Unusual Canine Cutaneous Melanoma Presenting Parietal Bone Metastasis: A Case Report
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
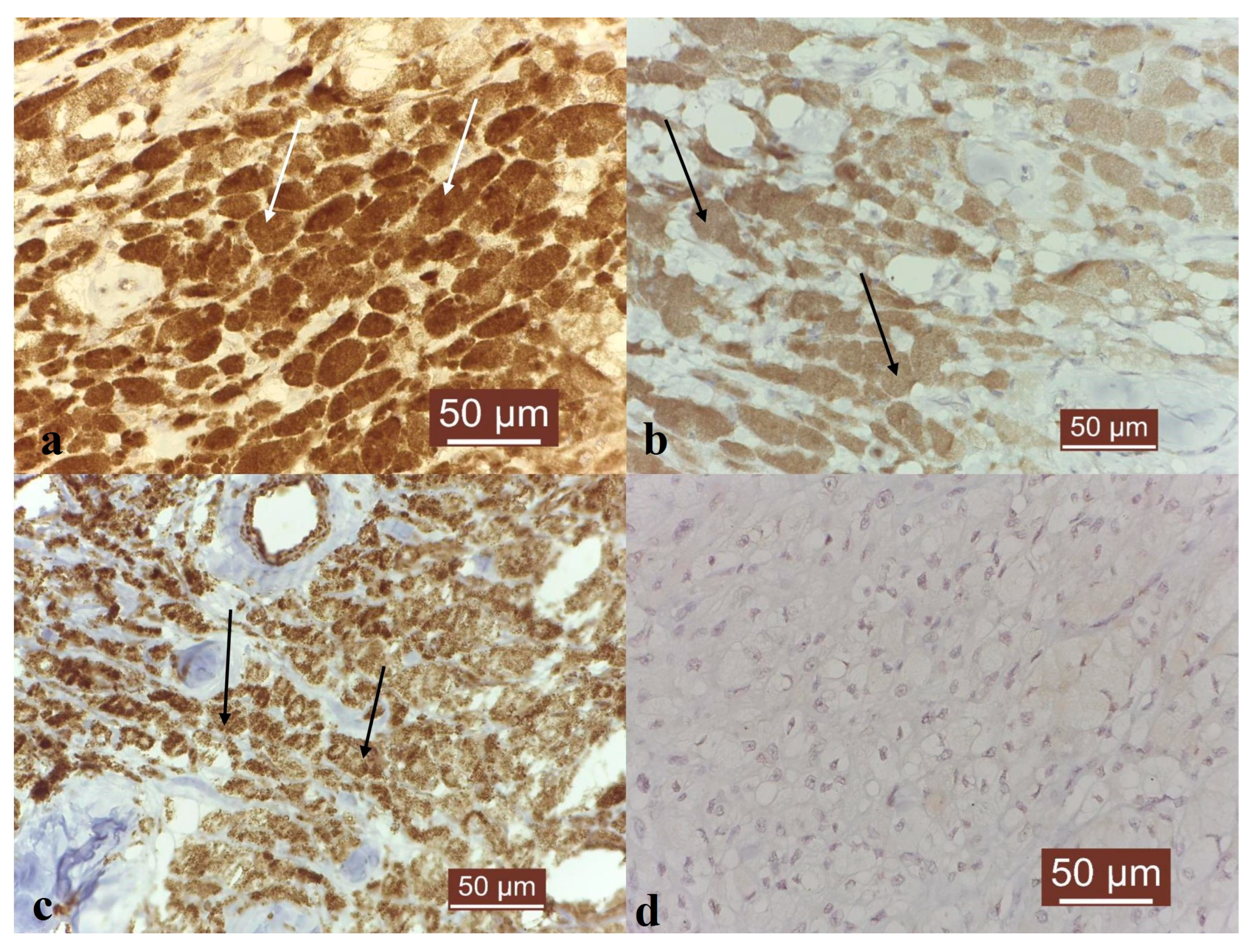
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nishiya, A.T.; Massoco, C.O.; Felizzola, C.R.; Perlmann, E.; Batschinski, K.; Tedardi, M.V.; Garcia, J.S.; Mendonça, P.P.; Teixeira, T.F.; Dagli, M.L.Z. Comparative Aspects of Canine Melanoma. Vet. Sci. 2016, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, T.F.; da Silva, T.C.; Cogliati, B.; Nagamine, M.K.; Dagli, M.L.Z. Retrospective study of melanocytic neoplasms in dogs and cats. Braz. J. Vet. Pathol. 2010, 3, 100–104. [Google Scholar]
- Meuten, J.D. Tumors in Domestic Animals, 5th ed.; Wiley-Blackwell: Raleigh, NC, USA, 2017. [Google Scholar]
- Liu, W.; Bennett, M.; Helm, T. Canine melanoma: A comparison with human pigmented epithelioid melanocytoma. Int. J. Dermatol. 2011, 50, 1542–1545. [Google Scholar] [CrossRef] [PubMed]
- Brockley, L.K.; Cooper, M.A.; Bennett, P.F. Malignant melanoma in 63 dogs: The effects of carboplatin chemotherapy on survival. N. Z. Vet. J. 2012, 61, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Fukunag-Kalabis, M.; Herlyn, M. Crosstalk in skin: Melanocytes, keratinocytes, stem cells and melanoma. J. Cell Commun. Signal. 2018, 10, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Smedley, C.R.; Sebastian, K.; Kiupel, M. Diagnosis and prognosis of canine melanocytic neoplasms. Vet. Sci. 2022, 9, 175. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Royal, A.B.; Villamil, J.A. Disseminated melanoma in a Dog with Involvement of Leptomeninges and Bone marrow. Vet. Pathol. 2009, 46, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Camerino, M.; Giacobino, D.; Manassero, L.; Iussich, S.; Riccardo, F.; Cavallo, F.; Tarone, L.; Olimpo, M.; Lardone, E.; Martano, M.; et al. Prognostic impact of bone invasion in canine oral malignant melanoma treated by surgery and anti-CSPG4 vaccination: A retrospective study on 68 cases (2010–2020). Vet. Comp. Oncol. 2022, 20, 189–197. [Google Scholar] [CrossRef]
- Napoli, S.; Scuderi, C.; Gattuso, G.; Di Bella, V.; Candido, S.; Basile, M.S.; Libra, M.; Falzone, L. Functional roles of matrix metalloproteinases and their inhibitors in melanoma. Cells 2020, 9, 1151. [Google Scholar] [CrossRef]
- Flickinger, I.; Rütgen, B.C.; Gerner, W.; Calice, I.; Tichy, A.; Saalmüller, A.; Kleiter, M. Radiation upregulates the expression of VEGF in a canine oral melanoma cell line. J. Vet. Sci. 2013, 14, 207–214. [Google Scholar] [CrossRef] [PubMed]
- AVMA Guidelines for the Euthanasia of Animals: 2020 Edition. Available online: https://www.avma.org/resources-tools/avma-policies/avma-guidelines-euthanasia-animals (accessed on 28 January 2020).
