Determining Frequency of Common Pulmonary Gross and Histopathological Findings in Feedyard Fatalities
Abstract
Simple Summary
Abstract
1. Introduction
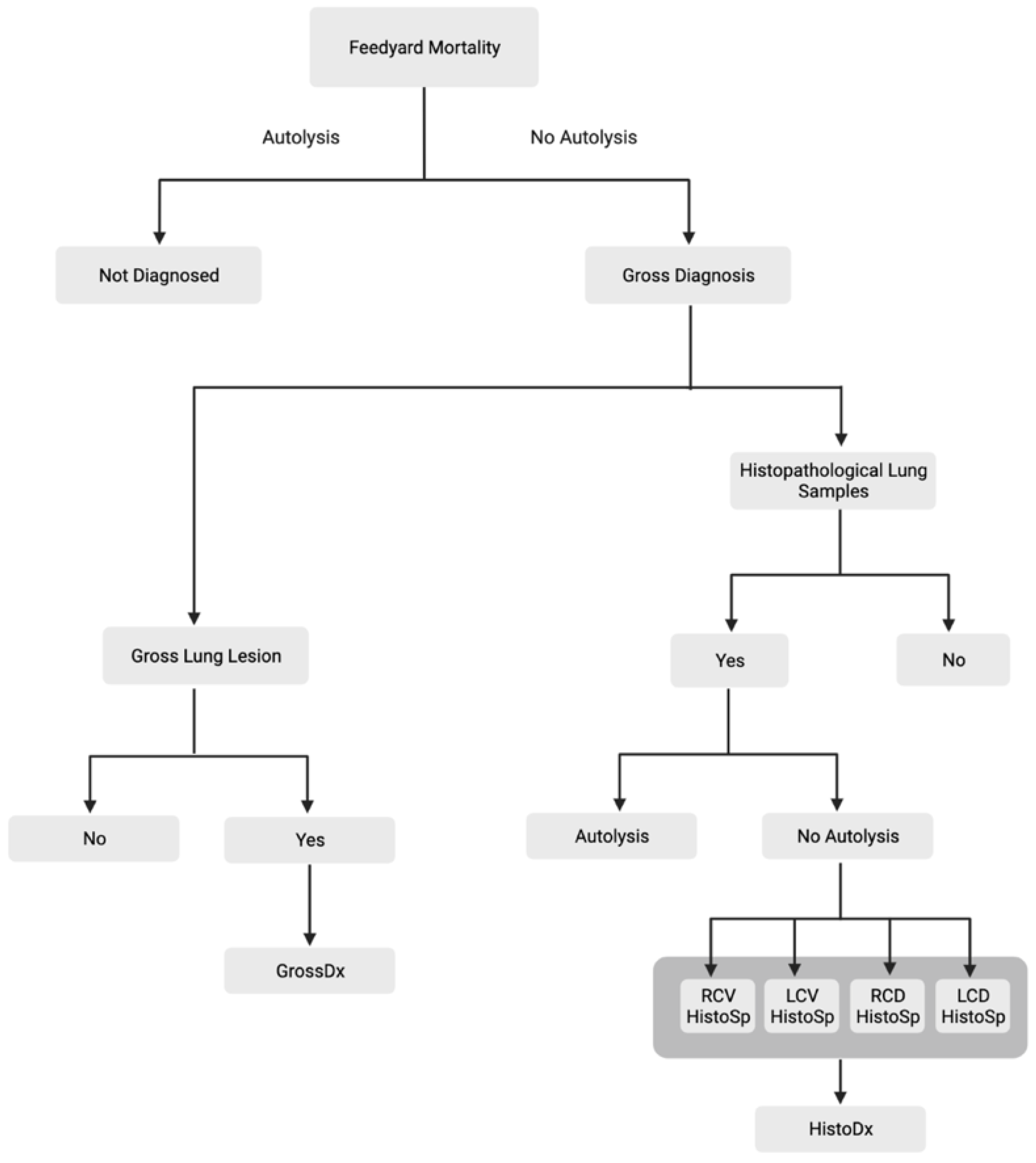
2. Materials and Methods
2.1. Experimental Design
2.2. Postmortem Evaluation
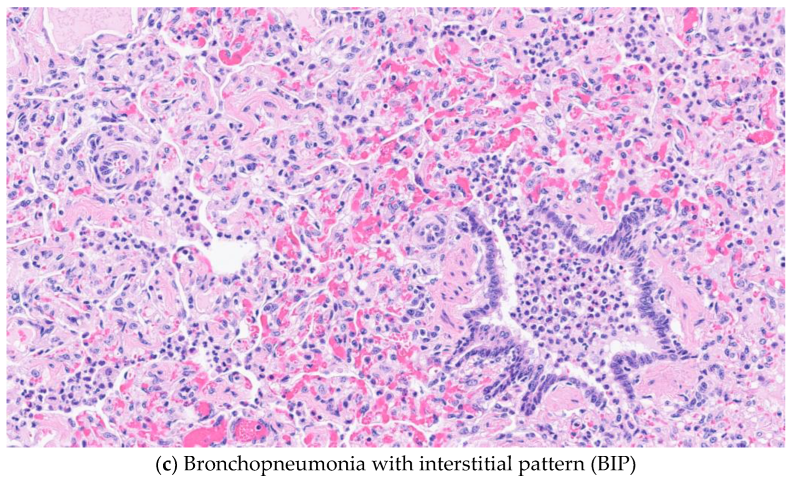
2.3. Histopathology
2.4. Statistical Analysis
3. Results
3.1. Gross Results
3.2. Histopathological Results
3.3. GrossDx versus HistoDx
3.4. HistoDx and HistoSp
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnson, K.K.; Pendell, D.L. Market Impacts of Reducing the Prevalence of Bovine Respiratory Disease in United States Beef Cattle Feedlots. Front. Vet. Sci. 2017, 4, 189. [Google Scholar] [CrossRef]
- Griffin, D. Economic Impact Associated with Respiratory Disease in Beef Cattle. Vet. Clin. N. Am. Food Anim. Pract. 1997, 13, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.J.; Tait, R.G.; Busby, W.D.; Reecy, J.M. An Evaluation of Bovine Respiratory Disease Complex in Feedlot Cattle: Impact on Performance and Carcass Traits Using Treatment Records and Lung Lesion Scores1,2. J. Anim. Sci. 2009, 87, 1821–1827. [Google Scholar] [CrossRef] [PubMed]
- Brooks, K.R.; Raper, K.C.; Ward, C.E.; Holland, B.P.; Krehbiel, C.R.; Step, D.L. Economic Effects of Bovine Respiratory Disease on Feedlot Cattle during Backgrounding and Finishing Phases 1. Prof. Anim. Sci. 2011, 27, 195–203. [Google Scholar] [CrossRef]
- Blakebrough-Hall, C.; Hick, P.; Mahony, T.J.; González, L.A. Factors Associated with Bovine Respiratory Disease Case Fatality in Feedlot Cattle. J. Anim. Sci. 2022, 100, skab361. [Google Scholar] [CrossRef] [PubMed]
- Engler, M.; Defoor, P.; King, C.; Gleghorn, J. The Impact of Bovine Respiratory Disease: The Current Feedlot Experience. Anim. Health Res. Rev. 2014, 15, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Babcock, A.H.; Renter, D.G.; White, B.J.; Dubnicka, S.R.; Scott, H.M. Temporal Distributions of Respiratory Disease Events within Cohorts of Feedlot Cattle and Associations with Cattle Health and Performance Indices. Prev. Vet. Med. 2010, 97, 198–219. [Google Scholar] [CrossRef]
