Correlation between Babesia Species Affecting Dogs in Taiwan and the Local Distribution of the Vector Ticks
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Animal and Tick Collections
2.2. Multiplex-Nested PCR Amplification of the B. gibsoni 18S rRNA Gene
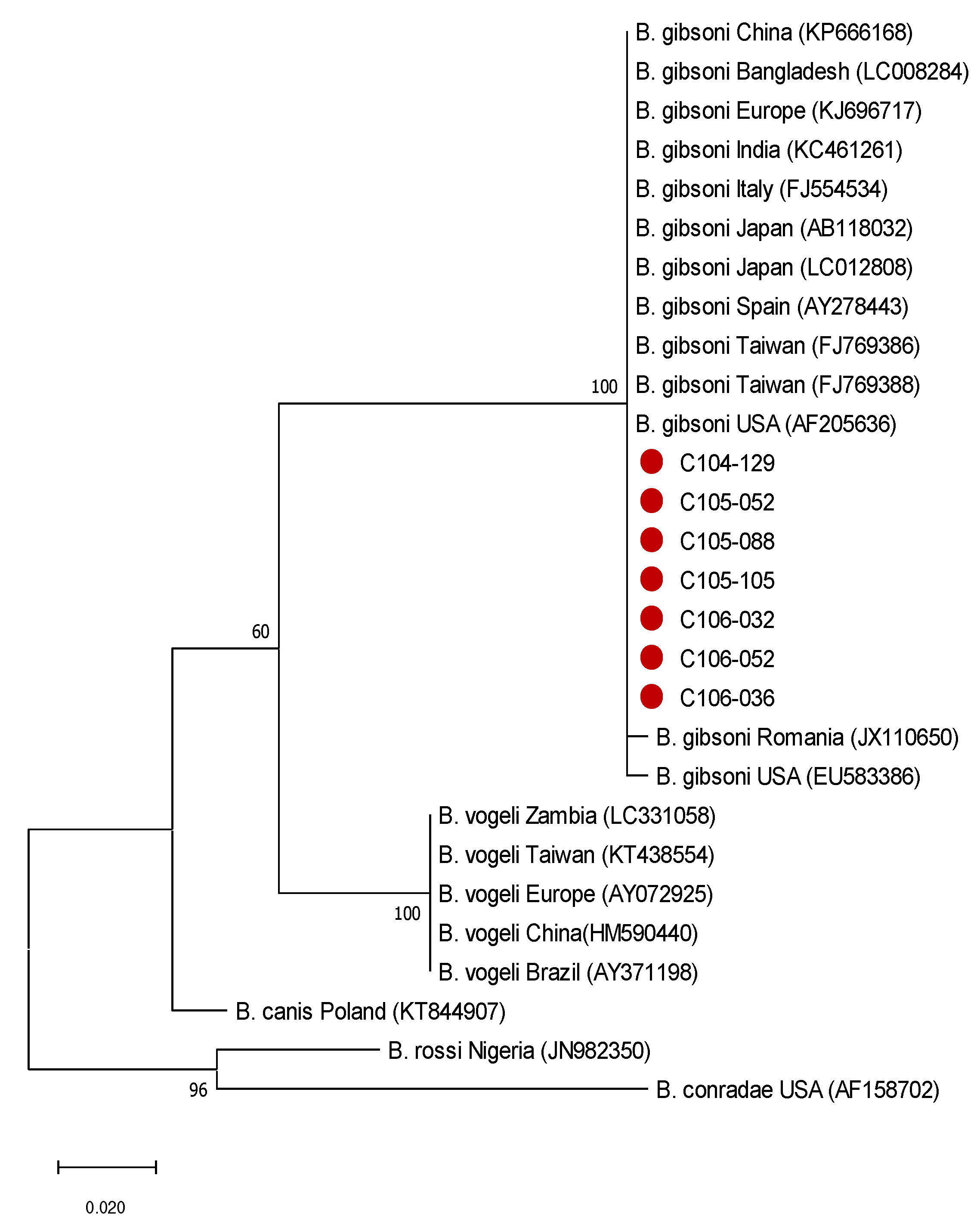
2.3. Phylogenetic Analysis of Babesia gibsoni from Seven Samples with Different Anaemic Severity
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sonenshine, D.E. Range expansion of tick disease vectors in North America: Implications for spread of tick-borne disease. Int. J. Environ. Res. Public Health 2018, 15, 478. [Google Scholar] [CrossRef]
- Birkenheuer, A.J.; Levy, M.G.; Breitschwerdt, E.B. Efficacy of combined atovaquone and azithromycin for therapy of chronic Babesia gibsoni (Asian genotype) infections in dogs. J. Vet. Intern. Med. 2004, 18, 494–498. [Google Scholar] [CrossRef]
- Jefferies, R.; Ryan, U.; Jardine, J.; Broughton, D.; Robertson, I.; Irwin, P. Blood, bull terriers and babesiosis: Further evidence for direct transmission of Babesia gibsoni in dogs. Aust. Vet. J. 2007, 85, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.C.; Chueh, L.L.; Lin, C.N.; Hsieh, L.E.; Su, B.L. The therapeutic efficacy of two anti-babesial strategies against Babesia gibsoni. Vet. Parasitol. 2012, 186, 159–164. [Google Scholar] [CrossRef]
- Liu, P.C.; Su, B.L. Causes of canine anemia in Taiwan: A five-year retrospective survey. Taiwan Vet. J. 2015, 41, 31–37. [Google Scholar] [CrossRef]
- Birkenheuer, A.J.; Buch, J.; Beall, M.J.; Braff, J.; Chandrashekar, R. Global distribution of canine Babesia species identified by a commercial diagnostic laboratory. Vet. Parasitol. Reg. Stud. Rep. 2022, 22, 100471. [Google Scholar] [CrossRef] [PubMed]
- Do, T.; Ngasaman, R.; Saechan, V.; Pitaksakulrat, O.; Liu, M.; Xuan, X.; Inpankaew, T. First Molecular Detection of Babesia gibsoni in Stray Dogs from Thailand. Pathogens 2021, 10, 639. [Google Scholar] [CrossRef]
