors in dogs. Am. J. Vet. Res. 2006, 67, 985–991. [Google Scholar] [CrossRef]
| Method | Main Target | Preparation | Equipment | Cost | Species |
|---|---|---|---|---|---|
| Computed Tomography [12,14,15,17] | Mammary tumours and metastatic lymph nodes | Under general anaesthesia Optional administration of contrast agent | Not portable | ++ | Companion animals |
| Positron Emission Tomography [3,8,24] | Mammary tumours and metastatic lymph nodes | Under general anaesthesia Administration of radioactive isotopes | Not portable | ++++ | Companion animals |
| Magnetic Resonance Imaging [3,4,31] | Mammary tumours | Under general anaesthesia Optional administration of contrast agent | Not portable | +++ | Companion animals |
| Doppler Examination [41,43,53] | Mammary tumours Mastitis | The hair of the region fully clipped | Portable | + | Companion animals (tumours) Farm animals (mastitis) |
| Contrast-enhanced ultrasonographic examination (CEUS) [62,66,10] | Mammary tumours Mastitis | The hair of the region fully clipped Administration of contrast agent | Portable | ++ | Companion animals (tumours) Farm animals (mastitis) |
| Three-Dimensional [77,78,87] | No report in mammary diseases | The hair of the region fully clipped | Portable | +++ | Farm animals (healthy) |
| Elastography [5,99,100] | Mammary tumours | The hair of the region fully clipped | Portable | ++ | Companion animals (tumours) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbagianni, M.S.; Gouletsou, P.G. Modern Imaging Techniques in the Study and Disease Diagnosis of the Mammary Glands of Animals. Vet. Sci. 2023, 10, 83. https://doi.org/10.3390/vetsci10020083
Barbagianni MS, Gouletsou PG. Modern Imaging Techniques in the Study and Disease Diagnosis of the Mammary Glands of Animals. Veterinary Sciences. 2023; 10(2):83. https://doi.org/10.3390/vetsci10020083
Chicago/Turabian StyleBarbagianni, Mariana S., and Pagona G. Gouletsou. 2023. "Modern Imaging Techniques in the Study and Disease Diagnosis of the Mammary Glands of Animals" Veterinary Sciences 10, no. 2: 83. https://doi.org/10.3390/vetsci10020083
APA StyleBarbagianni, M. S., & Gouletsou, P. G. (2023). Modern Imaging Techniques in the Study and Disease Diagnosis of the Mammary Glands of Animals. Veterinary Sciences, 10(2), 83. https://doi.org/10.3390/vetsci10020083