- Loneragan, G.H.; Gould, D.H.; Mason, G.L.; Garry, F.B.; Yost, G.S.; Miles, D.G.; Hoffman, B.W.; Mills, L.J. Involvement of Microbial Respiratory Pathogens in Acute Interstitial Pneumonia in Feedlot Cattle. Am. J. Vet. Res. 2001, 62, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Booker, C.W.; Abutarbush, S.M.; Morley, P.S.; Jim, G.K.; Pittman, T.J.; Schunicht, O.C.; Perrett, T.; Wildman, B.K.; Fenton, R.K.; Guichon, P.T.; et al. Microbiological and Histopathological Findings in Cases of Fatal Bovine Respiratory Disease of Feedlot Cattle in Western Canada. Can. Vet. J. 2008, 49, 473–481. [Google Scholar] [PubMed]
- Fulton, R.W.; Blood, K.S.; Panciera, R.J.; Payton, M.E.; Ridpath, J.F.; Confer, A.W.; Saliki, J.T.; Burge, L.T.; Welsh, R.D.; Johnson, B.J.; et al. Lung Pathology and Infectious Agents in Fatal Feedlot Pneumonias and Relationship with Mortality, Disease Onset, and Treatments. J. Vet. Diagn. Investig. 2009, 21, 464–477. [Google Scholar] [CrossRef]
- Gardner, B.A.; Dolezal, H.G.; Bryant, L.K.; Owens, F.N.; Smith, R.A. Health of Finishing Steers: Effects on Performance, Carcass Traits, and Meat Tenderness. J. Anim. Sci. 1999, 77, 3168. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.; del Ferreras, M.C.; Giráldez, F.J.; Benavides, J.; Pérez, V. Production Significance of Bovine Respiratory Disease Lesions in Slaughtered Beef Cattle. Animals 2020, 10, 1770. [Google Scholar] [CrossRef] [PubMed]
- Griffin, D.; Chengappa, M.M.; Kuszak, J.; McVey, D.S. Bacterial Pathogens of the Bovine Respiratory Disease Complex. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 381–394. [Google Scholar] [CrossRef]
- Duff, G.C.; Galyean, M.L. BOARD-INVITED REVIEW: Recent Advances in Management of Highly Stressed, Newly Received Feedlot Cattle. J. Anim. Sci. 2007, 85, 823–840. [Google Scholar] [CrossRef]
- Li, C.; Zaheer, R.; Kinnear, A.; Jelinski, M.; McAllister, T.A. Comparative Microbiomes of the Respiratory Tract and Joints of Feedlot Cattle Mortalities. Microorganisms 2022, 10, 134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hill, J.E.; Godson, D.L.; Ngeleka, M.; Fernando, C.; Huang, Y. The Pulmonary Virome, Bacteriological and Histopathological Findings in Bovine Respiratory Disease from Western Canada. Transbound. Emerg. Dis. 2020, 67, 924–934. [Google Scholar] [CrossRef] [PubMed]
- Ayroud, M.; Popp, J.D.; VanderKop, M.A.; Yost, G.S.; Haines, D.M.; Majak, W.; Karren, D.; Yanke, L.J.; McAllister, T.A. Characterization of Acute Interstitial Pneumonia in Cattle in Southern Alberta Feedyards. Can. Vet. J. 2000, 41, 8. [Google Scholar]