- Chou, S.J.; Wu, J.T.; Liao, P.J.; Huang, H.C.; Wang, K.T.; Chang, H.Y.; Hsieh, Y.C.; Lee, C.C.; Wang, J.H.; Chan, K.W. Epidemiological Survey of Tick-borne Disease of Stray Dogs in Yun-Chia-Nan Areas in Taiwan. Taiwan Vet. J. 2012, 38, 276–282. [Google Scholar]
- Yang, W.Y.; Reynolds, C.; Mestek, A.; Huang, G.C.; Lee, C.J.; Wang, S.L. A molecular and serological survey in Taiwan to determine the true risk of babesiosis in dogs not receiving regular tick prevention. Vet. Parasitol. Reg. Stud. Rep. 2022, 27, 100670. [Google Scholar] [CrossRef]
- Birkenheuer, A.J.; Levy, M.G.; Stebbins, M.; Poore, M.; Breitschwerdt, E. Serosurvey of anti-Babesia antibodies in stray dogs and American pit bull terriers and American staffordshire terriers from North Carolina. J. Am. Anim. Hosp. Assoc. 2003, 39, 551–557. [Google Scholar] [CrossRef]
- Ikadai, H.; Tanaka, H.; Shibahara, N.; Matsuu, A.; Uechi, M.; Itoh, N.; Oshiro, S.; Kudo, N.; Igarashi, I.; Oyamada, T. Molecular evidence of infections with Babesia gibsoni parasites in Japan and evaluation of the diagnostic potential of a loop-mediated isothermal amplification method. J. Clin. Microbiol. 2004, 42, 2465–2469. [Google Scholar] [CrossRef]
- Miyama, T.; Sakata, Y.; Shimada, Y.; Ogino, S.; Watanabe, M.; Itamoto, K.; Okuda, M.; Verdida, R.A.; Xuan, X.; Inokuma, H. Epidemiological survey of Babesia gibsoni infection in dogs in eastern Japan. J. Vet. Med. Sci. 2005, 67, 467–471. [Google Scholar] [CrossRef]
- Yao, D.W.; Jiang, J.Y.; Yu, Z.Z.; Yao, D.Q.; Yang, D.J.; Zhao, Y.B. Canine Babesiosis in China caused by Babesia gibsoni: A molecular approach. Iran. J. Parasitol. 2014, 9, 163–168. [Google Scholar]
- Lee, M.J.; Yu, D.H.; Yoon, J.S.; Li, Y.H.; Lee, J.H.; Chae, J.S.; Park, J. Epidemiologic and clinical surveys in dogs infected with Babesia gibsoni in South Korea. Vector Borne Zoonotic Diseases 2009, 9, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Iwakami, S.; Ichikawa, Y.; Inokuma, H. Molecular survey of Babesia gibsoni using Haemaphysalis longicornis collected from dogs and cats in Japan. J. Vet. Med. Sci. 2014, 76, 1313–1316. [Google Scholar] [CrossRef] [PubMed]