- Bocaneti, F.; Altamura, G.; Corteggio, A.; Solcan, C.; Velescu, E.; Borzacchiello, G. Expression of Cyclooxygenase-2 in naturally occurring bovine cutaneous fibropapillomas. Pol. J. Vet. Sci. 2015, 18, 655–658. [Google Scholar] [CrossRef] [PubMed]
- Zbytek, B.; Carlson, J.A.; Granese, J.; Ross, J.; Mihm, M.C.; Slominski, A. Current concepts of metastasis in melanoma. Expert Rev. Dermatol. 2008, 3, 569–585. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.H.; Goldschmidt, M.H.; McManus, P.M. A comparative review of Melanocytic Neoplasms. Vet. Pathol. 2002, 39, 651–678. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, S.; Alhefdhi, A.Y.; Almalik, O.; Anwar, I.; Mahmood, R.; Mahasin, Z.; Al-Tweigeri, T. Primary oral malignant melanoma metastasis to the brain and breast: A case report and literature review. Oncol. Lett. 2017, 14, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Leon, N.; Pacheco-Barcia, V.; Ballesteros, A.I.; Fraga, J.; Colomer, R.; Friera, A. Skeletal muscle and solitary bone metastasis from malignant melanoma: Multimodality imaging and oncological outcome. Melanoma Res. 2018, 28, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Sobczynska-Rak, A.; Polkowska, I.; Silmanowicz, P. Elevated vascular endothelial growth factor (VEGF) levels in the blood serum of dogs with malignant neoplasms of the oral cavity. Acta Vet. Hung. 2014, 62, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Pires, I.; Gomes, J.; Prada, J.; Pereira, D.; Queiroga, F.L. MMP-2 and MMP-9 Expression in Canine Cutaneous Melanocytic Tumours: Evidence of a Relationship with Tumoral Malignancy; Melanoma—From Early Detection, Book 2; IntechOpen: London, UK, 2013. [Google Scholar]
- Hofmann, U.B.; Westphal, J.R.; van Muijen, G.N.; Ruiter, D.J. Matrix metalloproteinases in human melanoma. J. Investig. Dermatol. 2000, 115, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Haqq, C.; Nosrati, M.; Sudilovsky, D.; Crothers, J.; Khodabakhsh, D.; Pulliam, B.L.; Federman, S.; Miller, J.R., III; Allen, R.E.; Singer, M.I.; et al. The gene expression signatures of melanoma progression. Proc. Natl. Acad. Sci. USA 2005, 102, 6092–6097. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Miyake, M.; Lawton, A.; Goodison, S.; Rosser, C.J. Matrix metalloproteinase-10 promotes tumour progression through regulation of angiogenic and apoptotic pathways in cervical tumours. BMC Cancer 2014, 14, 310. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hritcu, O.-M.; Bocaneti Daraban, F.; Bacusca, F.D.; Pasca, A.-S. Unusual Canine Cutaneous Melanoma Presenting Parietal Bone Metastasis: A Case Report. Vet. Sci. 2023, 10, 282. https://doi.org/10.3390/vetsci10040282
Hritcu O-M, Bocaneti Daraban F, Bacusca FD, Pasca A-S. Unusual Canine Cutaneous Melanoma Presenting Parietal Bone Metastasis: A Case Report. Veterinary Sciences. 2023; 10(4):282. https://doi.org/10.3390/vetsci10040282
Chicago/Turabian StyleHritcu, Ozana-Maria, Florentina Bocaneti Daraban, Fabian Dominic Bacusca, and Aurelian-Sorin Pasca. 2023. "Unusual Canine Cutaneous Melanoma Presenting Parietal Bone Metastasis: A Case Report" Veterinary Sciences 10, no. 4: 282. https://doi.org/10.3390/vetsci10040282
APA StyleHritcu, O.-M., Bocaneti Daraban, F., Bacusca, F. D., & Pasca, A.-S. (2023). Unusual Canine Cutaneous Melanoma Presenting Parietal Bone Metastasis: A Case Report. Veterinary Sciences, 10(4), 282. https://doi.org/10.3390/vetsci10040282