- Woolums, A.R. Baseline Management Practices and Animal Health Data Reported by US Feedlots Responding to a Survey Regarding Acute Interstitial Pneumonia. Bov. Pract. 2005, 39, 9. [Google Scholar] [CrossRef]
- Blood, D.C. Atypical Interstitial Pneumonia of Cattle. Can. Vet. J. 1962, 3, 40–47. [Google Scholar]
- Loneragan, G.H.; Gould, D.H.; Mason, G.L.; Garry, F.B.; Yost, G.S.; Lanza, D.L.; Miles, D.G.; Hoffman, B.W.; Mills, L.J. Association of 3-Methyleneindolenine, a Toxic Metabolite of 3-Methylindole, with Acute Interstitial Pneumonia in Feedlot Cattle. Am. J. Vet. Res. 2001, 62, 1525–1530. [Google Scholar] [CrossRef]
- Woolums, A.R.; Loneragan, G.H.; Hawkins, L.L.; Williams, S.M. A Survey of the Relationship Between Management Practices and Risk of Acute Interstitial Pneumonia at US Feedlots. Bov. Pract. 2005, 32, 125–133. [Google Scholar] [CrossRef]
- Haydock, L.A.J.; Fenton, R.K.; Sergejewich, L.; Veldhuizen, R.A.W.; Smerek, D.; Ojkic, D.; Caswell, J.L. Bronchopneumonia with Interstitial Pneumonia in Beef Feedlot Cattle: Characterization and Laboratory Investigation. Vet. Pathol. 2023, 60, 214–225. [Google Scholar] [CrossRef]
- Haydock, L.A.J.; Fenton, R.K.; Smerek, D.; Renaud, D.L.; Caswell, J.L. Bronchopneumonia with Interstitial Pneumonia in Feedlot Cattle: Epidemiologic Characteristics of Affected Animals. Vet. Pathol. 2023, 60, 226–234. [Google Scholar] [CrossRef]
- Griffin, D. Field Necropsy and Diagnostic Sample Submission. Am. Assoc. Bov. Pract. Conf. Proc. 2020, 53, 346–352. [Google Scholar] [CrossRef]
- Panciera, R.J.; Confer, A.W. Pathogenesis and Pathology of Bovine Pneumonia. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Andrews, G.A.; Kennedy, G.A. Respiratory Diagnostic Pathology. Vet. Clin. N. Am. Food Anim. Pract. 1997, 13, 515–547. [Google Scholar] [CrossRef] [PubMed]
- Woolums, A.R.; Mason, G.L.; Hawkins, L.L.; Brown, C.C.; Williams, S.M.; Gould, J.A.; Fox, J.J.; Sturgeon, S.D.; Anderson, J.L.; Duggan, F.E.; et al. Microbiologic Findings in Feedlot Cattle with Acute Interstitial Pneumonia. Am. J. Vet. Res. 2004, 65, 1525–1532. [Google Scholar] [CrossRef]