- Chao, L.L.; Liao, H.T.; Ho, T.Y.; Shih, C.M. First detection and molecular identification of Babesia gibsoni from Rhipicephalus sanguineus ticks. Acta Trop. 2017, 166, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Jongejan, F.; Su, B.L.; Yang, H.J.; Berger, L.; Bevers, J.; Liu, P.C.; Fang, J.C.; Cheng, Y.W.; Kraakman, C.; Plaxton, N. Molecular evidence for the transovarial passage of Babesia gibsoni in Haemaphysalis hystricis (Acari: Ixodidae) 9cks from Taiwan: A novel vector for canine babesiosis. Parasites Vectors 2018, 11, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Chao, L.L.; Hsieh, C.K.; Ho, T.Y.; Shih, C.M. First zootiological survey of hard ticks (Acari: Ixodidae) infesting dogs in northern Taiwan. Exp. Appl. Acarol. 2019, 77, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Zahler, M.; Schein, E.; Rinder, H.; Gothe, R. Characteristic genotypes discriminate between Babesia canis isolates of differing vector specigicity and pathogenicity to dogs. Parasitol. Res. 1998, 84, 544–548. [Google Scholar] [CrossRef]
- Liu, P.C.; Lin, C.N.; Su, B.L. Clinical characteristics of naturally Babesia gibsoni infected dogs: A study of 60 dogs. Vet. Parasitol. Reg. Stud. Rep. 2022, 28, 100675. [Google Scholar] [CrossRef]
- Salano-Gallego, L.; Trotta, M.; Carli, E.; Carcy, B.; Caldin, M.; Furlanello, T. Babesia canis canis and Babesia canis vogeli clinicopathological findings and DNA detection by means of PCR-RFLP in blood from Italian dogs suspected of tick-borne disease. Vet. Paratitol. 