- Vale, J.A.; Apley, M.D.; Reinhardt, C.D.; Bartle, S.J.; Thomson, D.U. Pathologies of Acute Interstitial Pneumonia in Feedlot Cattle. Am. J. Anim. Vet. 2016, 11, 1–7. [Google Scholar] [CrossRef]
- Chien, R.C.; Sorensen, N.J.; Payton, M.E.; Confer, A.W. Comparative Histopathology of Bovine Acute Interstitial Pneumonia and Bovine Respiratory Syncytial Virus-Associated Interstitial Pneumonia. J. Comp. Pathol. 2022, 192, 23–32. [Google Scholar] [CrossRef]
- Klima, C.L.; Zaheer, R.; Cook, S.R.; Booker, C.W.; Hendrick, S.; Alexander, T.W.; McAllister, T.A. Pathogens of Bovine Respiratory Disease in North American Feedlots Conferring Multidrug Resistance via Integrative Conjugative Elements. J. Clin. Microbiol. 2014, 52, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.W.; Nagy, E.; Armstrong, D. The Associations of Viral and Mycoplasmal Antibody Titers with Respiratory Disease and Weight Gain in Feedlot Calves. Can. Vet. J. 1999, 40, 9. [Google Scholar]
- Woolums, A.R. Feedlot Acute Interstitial Pneumonia. Vet. Clin. N. Am. Food Anim. Pract. 2015, 31, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Haydock, L.A.J.; Fenton, R.K.; Sergejewich, L.; Squires, E.J.; Caswell, J.L. Acute Interstitial Pneumonia and the Biology of 3-Methylindole in Feedlot Cattle. Anim. Health Res. Rev. 2022, 23, 72–81. [Google Scholar] [CrossRef]





| Category | Count | Percent Total |
|---|---|---|
| Sex | ||
| Heifer | 279 | 69.40% |
| Steer | 123 | 30.60% |
| Origin/Breed Type | ||
| Native | 374 | 93.00% |
| Beef × Dairy | 24 | 6.00% |
| Holstein | 3 | 0.70% |
| Mexican | 1 | 0.20% |
| Arrival Weight (kg) | ||
| 181 | 3 | 0.70% |
| 227 | 42 | 10.40% |
| 272 | 81 | 20.10% |
| 318 | 152 | 37.80% |
| 363 | 96 | 23.90% |
| 408 | 17 | 4.20% |
| 454 | 6 | 1.50% |
| Days on Feed | ||
| 0–50 | 114 | 28.40% |
| 51–100 | 107 | 26.60% |
| 101–150 | 122 | 30.30% |
| 151–200 | 51 | 12.70% |
| >200 | 5 | 1.20% |
| Week | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Feedyard 1 | Total | 0 | 5 | 17 | 22 | 12 | 7 | 14 | 12 | 25 | 114 |
| Gross Dx | 0 | 5 | 9 | 22 | 10 | 7 | 13 | 12 | 24 | 102 | |
| HistoDx | 0 | 5 | 10 | 9 | 11 | 7 | 10 | 11 | 10 | 73 | |
| Feedyard 2 | Total | 1 | 5 | 6 | 11 | 10 | 3 | 2 | 2 | 6 | 46 |
| GrossDx | 1 | 1 | 5 | 10 | 8 | 3 | 2 | 0 | 5 | 35 | |
| HistoDx | 1 | 2 | 5 | 10 | 7 | 2 | 1 | 2 | 5 | 35 | |
| Feedyard 3 | Total | 1 | 11 | 8 | 9 | 5 | 4 | 13 | 10 | 10 | 71 |
| GrossDx | 1 | 9 | 6 | 7 | 5 | 3 | 10 | 9 | 9 | 59 | |
| HistoDx | 0 | 9 | 6 | 7 | 3 | 4 | 9 | 9 | 10 | 57 | |
| Feedyard 4 | Total | 2 | 6 | 4 | 5 | 5 | 2 | 1 | 2 | 2 | 29 |
| GrossDx | 2 | 5 | 4 | 3 | 5 | 2 | 1 | 2 | 2 | 26 | |
| HistoDx | 2 | 5 | 4 | 4 | 4 | 1 | 1 | 1 | 2 | 24 | |
| Feedyard 5 | Total | 6 | 14 | 12 | 12 | 6 | 4 | 8 | 9 | 5 | 76 |
| GrossDx | 6 | 12 | 10 | 8 | 5 | 4 | 5 | 7 | 5 | 62 | |
| HistoDx | 5 | 9 | 9 | 9 | 6 | 4 | 8 | 8 | 4 | 62 | |
| Feedyard 6 | Total | 3 | 3 | 13 | 7 | 14 | 9 | 11 | 12 | 9 | 81 |
| GrossDx | 3 | 3 | 10 | 9 | 13 | 8 | 9 | 11 | 9 | 75 | |
| HistoDx | 3 | 3 | 9 | 7 | 9 | 8 | 10 | 9 | 9 | 67 | |
| Histopathological Diagnoses | ||||
|---|---|---|---|---|
| Gross Diagnosis | AIP | BIP | BP | Undifferentiated |
| AIP | 3 | 8 | 1 | 1 |
| BIP | 10 | 44 | 32 | 3 |
| BP | 6 | 13 | 21 | 18 |
| Undifferentiated | 3 | 2 | 5 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, P.H.; White, B.J.; Finley, A.; Bortoluzzi, E.M.; Depenbusch, B.E.; Mancke, M.; Brown, R.E.; Jensen, M.; Lancaster, P.A.; Larson, R.L. Determining Frequency of Common Pulmonary Gross and Histopathological Findings in Feedyard Fatalities. Vet. Sci. 2023, 10, 228. https://doi.org/10.3390/vetsci10030228
Schmidt PH, White BJ, Finley A, Bortoluzzi EM, Depenbusch BE, Mancke M, Brown RE, Jensen M, Lancaster PA, Larson RL. Determining Frequency of Common Pulmonary Gross and Histopathological Findings in Feedyard Fatalities. Veterinary Sciences. 2023; 10(3):228. https://doi.org/10.3390/vetsci10030228
Chicago/Turabian StyleSchmidt, Paige H., Brad J. White, Abigail Finley, Eduarda M. Bortoluzzi, Brandon E. Depenbusch, Maddie Mancke, Rachel E. Brown, Makenna Jensen, Phillip A. Lancaster, and Robert L. Larson. 2023. "Determining Frequency of Common Pulmonary Gross and Histopathological Findings in Feedyard Fatalities" Veterinary Sciences 10, no. 3: 228. https://doi.org/10.3390/vetsci10030228
APA StyleSchmidt, P. H., White, B. J., Finley, A., Bortoluzzi, E. M., Depenbusch, B. E., Mancke, M., Brown, R. E., Jensen, M., Lancaster, P. A., & Larson, R. L. (2023). Determining Frequency of Common Pulmonary Gross and Histopathological Findings in Feedyard Fatalities. Veterinary Sciences, 10(3), 228. https://doi.org/10.3390/vetsci10030228