2008, 157, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Rawangchue, T.; Sungpradit, S. Clinicopathological and molecular profiles of Babesia vogeli infection and Ehrlichia canis coinfection. Vet. World 2020, 13, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, J.; Kelly, P.; Zheng, X.; Li, M.; You, J.; Huang, K.; Qiu, H.; Wang, Y.; Zhang, R.; et al. first description of pathogenicity of Babesia vogeli in experimentally infected dogs. Vet. Parasitol. 2018, 253, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Birkenheuer, A.J.; Levy, M.G.; Breitschwerdt, E.B. Development and evaluation of a seminested PCR for detection and differentiation of Babesia gibsoni (Asian Genotype) and Babesia canis DNA in canine blood samples. J. Clin. Microbiol. 2003, 41, 4172–4177. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Flipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rate base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Yamaguti, N.; Tipton, V.J.; Keegan, H.L.; Toshioka, S. Ticks of Japan, Korea, and the Ryuku Islands. Brigham Young Univ. Sci. Bull. 1971, 15, 77–83. [Google Scholar]
- Yuasa, Y.; Tsai, Y.L.; Chang, C.C.; Hsu, T.H.; Chou, C.C. The prevalence of Anaplasma platys and a potential novel Anaplasma species exceed that of Ehrlichia canis in asymptomatic dogs and Rhipicephalus sanguineus in Taiwan. J. Vet. Med. Sci. 2017, 17, 2–24. [Google Scholar] [CrossRef]
- Dantas-Torres, F. Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasites Vectors 2010, 3, 1–11. [Google Scholar] [CrossRef]
- Chao, L.L.; Shih, C.M. Molecular analysis of Rhipicephalus sanguineus (Acari: Ixodidae), an incriminated vector tick for Babesia vogeli in Taiwan. Exp. Appl. Acarol. 2016, 70, 469–481. [Google Scholar] [CrossRef]
- Chao, L.L.; Yeh, S.T.; Hsieh, C.K.; Shih, C.M. First detection and molecular identification of Babesia vogeli from Rhipicephalus sanguineus (Acari: Ixodidae) in Taiwan. Exp. Appl. Acarol. 2016, 68, 539–551. [Google Scholar] [CrossRef] [PubMed]
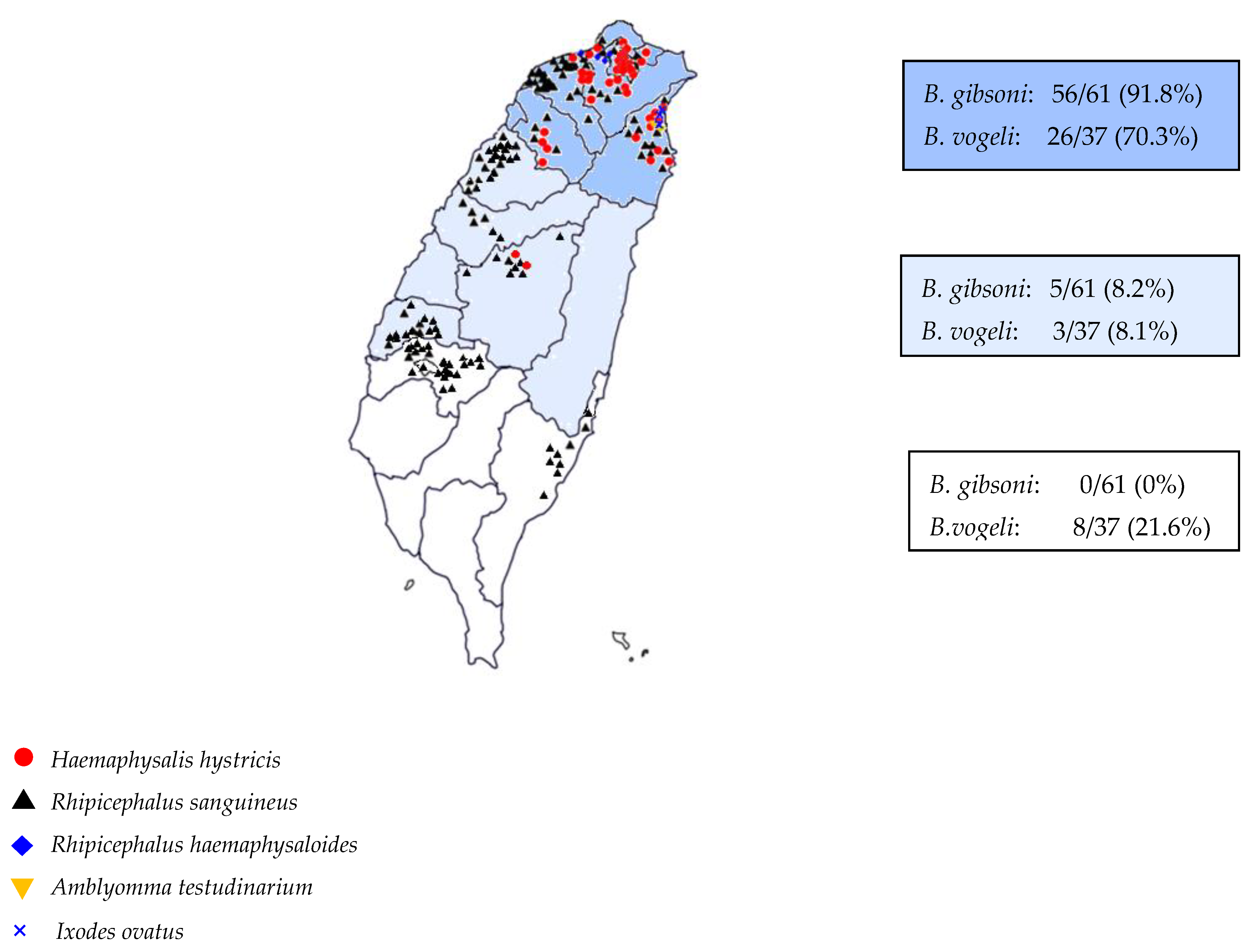
| Total | R. sanguineus a | H. hystricis b | R. haemaphysaloides c | I. ovatus d | R. sanguineus a + H. hystricis b | R. sanguineus a + H. hystricis b + A. testudinarium e | H. hystrici b + A. testudinarium e | R. sanguineus a + R. haemaphysaloides c | R. sanguineus a + H. hystricis b + I. ovatus d | |
|---|---|---|---|---|---|---|---|---|---|---|
| Northern | 261 | 185 | 53 | 0 | 1 | 12 | 5 | 3 | 1 | 1 |
| (70.9%) | (20.3%) | (0%) | (0.4%) | (4.6%) | (1.9%) | (1.1%) | (0.4%) | (0.4%) | ||
| Central | 83 | 78 | 3 | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| (94%) | (3.6%) | (1.2%) | (0%) | (0%) | (0%) | (0%) | (1.2%) | (0%) | ||
| Southern | 44 | 44 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| (100%) | (0%) | (0%) | (0%) | (0%) | (0%) | (0%) | (0%) | (0%) |
| (a) | |||||
|---|---|---|---|---|---|
| Dogs Infested with Tick Species | p-Value | Relative Risk (95% CI) | Odds Ratio (95% CI) | ||
| H. hystricis | Others | ||||
| B. gibsoni | <0.001 | 6.042 (3.93–9.28) | 12.23 (6.2–24.15) | ||
| + | 27 | 22 | |||
| − | 29 | 289 | |||
| B. vogeli | 0.326 | ||||
| + | 3 | 31 | |||
| − | 53 | 270 | |||
| (b) | |||||
| Dogs Infested with Tick Species | p-Value | Relative Risk (95% CI) | Odds Ratio (95% CI) | ||
| R. sanguineus | Others | ||||
| B. gibsoni | <0.001 | 0.456 (0.33–0.64) | 0.076 (0.04–0.15) | ||
| + | 21 | 30 | |||
| − | 287 | 31 | |||
| B. vogeli | 0.481 | ||||
| + | 31 | 4 | |||
| − | 276 | 57 | |||
| Item | Total | Nonanemia | Mild | Moderate | Severe | Reference | |
|---|---|---|---|---|---|---|---|
| Number of dogs | 61 | 8 (13.1%) | 18 (29.5%) | 23 (37.7%) | 12 (19.7%) | ||
| Red blood cell count | M/μL | 4.62 ± 1.28 | 6.67 ± 0.85 | 5.20 ± 0.56 | 4.02 ± 0.58 | 2.62 ± 0.40 | 5.5–8.5 |
| Hematocrit | % | 28.7 ± 9.1 | 44.6 ± 7.4 | 33.4 ± 2.2 | 25.2 ± 2.9 | 17.14 ± 2.15 | 37–55 |
| Hemoglobin | g/dL | 10.38 ± 2.80 | 14.66 ± 2.15 | 11.81 ± 1.24 | 9.00 ± 1.15 | 6.13 ± 0.80 | 12.0–18.0 |
| Mean corpuscular volume | fL | 62.9 ± 3.94 | 64.3 ± 4.38 | 62.2 ± 2.07 | 62.5 ± 4.44 | 65.87 ± 5.84 | 60–72 |
| Mean corpuscular hemoglobin | Pg | 22.6 ± 1.38 | 21.9 ± 1.68 | 22.6 ± 0.94 | 22.6 ± 1.57 | 23.62 ± 1.52 | 19.5–25.5 |
| Mean corpuscular hemoglobin concentration | g/dL | 35.9 ± 1.51 | 34.2 ± 1.07 | 36.4 ± 1.41 | 36.0 ± 1.50 | 35.82 ± 1.34 | 32–38.5 |
| Red cell distribution width | % | 17.4 ± 1.67 | 17.7 ± 2.36 | 18.3 ± 5.35 | 17.5 ± 2.00 | 17.94 ± 1.39 | 12.0–17.5 |
| White blood cell count | /μL | 13,150 ± 5434 | 16,847 ± 6765 | 12,394 ± 5016 | 12,939 ± 4986.9 | 12,000 ± 4889 | 6000–17,000 |
| Neutrophil | /μL | 8967 ± 4878 | 11,292 ± 6525 | 8384 ± 4385 | 8869 ± 4636 | 8581 ± 4167 | 2950–11,640 |
| Lymphocyte | /μL | 2277 ± 1307 | 2809 ± 2705 | 2027 ± 893 | 2236 ± 993 | 1208 ± 790 | 1050–5100 |
| Monocyte | /μL | 1435 ± 955 | 2379 ± 1350 | 1539 ± 945 | 1191 ± 631 | 851 ± 562 | 160–1120 |
| Eosinophil | /μL | 399 ± 538 | 373 ± 465 | 424 ± 603 | 473 ± 582 | 132 ± 179 | 60–1230 |
| Platelets | K/μL | 169 ± 94 | 158 ± 68.0 | 185 ± 102.3 | 195 ± 106 | 155 ± 225 | 200–500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, B.-L.; Liu, P.-C.; Fang, J.-C.; Jongejan, F. Correlation between Babesia Species Affecting Dogs in Taiwan and the Local Distribution of the Vector Ticks. Vet. Sci. 2023, 10, 227. https://doi.org/10.3390/vetsci10030227
Su B-L, Liu P-C, Fang J-C, Jongejan F. Correlation between Babesia Species Affecting Dogs in Taiwan and the Local Distribution of the Vector Ticks. Veterinary Sciences. 2023; 10(3):227. https://doi.org/10.3390/vetsci10030227
Chicago/Turabian StyleSu, Bi-Ling, Pin-Chen Liu, Jou-Chien Fang, and Frans Jongejan. 2023. "Correlation between Babesia Species Affecting Dogs in Taiwan and the Local Distribution of the Vector Ticks" Veterinary Sciences 10, no. 3: 227. https://doi.org/10.3390/vetsci10030227
APA StyleSu, B.-L., Liu, P.-C., Fang, J.-C., & Jongejan, F. (2023). Correlation between Babesia Species Affecting Dogs in Taiwan and the Local Distribution of the Vector Ticks. Veterinary Sciences, 10(3), 227. https://doi.org/10.3390/vetsci10030227






